The Use of Positron-Emission Tomography–Magnetic Resonance Imaging to Improve the Local Staging of Disease in Myxofibrosarcoma: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
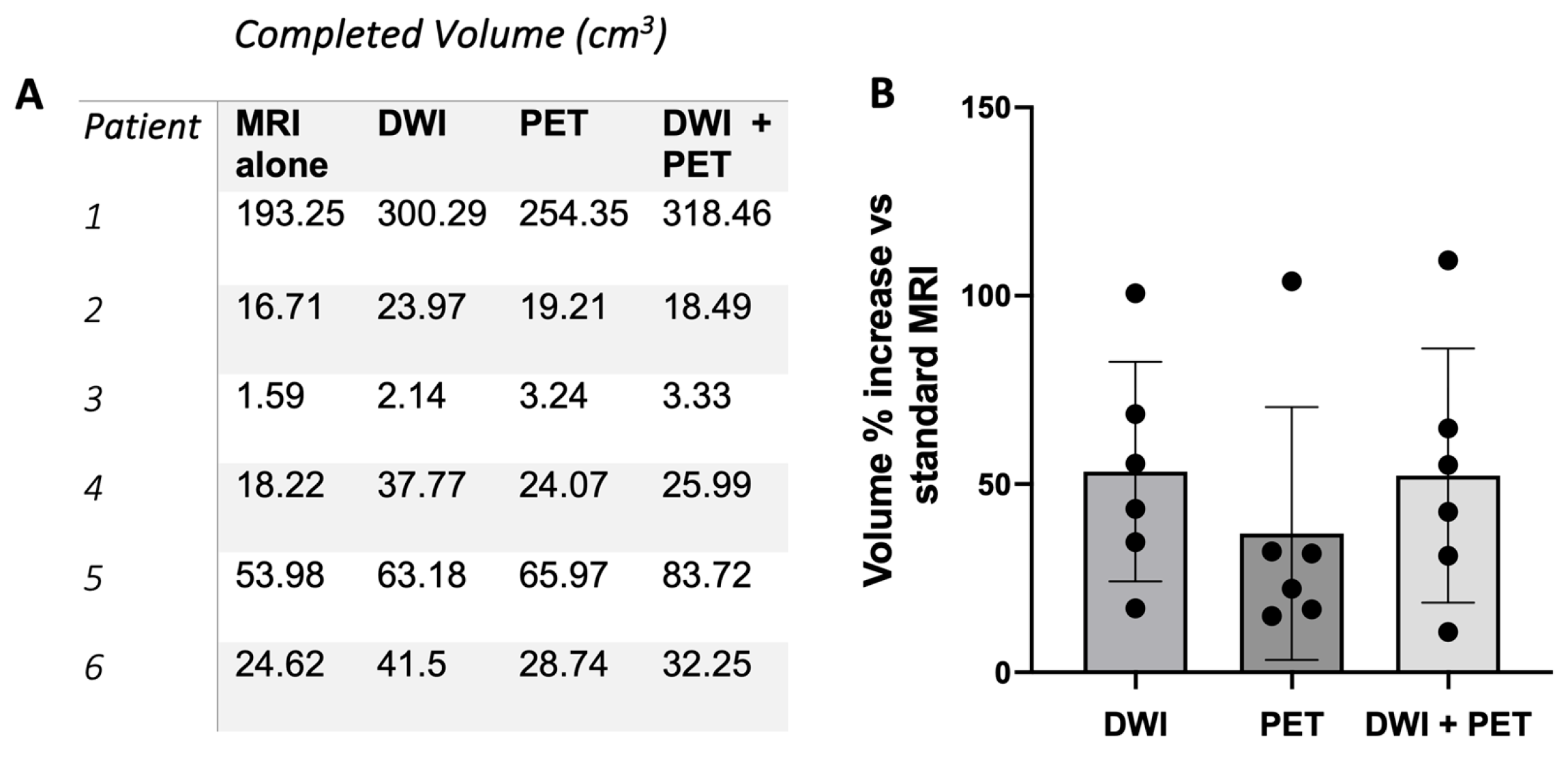
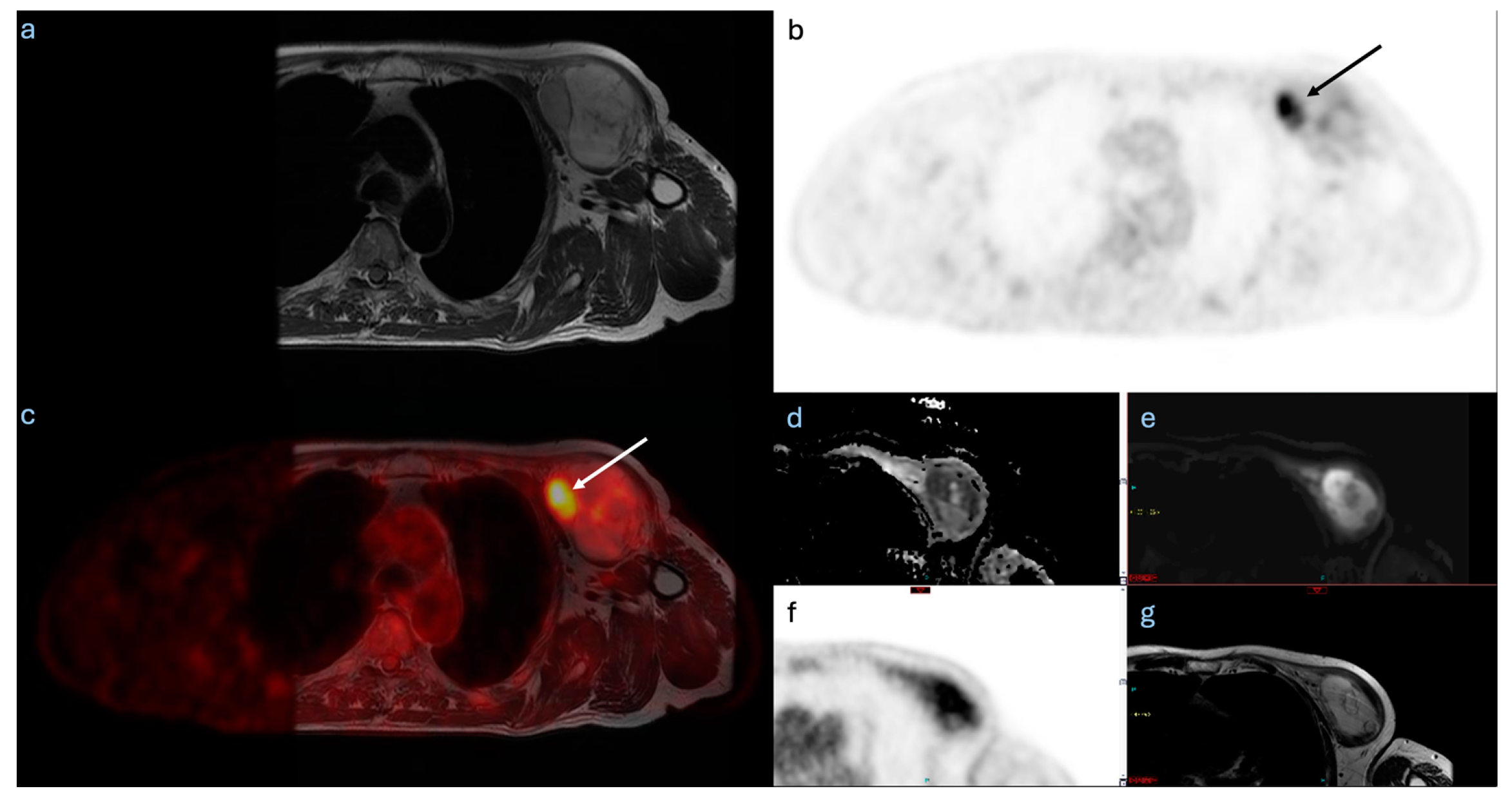
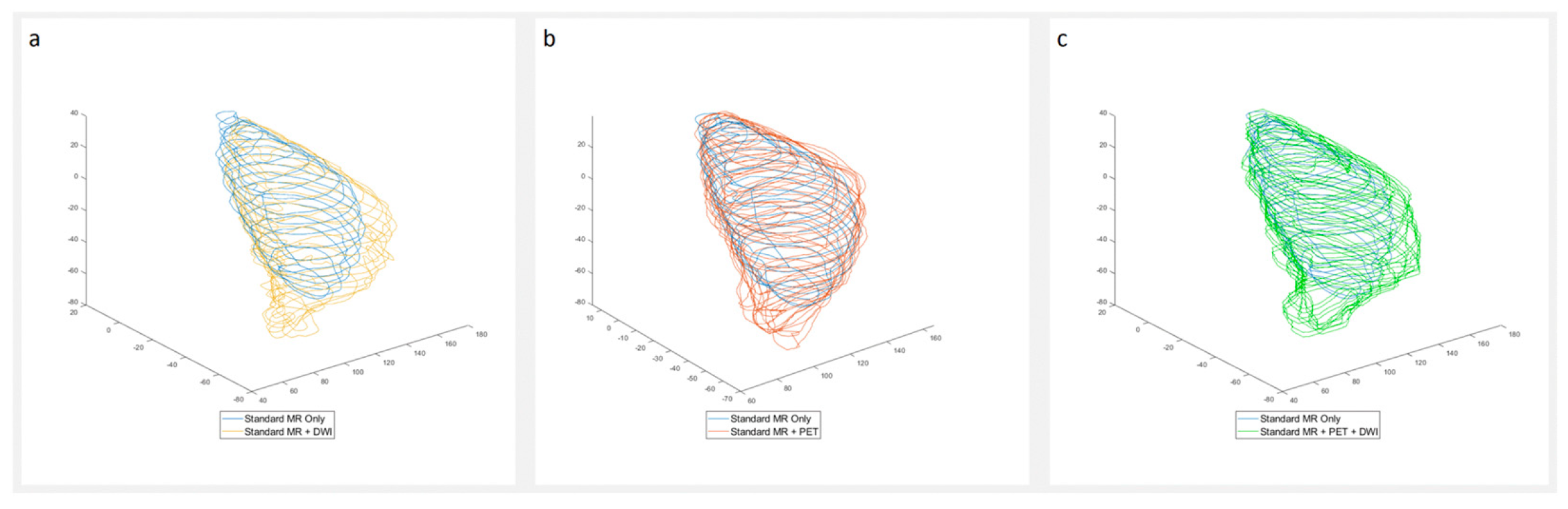
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| DWI | Diffusion-Weighted Imaging |
| FDG | [1⁸F] Fluorodeoxyglucose |
| ICG | Indocyanine Green |
| MFS | Myxofibrosarcoma |
| MRI | Magnetic Resonance Imaging |
| NIRF | Near-Infrared Fluorescence |
| PET | Positron Emission Tomography |
| STS | Soft-Tissue Sarcoma |
| SUV | Standardised Uptake Value |
References
- Orabona, G.D.; Iaconetta, G.; Abbate, V.; Piombino, P.; Romano, A.; Maglitto, F.; Salzano, G.; Califano, L. Head and neck myxofibrosarcoma: A case report and review of the literature. J. Med. Case Rep. 2014, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I.; Spazzoli, B.; Stevens, J.; Hansa, A.; Spelman, T.; Pang, G.; Guiney, M.; Powell, G.; Choong, P.; Di Bella, C. Oncologic outcomes in myxofibrosarcomas: The role of a multidisciplinary approach and surgical resection margins. ANZ J. Surg. 2023, 93, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Wada, T.; Nagoya, S.; Sasaki, M.; Matsumura, T.; Yamaguchi, T.; Hasegawa, T.; Yamashita, T. MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skelet. Radiol. 2008, 37, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Ghazala, C.G.; Agni, N.R.; Ragbir, M.; Dildey, P.; Lee, D.; Rankin, K.S.; Beckingsale, T.B.; Gerrand, C.H. Myxofibrosarcoma of the extremity and trunk. Bone Jt. J. 2016, 98, 1682–1688. [Google Scholar] [CrossRef]
- Haglund, K.E.; Raut, C.P.; Nascimento, A.F.; Wang, Q.; George, S.; Baldini, E.H. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 361–367. [Google Scholar] [CrossRef]
- Odei, B.; Rwigema, J.-C.; Eilber, F.R.; Eilber, F.C.; Selch, M.; Singh, A.; Chmielowski, B.; Nelson, S.D.; Wang, P.-C.; Steinberg, M.; et al. Predictors of Local Recurrence in Patients With Myxofibrosarcoma. Am. J. Clin. Oncol. 2018, 41, 827–831. [Google Scholar] [CrossRef]
- Yurtbay, A.; Coşkun, H.S.; Say, F.; Dabak, N. Is the Thickness of the Margin Associated with Local Recurrence and Survival in Patients with Myxofibrosarcoma? Clin. Orthop. Relat. Res. 2023, 481, 2125–2136. [Google Scholar] [CrossRef]
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Spinnato, P.; Clinca, R. MRI Tail Sign in Soft-Tissue Sarcoma. Radiology 2021, 299, 276. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.A.; Landa, J.; Hwang, S.; Zabor, E.C.; Moskowitz, C.S.; Agaram, N.P.; Panicek, D.M. Myxofibrosarcoma: Prevalence and diagnostic value of the “tail sign” on magnetic resonance imaging. Skelet. Radiol. 2013, 42, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Hong, S.H.; Kang, Y.; Choi, J.-Y.; Moon, K.C.; Kim, H.-S.; Han, I.; Yi, M.; Kang, H.S. MR imaging of myxofibrosarcoma and undifferentiated sarcoma with emphasis on tail sign; diagnostic and prognostic value. Eur. Radiol. 2014, 24, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Gundle, K.R.; Kafchinski, L.; Gupta, S.; Griffin, A.M.; Dickson, B.C.; Chung, P.W.; Catton, C.N.; O’sullivan, B.; Wunder, J.S.; Ferguson, P.C. Analysis of Margin Classification Systems for Assessing the Risk of Local Recurrence After Soft Tissue Sarcoma Resection. J. Clin. Oncol. 2018, 36, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Stevenson, J.; Parry, M.; Tsuda, Y.; Tsoi, K.; Jeys, L. What is an adequate margin for infiltrative soft-tissue sarcomas? Eur. J. Surg. Oncol. 2020, 46, 277–281. [Google Scholar] [CrossRef]
- Willeumier, J.J.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.; Pollock, R.; Aston, W.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; Wunder, J.S.; et al. Individualised risk assessment for local recurrence and distant metastases in a retrospective transatlantic cohort of 687 patients with high-grade soft tissue sarcomas of the extremities: A multistate model. BMJ Open 2017, 7, e012930. [Google Scholar] [CrossRef]
- Brookes, M.J.; Chan, C.D.; Nicoli, F.; Crowley, T.P.; Ghosh, K.M.; Beckingsale, T.; Saleh, D.; Dildey, P.; Gupta, S.; Ragbir, M.; et al. Intraoperative Near-Infrared Fluorescence Guided Surgery Using Indocyanine Green (ICG) for the Resection of Sarcomas May Reduce the Positive Margin Rate: An Extended Case Series. Cancers 2021, 13, 6284. [Google Scholar] [CrossRef]
- Zhang, Q.; Xi, Y.; Li, D.; Yuan, Z.; Dong, J. The utility of 18F-FDG PET and PET/CT in the diagnosis and staging of chondrosarcoma: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 229. [Google Scholar] [CrossRef]
- Mendoza, H.; Nosov, A.; Pandit-Taskar, N. Molecular imaging of sarcomas with FDG PET. Skelet. Radiol. 2022, 52, 461–475. [Google Scholar] [CrossRef]
- Botsikas, D.; Kalovidouri, A.; Becker, M.; Copercini, M.; Djema, D.A.; Bodmer, A.; Monnier, S.; Becker, C.D.; Montet, X.; Delattre, B.M.A.; et al. Clinical utility of 18F-FDG-PET/MR for preoperative breast cancer staging. Eur. Radiol. 2015, 26, 2297–2307. [Google Scholar] [CrossRef]
- Fowler, A.M.; Strigel, R.M. Clinical advances in PET-MRI for breast cancer. Lancet Oncol. 2022, 23, e32–e43. [Google Scholar] [CrossRef]
- Grueneisen, J.; Nagarajah, J.; Buchbender, C.; Hoffmann, O.; Schaarschmidt, B.M.; Poeppel, T.; Forsting, M.; Quick, H.H.; Umutlu, L.; Kinner, S. Positron Emission Tomography/Magnetic Resonance Imaging for Local Tumor Staging in Patients with Primary Breast Cancer: A Comparison with Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging. Investig. Radiol. 2015, 50, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Goorts, B.; Vöö, S.; van Nijnatten, T.J.A.; Kooreman, L.F.S.; de Boer, M.; Keymeulen, K.B.M.I.; Aarnoutse, R.; Wildberger, J.E.; Mottaghy, F.M.; Lobbes, M.B.I.; et al. Hybrid 18F-FDG PET/MRI might improve locoregional staging of breast cancer patients prior to neoadjuvant chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.; Jena, A.; Goel, R.; Sarin, R.; Kaul, S. Simultaneous whole-body 18F-FDG PET-MRI in primary staging of breast cancer: A pilot study. Eur. J. Radiol. 2014, 83, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Galgano, S.J.; Calderone, C.E.; Xie, C.; Smith, E.N.; Porter, K.K.; McConathy, J.E. Applications of PET/MRI in Abdominopelvic Oncology. RadioGraphics 2021, 41, 1750–1765. [Google Scholar] [CrossRef]
- Hong, J.H.; Jee, W.-H.; Jung, C.-K.; Jung, J.-Y.; Shin, S.H.; Chung, Y.-G. Soft tissue sarcoma: Adding diffusion-weighted imaging improves MR imaging evaluation of tumor margin infiltration. Eur. Radiol. 2018, 29, 2589–2597. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Chung, H.W.; Lee, M.H.; Lee, S.H.; Shin, M.J. Usefulness of diffusion-weighted MR imaging for differentiating between benign and malignant superficial soft tissue tumours and tumour-like lesions. Br. J. Radiol. 2016, 89, 20150929. [Google Scholar] [CrossRef]
- Erfanian, Y.; Grueneisen, J.; Kirchner, J.; Wetter, A.; Podleska, L.E.; Bauer, S.; Poeppel, T.; Forsting, M.; Herrmann, K.; Umutlu, L. Integrated 18F-FDG PET/MRI compared to MRI alone for identification of local recurrences of soft tissue sarcomas: A comparison trial. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1823–1831. [Google Scholar] [CrossRef]
- Berner, J.E.; Crowley, T.P.; Teelucksingh, S.; Lee, D.; Ghosh, K.M.; Beckingsale, T.B.; Rankin, K.S.; Ragbir, M. The importance of clear margins in myxofibrosarcoma: Improving local control by means of staged resection and reconstruction. Eur. J. Surg. Oncol. 2021, 47, 2627–2632. [Google Scholar] [CrossRef]
- Crombé, A.; Marcellin, P.-J.; Buy, X.; Stoeckle, E.; Brouste, V.; Italiano, A.; Le Loarer, F.; Kind, M. Soft-Tissue Sarcomas: Assessment of MRI Features Correlating with Histologic Grade and Patient Outcome. Radiology 2019, 291, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Sedaghat, S. Inferring malignancy grade of soft tissue sarcomas from magnetic resonance imaging features: A systematic review. Eur. J. Radiol. 2024, 177, 111548. [Google Scholar] [CrossRef] [PubMed]
- Spinnato, P.; Clinca, R.; Vara, G.; Cesari, M.; Ponti, F.; Facchini, G.; Longhi, A.; Donati, D.M.; Bianchi, G.; Sambri, A. MRI Features as Prognostic Factors in Myxofibrosarcoma: Proposal of MRI Grading System. Acad. Radiol. 2021, 28, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Prosch, H.; Beer, L.; Tamandl, D.; Beyer, T.; Hoeller, C.; Berzaczy, D.; Raderer, M.; Preusser, M.; Hochmair, M.; et al. PET/MRI versus PET/CT in oncology: A prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 51–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pringle, T.A.; Chan, C.D.; Luli, S.; Blair, H.J.; Rankin, K.S.; Knight, J.C. Synthesis and In Vivo Evaluation of a Site-specifically Labeled Radioimmunoconjugate for Dual-Modal (PET/NIRF) Imaging of MT1-MMP in Sarcomas. Bioconjug. Chem. 2022, 33, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Nishio, J.; Nakayama, S. Biology and Management of High-Grade Myxofibrosarcoma: State of the Art and Future Perspectives. Diagnostics 2023, 13, 3022. [Google Scholar] [CrossRef]
- Gilg, M.M.; Sunitsch, S.; Leitner, L.; Bergovec, M.; Szkandera, J.; Leithner, A.; Liegl-Atzwanger, B. Tumor-associated mortality and prognostic factors in myxofibrosarcoma-A retrospective review of 109 patients. Orthop. Traumatol. Surg. Res. 2020, 106, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, Z.; Qiu, J.; Lu, W. Performance and application of the total-body PET/CT scanner: A literature review. EJNMMI Res. 2024, 14, 38. [Google Scholar] [CrossRef]
| Patient | Gender | Age at Scan | Time from Scan to Surgery (Days) | Margin Status | Re-Excision | Local Recurrence |
|---|---|---|---|---|---|---|
| 1 | M | 63 | 11 | Positive | Y | N |
| 2 | M | 65 | 2 | Negative | N | N |
| 3 | M | 67 | 3 | Close | Y | N |
| 4 | M | 54 | 1 | Close | Y | N |
| 5 | M | 77 | 8 | Close | Y | Y |
| 6 | M | 81 | 8 | Close | Y | N |
| Case | Ki67 | Mitotic Rate | SUV Max |
|---|---|---|---|
| 1 | 66 | 35 | 7 |
| 2 | 50 | 29 | 5.4 |
| 3 | 30 | 14 | 2.5 |
| 4 | 50 | 26 | 6 |
| 5 | 80 | 110 | 29.3 |
| 6 | 50 | 23 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.D.; Brookes, M.J.; Ali, T.; Howell, E.; Dildey, P.; Firbank, M.; Pearson, R.; Sloan, P.; Lowes, S.; Sinha, R.; et al. The Use of Positron-Emission Tomography–Magnetic Resonance Imaging to Improve the Local Staging of Disease in Myxofibrosarcoma: A Feasibility Study. Diagnostics 2025, 15, 1039. https://doi.org/10.3390/diagnostics15081039
Chan CD, Brookes MJ, Ali T, Howell E, Dildey P, Firbank M, Pearson R, Sloan P, Lowes S, Sinha R, et al. The Use of Positron-Emission Tomography–Magnetic Resonance Imaging to Improve the Local Staging of Disease in Myxofibrosarcoma: A Feasibility Study. Diagnostics. 2025; 15(8):1039. https://doi.org/10.3390/diagnostics15081039
Chicago/Turabian StyleChan, Corey D., Marcus J. Brookes, Tamir Ali, Elizabeth Howell, Petra Dildey, Michael Firbank, Rachel Pearson, Philip Sloan, Simon Lowes, Raj Sinha, and et al. 2025. "The Use of Positron-Emission Tomography–Magnetic Resonance Imaging to Improve the Local Staging of Disease in Myxofibrosarcoma: A Feasibility Study" Diagnostics 15, no. 8: 1039. https://doi.org/10.3390/diagnostics15081039
APA StyleChan, C. D., Brookes, M. J., Ali, T., Howell, E., Dildey, P., Firbank, M., Pearson, R., Sloan, P., Lowes, S., Sinha, R., Tuckett, J., Ragbir, M., Beckingsale, T., Hide, G., Gerrand, C., Rankin, K. S., & Petrides, G. S. (2025). The Use of Positron-Emission Tomography–Magnetic Resonance Imaging to Improve the Local Staging of Disease in Myxofibrosarcoma: A Feasibility Study. Diagnostics, 15(8), 1039. https://doi.org/10.3390/diagnostics15081039







