Multimodality Imaging in Apical Hypertrophic Cardiomyopathy: Can Echocardiography Learn from Cardiac Magnetic Resonance?
Abstract
1. Introduction
2. Overview of ApHCM
3. Multimodal Imaging: TTE and CMRi as Main Actors
4. Echocardiography
4.1. Obstruction
4.2. Apical Obliteration
4.3. Speckle Tracking Imaging
4.4. Perfusion
5. Differential Diagnosis and Interpretative Pitfalls
6. Cardiac Magnetic Resonance Imaging
6.1. Cine-MR and Morphology
6.2. Feature Tracking and Strain
6.3. Tissue Characterization
6.3.1. T1 Mapping and ECV
6.3.2. T2 Mapping
6.3.3. Late Gadolinium Enhancement
6.4. Perfusion and Microvascularity
Intracavitary Dynamics and 4D-Flow
6.5. Practical Limitations and Technological Developments
7. Diagnosis and Novel Diagnostic Criteria
8. What Echocardiography Can Learn from CMRi and Future Perspectives
9. Clinical Implications
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCM | hypertrophic cardiomyopathy |
| LV | left ventricle |
| ApHCM | apical hypertrophic cardiomyopathy |
| TTE | transthoracic echocardiography |
| CMRi | cardiac magnetic resonance imaging |
| ECG | electrocardiogram |
| EF | ejection fraction |
| LVOT | left ventricular outflow tract |
| MVO | mid-ventricular obstruction |
| LVOTO | left ventricular outflow tract obstruction |
| CT | computed tomography |
| SPECT | single-photon emission computed tomography |
| PET | positron emission tomography |
| EACVI | European Association of Cardiovascular Imaging |
| ASE | American Society of Echocardiography |
| TEE | transesophageal echocardiography |
| CW | continuous-wave |
| GLS | global longitudinal strain |
| 3D | three-dimensional |
| SSFP | steady-state free-precession |
| SAM | systolic anterior motion |
| LGE | late gadolinium enhancement |
| ECV | extracellular volume |
| PSIR | phase-sensitive inversion recovery |
| MBF | myocardial blood flow |
| MPR | myocardial perfusion reserve |
| 4D-FLOW | four-dimensional flow |
| VFM | vector flow mapping |
| BSI | blood speckle |
References
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Coleman, J.A.; Ashkir, Z.; Raman, B.; Bueno-Orovio, A. Mechanisms and prognostic impact of myocardial ischaemia in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2023, 39, 1979–1996. [Google Scholar] [CrossRef]
- Aro, A.L.; Nair, S.G.; Reinier, K.; Jayaraman, R.; Stecker, E.C.; Uy-Evanado, A.; Rusinaru, C.; Jui, J.; Chugh, S.S. Population Burden of Sudden Death Associated with Hypertrophic Cardiomyopathy. Circulation 2017, 136, 1665–1667. [Google Scholar] [CrossRef] [PubMed]
- Dicorato, M.M.; Citarelli, G.; Mangini, F.; Alemanni, R.; Albanese, M.; Cicco, S.; Greco, C.A.; Forleo, C.; Basile, P.; Carella, M.C.; et al. Integrative Approaches in the Management of Hypertrophic Cardiomyopathy: A Comprehensive Review of Current Therapeutic Modalities. Biomedicines 2025, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, C.; Ye, J.; Liu, J. Clinical characteristics and long-term outcomes in patients with apical hypertrophic cardiomyopathy. ESC Heart Fail. 2025, 12, 2887–2897. [Google Scholar] [CrossRef] [PubMed]
- Dentamaro, I.; Dicorato, M.M.; Falagario, A.; Cicco, S.; Dentamaro, S.; Correale, M.; Manuppelli, V.; Citarelli, G.; Mangini, F.; Fiore, C.; et al. The Evolving Role of Cardiac Imaging in Hypertrophic Cardiomyopathy: Diagnosis, Prognosis, and Clinical Practice. Biomedicines 2025, 13, 2138. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Ishimura, T.; Nishiyama, S.; Nagasaki, F.; Nakanishi, S.; Takatsu, F.; Nishijo, T.; Umeda, T.; Machii, K. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): Ventriculographic and echocardiographic features in 30 patients. Am. J. Cardiol. 1979, 44, 401–412. [Google Scholar] [CrossRef]
- Cardim, N.; Haugaa, K.; Mohiddin, S.A.; Hinojar, R.; Hirsch, A.; Szabo, L.; Podlesnikar, T.; Dall’ARmellina, E.; Cameli, M.; Mandoli, G.E.; et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy in 2025. A Clinical Consensus Statement of the European Association of Cardiovascular Imaging (EACVI) of the ESC. Eur. Heart J. Cardiovasc. Imaging 2025, jeaf282. [Google Scholar] [CrossRef]
- Sakamoto, T. Apical hypertrophic cardiomyopathy (apical hypertrophy): An overview. J. Cardiol. 2001, 37 (Suppl. 1), 161–178. [Google Scholar]
- Yang, K.; Song, Y.Y.; Chen, X.Y.; Wang, J.X.; Li, L.; Yin, G.; Zheng, Y.C.; Wei, M.D.; Lu, M.J.; Zhao, S.H. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: Prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1341–1350. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.; Link, M.S.; Maron, M.S. Hypertrophic Cardiomyopathy with Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J. Am. Coll. Cardiol. 2017, 69, 761–773. [Google Scholar] [CrossRef]
- Kitaoka, H.; Doi, Y.; Casey, S.A.; Hitomi, N.; Furuno, T.; Maron, B.J. Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am. J. Cardiol. 2003, 92, 1183–1186. [Google Scholar] [CrossRef]
- Eriksson, M.J.; Sonnenberg, B.; Woo, A.; Rakowski, P.; Parker, T.G.; Wigle, E.; Rakowski, H. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Paluszkiewicz, J.; Krasinska, B.; Milting, H.; Gummert, J.; Pyda, M. Apical hypertrophic cardiomyopathy: Diagnosis, medical and surgical treatment. Kardiochir. Torakochirurgia Pol. 2018, 15, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Bernard, S.; Tripathi, N.; Patel, Y.; Modi, V.; Axel, L.; Talebi, S.; Ghoshhajra, B.B.; Sanborn, D.Y.; Saric, M.; et al. Apical Aneurysms and Mid-Left Ventricular Obstruction in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2023, 16, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Z.J.; Montazeri, M.; Bataiosu, R.; Hoss, S.; Adler, A.; Nguyen, E.T.; Rakowski, H.; Chan, R.H. Clinical Characteristics and Prognostic Importance of Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2022, 15, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Hoey, E.T.; Teoh, J.K.; Das, I.; Ganeshan, A.; Simpson, H.; Watkin, R.W. The emerging role of cardiovascular MRI for risk stratification in hypertrophic cardiomyopathy. Clin. Radiol. 2014, 69, 221–230. [Google Scholar] [CrossRef]
- Hughes, R.K.; Augusto, J.B.; Knott, K.; Davies, R.; Shiwani, H.; Seraphim, A.; Malcolmson, J.W.; Khoury, S.; Joy, G.; Mohiddin, S.; et al. Apical Ischemia Is a Universal Feature of Apical Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2023, 16, e014907. [Google Scholar] [CrossRef]
- Hamza, I.; Odigie-Okon, E.; Xie, T.; Ahmad, M. Apical Hypertrophic Cardiomyopathy: A Clinical & Multimodality Imaging Assessment. Echocardiography 2025, 42, e70235. [Google Scholar] [CrossRef]
- Pradeep Kundur, S.; Malik, A.; Sivalokanathan, S. The Utility of Nuclear Imaging in Hypertrophic Cardiomyopathy: A Narrative Review. J. Clin. Med. 2025, 14, 2183. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef]
- Hughes, R.K.; Knott, K.D.; Malcolmson, J.; Augusto, J.B.; Mohiddin, S.A.; Kellman, P.; Moon, J.C.; Captur, G. Apical Hypertrophic Cardiomyopathy: The Variant Less Known. J. Am. Heart Assoc. 2020, 9, e015294. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.K.; Shiwani, H.; Rosmini, S.; Augusto, J.B.; Burke, L.; Jiang, Y.; Pierce, I.; Joy, G.; Castelletti, S.; Orini, M.; et al. Improved Diagnostic Criteria for Apical Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2024, 17, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Kubo, T.; Inoue, S.; Nomura, S.; Yamada, T.; Tobita, T.; Kuramoto, Y.; Miyashita, Y.; Asano, Y.; Ochi, Y.; et al. Unveiling Clinical and Genetic Distinctions in Pure-Apical Versus Distal-Dominant Apical Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2025, 14, e038208. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Porter, T.R.; Mulvagh, S.L.; Abdelmoneim, S.S.; Becher, H.; Belcik, J.T.; Bierig, M.; Choy, J.; Gaibazzi, N.; Gillam, L.D.; Janardhanan, R.; et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J. Am. Soc. Echocardiogr. 2018, 31, 241–274. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Abdelmoneim, S.; Belcik, J.T.; McCulloch, M.L.; Mulvagh, S.L.; Olson, J.J.; Porcelli, C.; Tsutsui, J.M.; Wei, K. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: A focused update from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Saccheri, M.C.; Cianciulli, T.F.; Morita, L.A.; Méndez, R.J.; Beck, M.A.; Guerra, J.E.; Cozzarin, A.; Puente, L.J.; Balletti, L.R.; Lax, J.A. Speckle tracking echocardiography to assess regional ventricular function in patients with apical hypertrophic cardiomyopathy. World J. Cardiol. 2017, 9, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Po, J.R.; Kim, B.; Aslam, F.; Arabadjian, M.; Winson, G.; Cantales, D.; Kushner, J.; Kornberg, R.; Sherrid, M.V. Doppler Systolic Signal Void in Hypertrophic Cardiomyopathy: Apical Aneurysm and Severe Obstruction without Elevated Intraventricular Velocities. J. Am. Soc. Echocardiogr. 2015, 28, 1462–1473. [Google Scholar] [CrossRef]
- Nakamura, T.; Kitamura, H.; Furukawa, K.; Matsubara, K.; Katahira, T.; Okamuro, S.; Tsuji, Y.; Takahashi, T.; Kunishige, H.; Katsume, H. Intraventricular flow dynamics in hypertrophic cardiomyopathy with midventricular obstruction investigated by Doppler echocardiography. J. Cardiol. 1989, 19, 455–471. [Google Scholar]
- Rakowski, H.; Sasson, Z.; Wigle, E.D. Echocardiographic and Doppler assessment of hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 1988, 1, 31–47. [Google Scholar] [CrossRef]
- Mihos, C.G.; Horvath, S.A.; Fernandez, R.; Escolar, E. Left ventricular strain and myocardial work in apical hypertrophic cardiomyopathy. J. Thorac. Dis. 2023, 15, 3197–3207. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; van der Bijl, P.; El Mahdiui, M.; Bax, J.J.; Delgado, V.; Marsan, N.A. Myocardial Work in Nonobstructive Hypertrophic Cardiomyopathy: Implications for Outcome. J. Am. Soc. Echocardiogr. 2020, 33, 1201–1208. [Google Scholar] [CrossRef]
- Hoffmann, R.; von Bardeleben, S.; Cate, F.T.; Borges, A.C.; Kasprzak, J.; Firschke, C.; Lafitte, S.; Al-Saadi, N.; Kuntz-Hehner, S.; Engelhardt, M.; et al. Assessment of systolic left ventricular function: A multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur. Heart J. 2005, 26, 607–616. [Google Scholar] [CrossRef]
- Pathan, F.; Marwick, T.H. Myocardial perfusion imaging using contrast echocardiography. Prog. Cardiovasc. Dis. 2015, 57, 632–643. [Google Scholar] [CrossRef]
- White, R.D.; Obuchowski, N.A.; Gunawardena, S.; Lipchik, E.O.; Lever, H.M.; Van Dyke, C.W.; Lytle, B.W. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy: Presurgical and postsurgical evaluation by computed tomography magnetic resonance imaging. Am. J. Card. Imaging 1996, 10, 1–13. [Google Scholar] [PubMed]
- Chu, L.C.; Porter, K.K.; Corona-Villalobos, C.P.; Gülsün, M.A.; Shea, S.M.; Markl, M.; Abraham, T.P.; Bluemke, D.A.; Kamel, I.R.; Zimmerman, S.L. Evaluation of Left Ventricular Outflow Tract Obstruction with Four-Dimensional Phase Contrast Magnetic Resonance Imaging in Patients with Hypertrophic Cardiomyopathy-A Pilot Study. J. Comput. Assist. Tomogr. 2016, 40, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.W.; Merchant, N. MRI of hypertrophic cardiomyopathy: Part I, MRI appearances. Am. J. Roentgenol. 2007, 189, 1335–1343. [Google Scholar] [CrossRef]
- Vasquez, N.; Ostrander, B.T.; Lu, D.Y.; Ventoulis, I.; Haileselassie, B.; Goyal, S.; Greenland, G.V.; Vakrou, S.; Olgin, J.E.; Abraham, T.P.; et al. Low Left Atrial Strain Is Associated with Adverse Outcomes in Hypertrophic Cardiomyopathy Patients. J. Am. Soc. Echocardiogr. 2019, 32, 593–603.e1. [Google Scholar] [CrossRef]
- Mangini, F.; Scarcia, M.; Biederman, R.W.W.; Calbi, R.; Spinelli, F.; Casavecchia, G.; Brunetti, N.D.; Gravina, M.; Fiore, C.; Suma, S.; et al. Cardiac magnetic resonance imaging in the evaluation and management of mitral valve prolapse—A comprehensive review. Echocardiography 2024, 41, e15894. [Google Scholar] [CrossRef]
- Cavus, E.; Muellerleile, K.; Schellert, S.; Schneider, J.; Tahir, E.; Chevalier, C.; Jahnke, C.; Radunski, U.K.; Adam, G.; Kirchhof, P.; et al. CMR feature tracking strain patterns and their association with circulating cardiac biomarkers in patients with hypertrophic cardiomyopathy. Clin. Res. Cardiol. 2021, 110, 1757–1769. [Google Scholar] [CrossRef]
- Li, Z.L.; He, S.; Xia, C.C.; Peng, W.L.; Li, L.; Liu, K.L.; Zhang, J.G.; Pu, J.; Guo, Y.K. Global longitudinal diastolic strain rate as a novel marker for predicting adverse outcomes in hypertrophic cardiomyopathy by cardiac magnetic resonance tissue tracking. Clin. Radiol. 2021, 76, e19–e78. [Google Scholar] [CrossRef]
- Pu, C.; Fei, J.; Lv, S.; Wu, Y.; He, C.; Guo, D.; Mabombo, P.U.; Chooah, O.; Hu, H. Global Circumferential Strain by Cardiac Magnetic Resonance Tissue Tracking Associated with Ventricular Arrhythmias in Hypertrophic Cardiomyopathy Patients. Front. Cardiovasc. Med. 2021, 8, 670361. [Google Scholar] [CrossRef]
- Sharifian, M.; Rezaeian, N.; Asadian, S.; Mohammadzadeh, A.; Nahardani, A.; Kasani, K.; Toloueitabar, Y.; Farahmand, A.M.; Hosseini, L. Efficacy of Novel Noncontrast Cardiac Magnetic Resonance Methods in Indicating Fibrosis in Hypertrophic Cardiomyopathy. Cardiol. Res. Pract. 2021, 2021, 9931136. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Gravina, M.; Casavecchia, G.; Mangini, F.; Mautone, F.; Ruggeri, D.; Guglielmi, G.; Macarini, L.; Brunetti, N.D. Magnetic resonance mapping for the assessment of cardiomyopathies and myocardial disease. Int. J. Cardiol. 2024, 415, 132440. [Google Scholar] [CrossRef]
- Forleo, C.; Carella, M.C.; Basile, P.; Mandunzio, D.; Greco, G.; Napoli, G.; Carulli, E.; Dicorato, M.M.; Dentamaro, I.; Santobuono, V.E.; et al. The Role of Magnetic Resonance Imaging in Cardiomyopathies in the Light of New Guidelines: A Focus on Tissue Mapping. J. Clin. Med. 2024, 13, 2621. [Google Scholar] [CrossRef]
- Xu, J.; Zhuang, B.; Sirajuddin, A.; Li, S.; Huang, J.; Yin, G.; Song, L.; Jiang, Y.; Zhao, S.; Lu, M. MRI T1 Mapping in Hypertrophic Cardiomyopathy: Evaluation in Patients Without Late Gadolinium Enhancement and Hemodynamic Obstruction. Radiology 2020, 294, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.K.; Knott, K.D.; Malcolmson, J.; Augusto, J.B.; Kellman, P.; Moon, J.C.; Captur, G. Advanced Imaging Insights in Apical Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2020, 13 Pt 2, 624–630. [Google Scholar] [CrossRef]
- Maurizi, N.; Antiochos, P.; Monney, P. Takotsubo cardiomyopathy mimicking an apical hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2024, 40, 1389–1391. [Google Scholar] [CrossRef]
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634. [Google Scholar] [CrossRef] [PubMed]
- Kellman, P.; Arai, A.E.; McVeigh, E.R.; Aletras, A.H. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn. Reson. Med. 2002, 47, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Holtackers, R.J.; Van De Heyning, C.M.; Chiribiri, A.; Wildberger, J.E.; Botnar, R.M.; Kooi, M.E. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: A review of current techniques. J. Cardiovasc. Magn. Reson. 2021, 23, 96. [Google Scholar] [CrossRef]
- Basile, P.; Soldato, N.; Pedio, E.; Siena, P.; Carella, M.C.; Dentamaro, I.; Khan, Y.; Baggiano, A.; Mushtaq, S.; Forleo, C.; et al. Cardiac magnetic resonance reveals concealed structural heart disease in patients with frequent premature ventricular contractions and normal echocardiography: A systematic review. Int. J. Cardiol. 2024, 412, 132306. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, S.; Yu, S.; Wu, G.; Wang, D.; Liu, L.; Song, J.; Zhu, Y.; Kang, L.; Wang, J.; et al. Patterns of Replacement Fibrosis in Hypertrophic Cardiomyopathy. Radiology 2022, 302, 298–306. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Dicorato, M.M.; Basile, P.; Naccarati, M.L.; Carella, M.C.; Dentamaro, I.; Falagario, A.; Cicco, S.; Forleo, C.; Guaricci, A.I.; Ciccone, M.M.; et al. Predicting New-Onset Atrial Fibrillation in Hypertrophic Cardiomyopathy: A Review. J. Clin. Med. 2025, 14, 2018. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Ma, X.; Zhao, K.; Yang, K.; Yu, S.; Yin, G.; Dong, Z.; Song, Y.; Cui, C.; et al. Assessment of late gadolinium enhancement in hypertrophic cardiomyopathy improves risk stratification based on current guidelines. Eur. Heart J. 2023, 44, 4781–4792. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, J.; Xu, J.; Zhuang, B.; Wu, B.; Wei, B. Patients who do not fulfill criteria for hypertrophic cardiomyopathy but have unexplained giant T-wave inversion: A cardiovascular magnetic resonance mid-term follow-up study. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2021, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Adler, A.; Fardfini, K.; Hoss, S.; Hanneman, K.; Rowin, E.J.; Maron, M.S.; Maron, B.J.; Rakowski, H.; Chan, R.H. Progression of Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Cardiac Magnetic Resonance Study. JACC Cardiovasc. Imaging 2021, 14, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Chiribiri, A.; Leuzzi, S.; Conte, M.R.; Bongioanni, S.; Bratis, K.; Olivotti, L.; De Rosa, C.; Lardone, E.; Di Donna, P.; Villa, A.; et al. Rest perfusion abnormalities in hypertrophic cardiomyopathy: Correlation with myocardial fibrosis and risk factors for sudden cardiac death. Clin. Radiol. 2015, 70, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.C.; Chang, S.A.; Jang, S.Y.; Kim, S.M.; Park, S.J.; Choi, J.O.; Park, S.W.; Jeon, E.S.; Choe, Y.H. Prevalence and clinical significance of cardiovascular magnetic resonance adenosine stress-induced myocardial perfusion defect in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 30. [Google Scholar] [CrossRef]
- Ismail, T.F.; Hsu, L.Y.; Greve, A.M.; Gonçalves, C.; Jabbour, A.; Gulati, A. Coronary microvascular ischemia in hypertrophic cardiomyopathy—A pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2014, 16, 49. [Google Scholar] [CrossRef]
- Camaioni, C.; Knott, K.D.; Augusto, J.B.; Seraphim, A.; Rosmini, S.; Ricci, F.; Boubertakh, R.; Xue, H.; Hughes, R.; Captur, G.; et al. Inline perfusion mapping provides insights into the disease mechanism in hypertrophic cardiomyopathy. Heart 2020, 106, 824–829. [Google Scholar] [CrossRef]
- Crandon, S.; Elbaz, M.S.M.; Westenberg, J.J.M.; van der Geest, R.J.; Plein, S.; Garg, P. Clinical applications of intra-cardiac four-dimensional flow cardiovascular magnetic resonance: A systematic review. Int. J. Cardiol. 2017, 249, 486–493. [Google Scholar] [CrossRef]
- El Mathari, S.; van Ooij, P.; Merton, R.; Schrauben, E.; Hopman, L.; Nederveen, A.; Götte, M.; Kluin, J. Feasibility of 4D-flow CMR for haemodynamic characterization in hypertrophic cardiomyopathy after septal myectomy with and without anterior mitral valve leaflet extension. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 40, ivae210. [Google Scholar] [CrossRef]
- Zhao, K.; Zhu, Y.; Chen, X.; Yang, S.; Yan, W.; Yang, K.; Song, Y.; Cui, C.; Xu, X.; Zhu, Q.; et al. Machine Learning in Hypertrophic Cardiomyopathy: Nonlinear Model From Clinical and CMR Features Predicting Cardiovascular Events. JACC Cardiovasc. Imaging 2024, 17, 880–893. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, L.H.; Plein, S.; Garg, P.; van der Geest, R.J. Deep learning based automated left ventricle segmentation and flow quantification in 4D flow cardiac MRI. J. Cardiovasc. Magn. Reson. 2024, 26, 100003. [Google Scholar] [CrossRef]
- Flett, A.S.; Maestrini, V.; Milliken, D.; Fontana, M.; Treibel, T.A.; Harb, R. Diagnosis of apical hypertrophic cardiomyopathy: T-wave inversion and relative but not absolute apical left ventricular hypertrophy. Int. J. Cardiol. 2015, 183, 143–148. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.H.; Won, K.B.; Yoon, H.J.; Park, H.S.; Cho, Y.K. Significance of apical cavity obliteration in apical hypertrophic cardiomyopathy. Heart 2016, 102, 1215–1220. [Google Scholar] [CrossRef]
- Filomena, D.; Vandenberk, B.; Dresselaers, T.; Willems, R.; Van Cleemput, J.; Olivotto, I.; Robyns, T.; Bogaert, J. Apical papillary muscle displacement is a prevalent feature and a phenotypic precursor of apical hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1009–1016. [Google Scholar] [CrossRef]
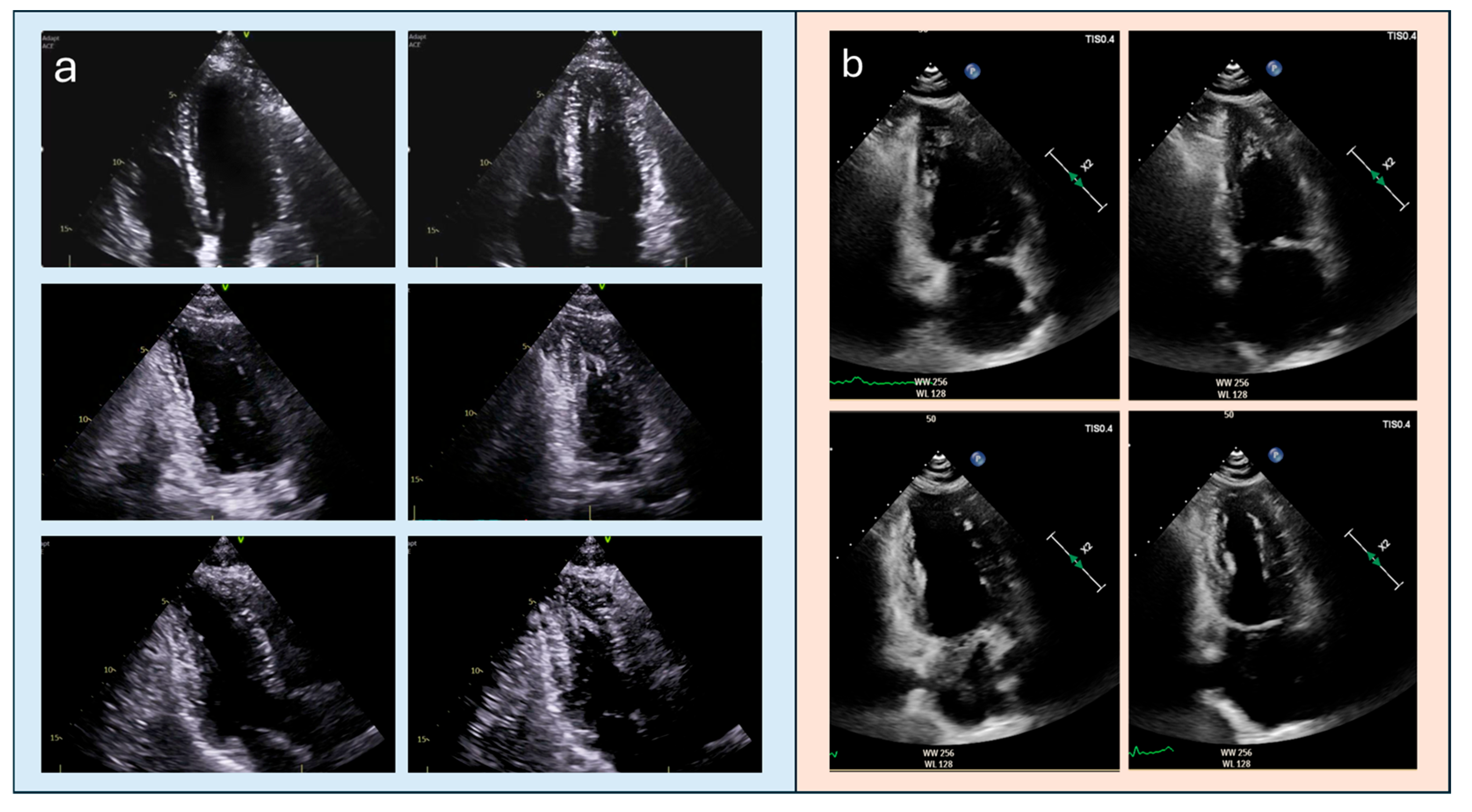
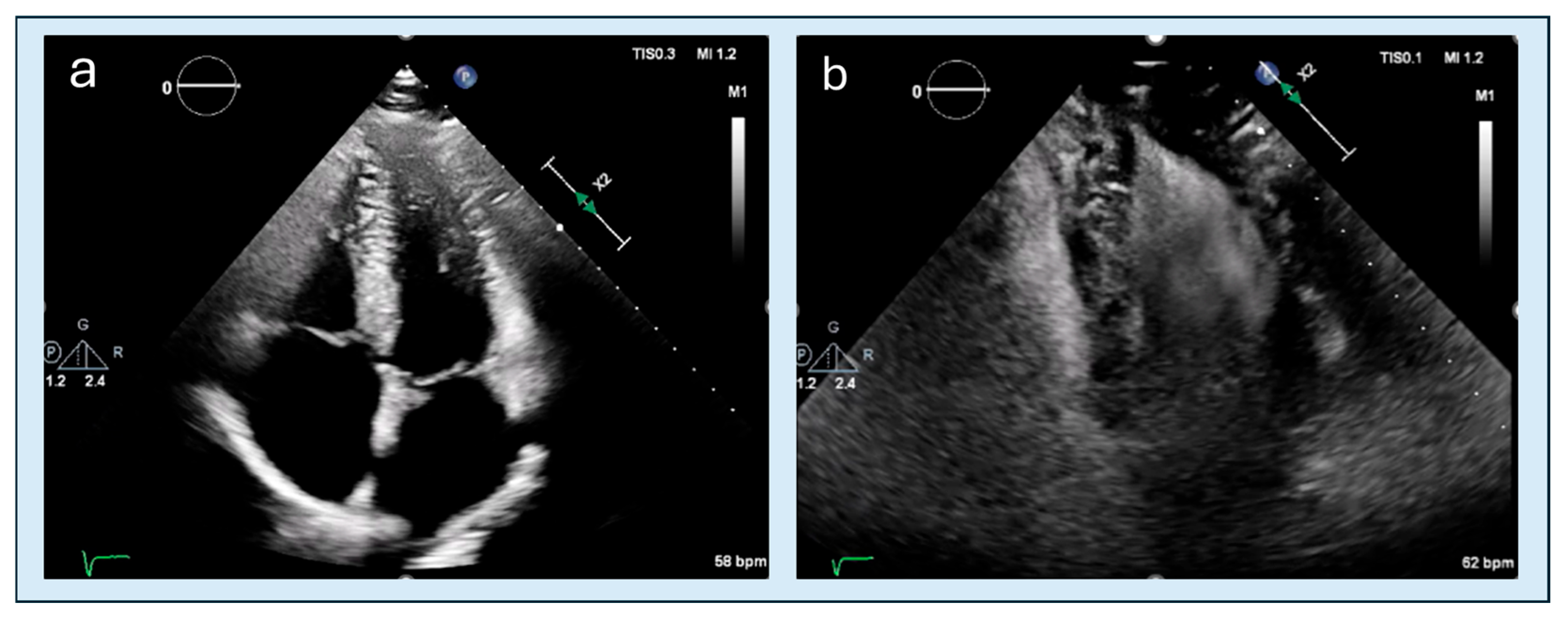
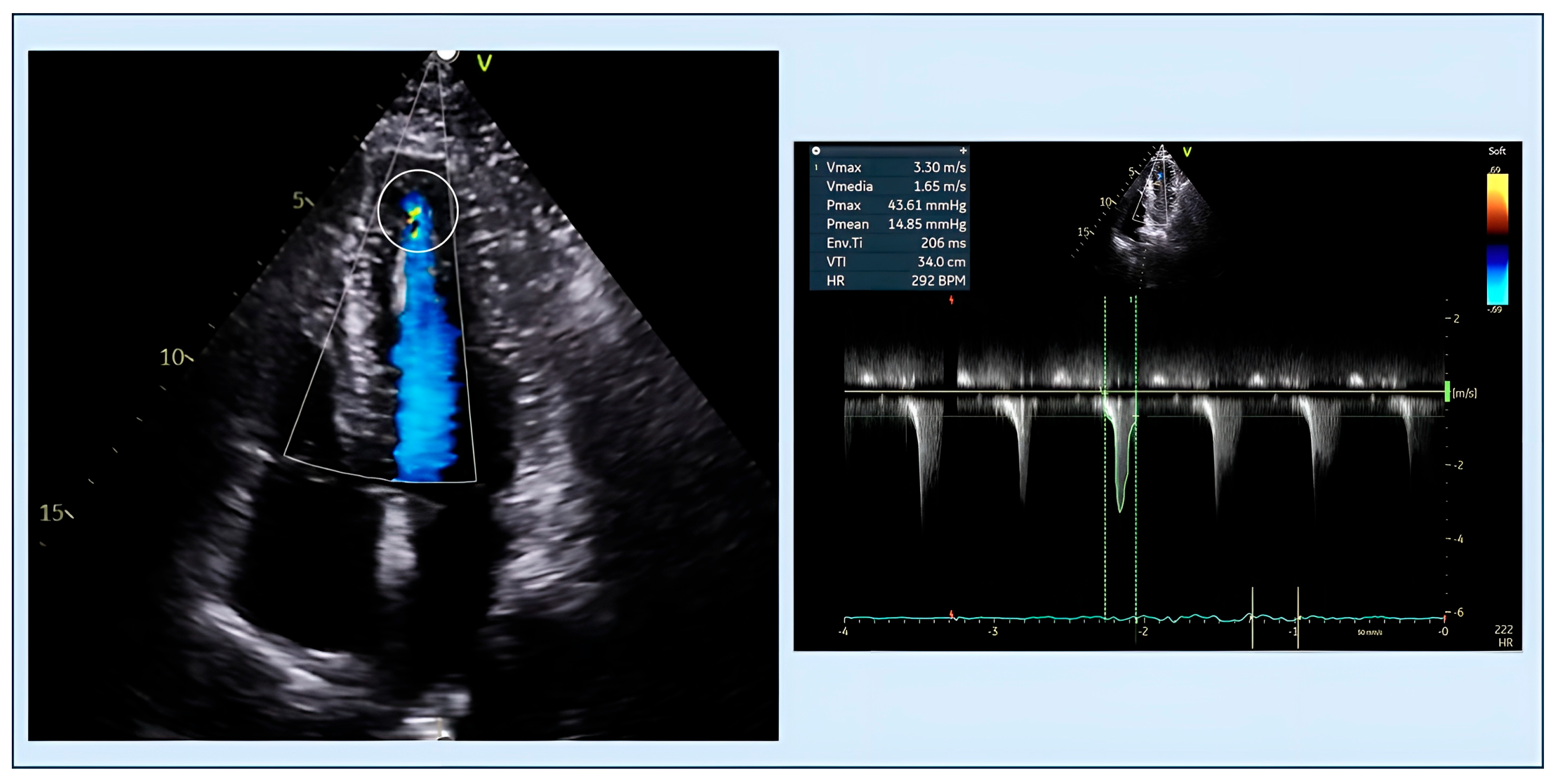
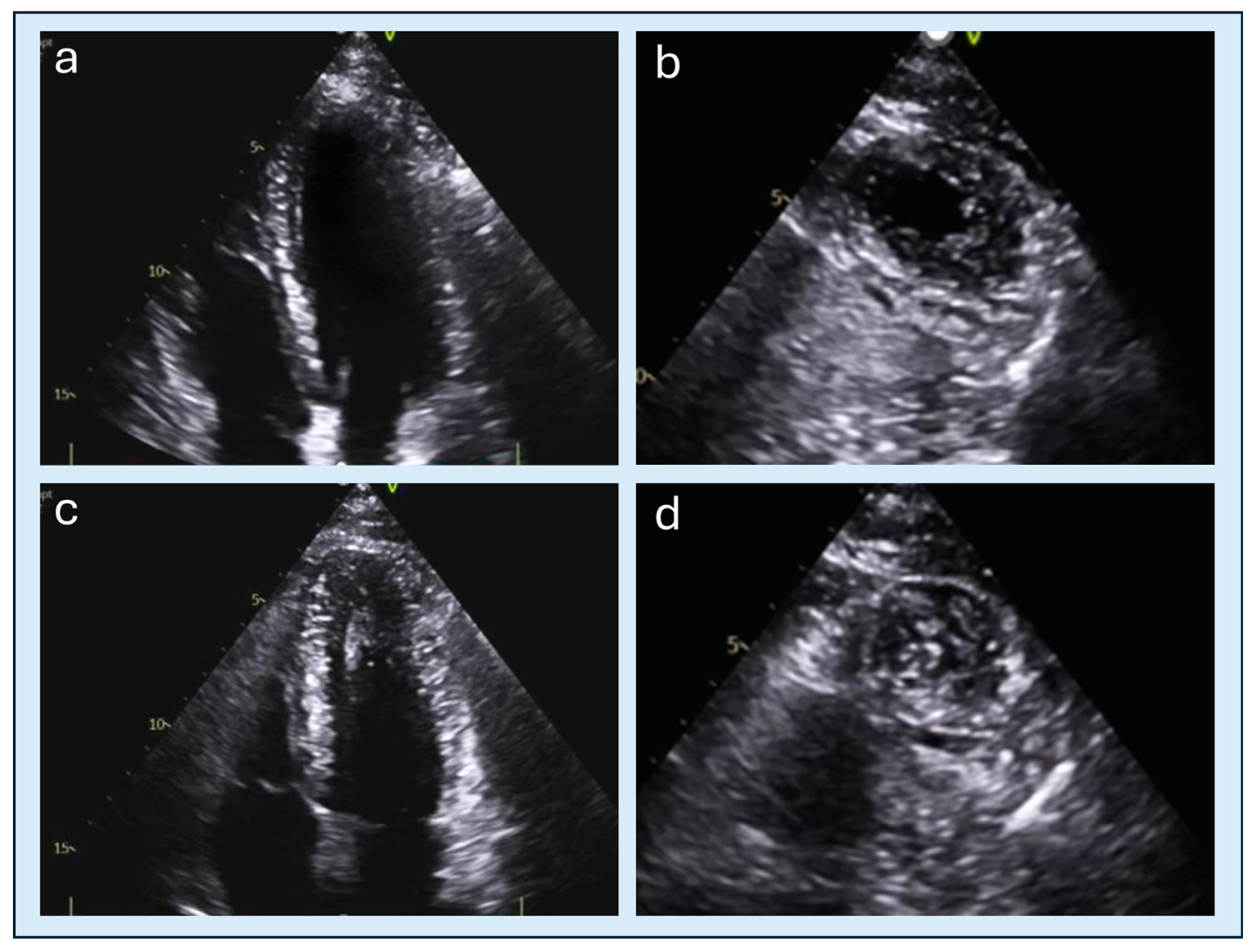
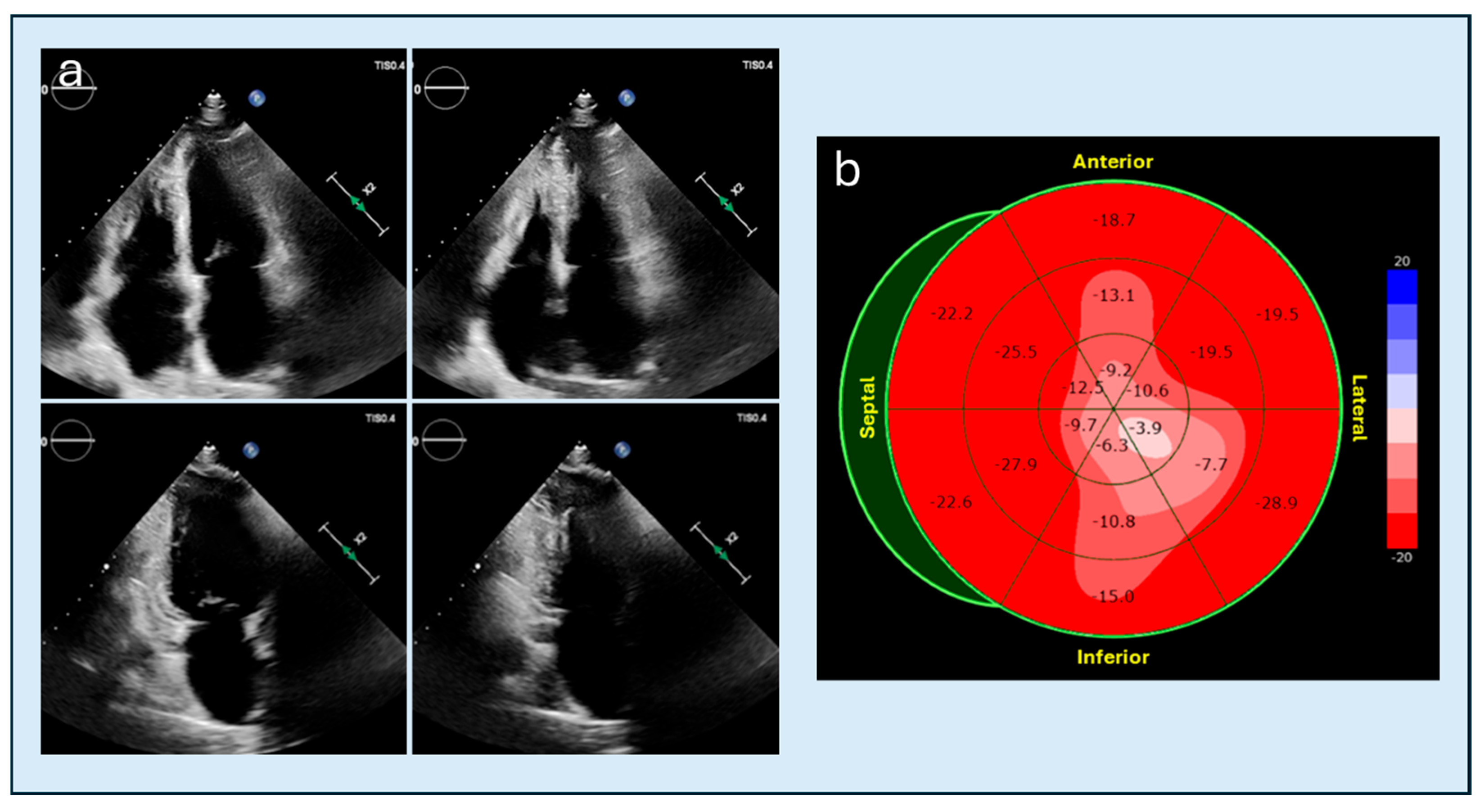
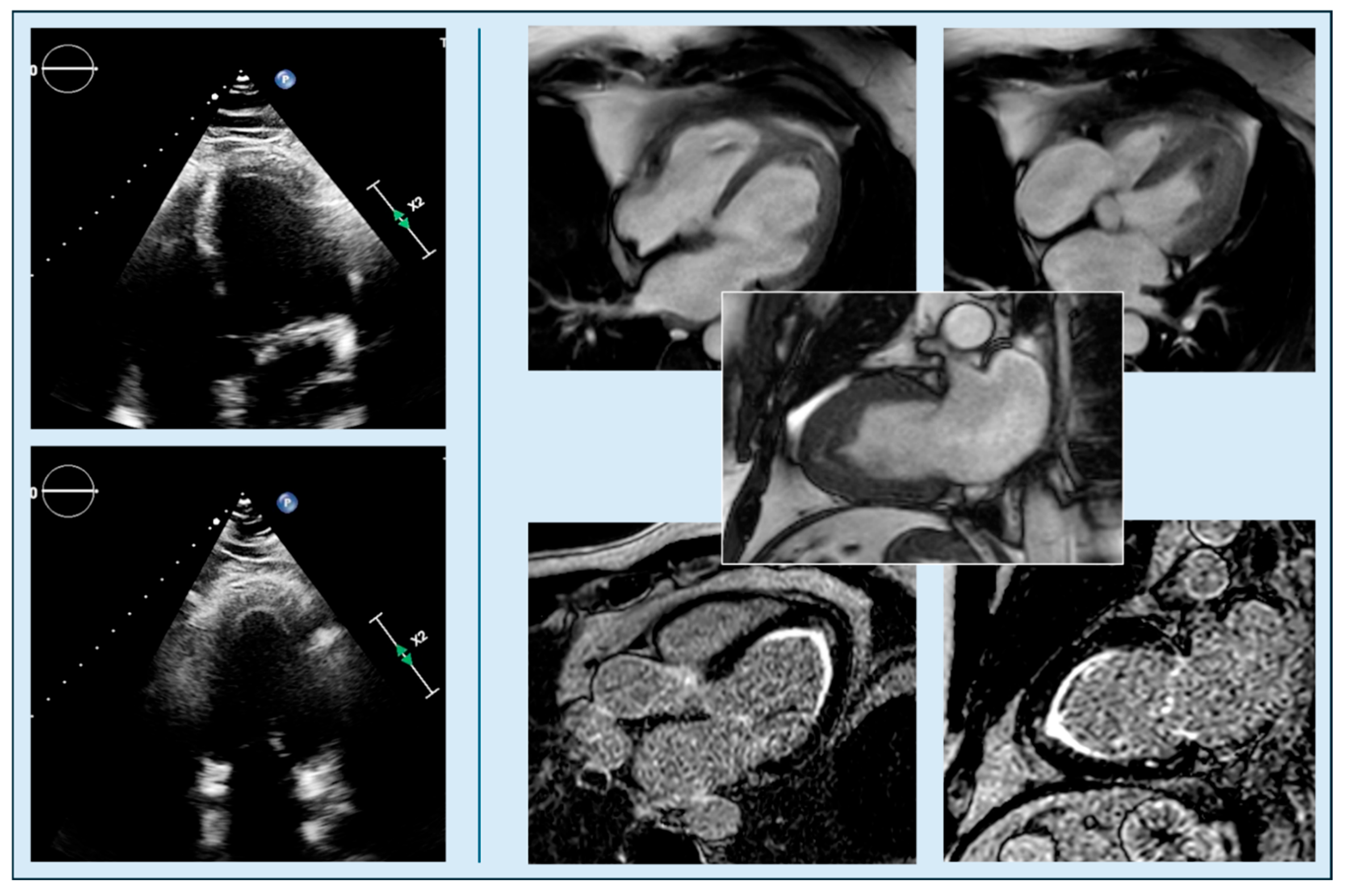
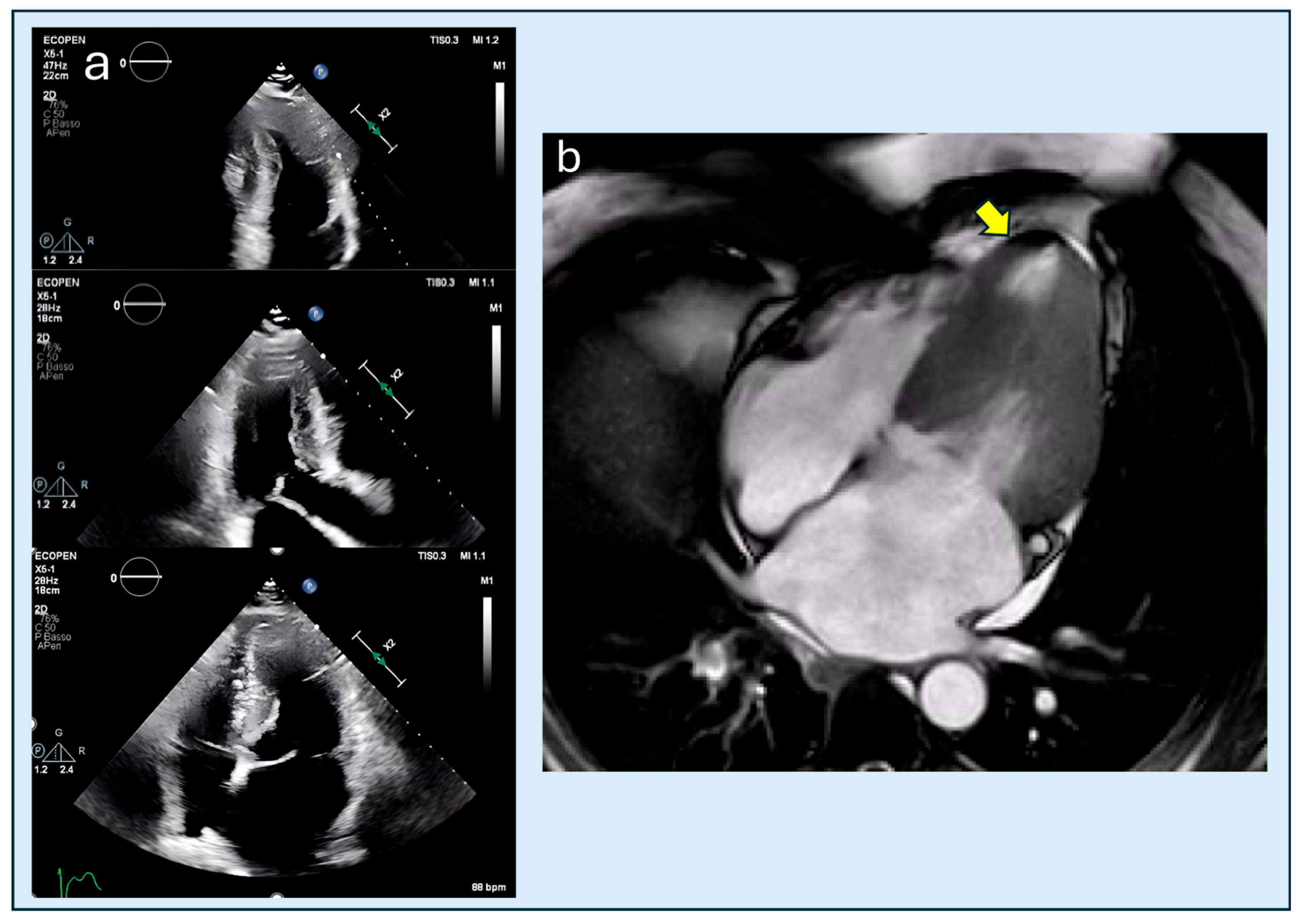
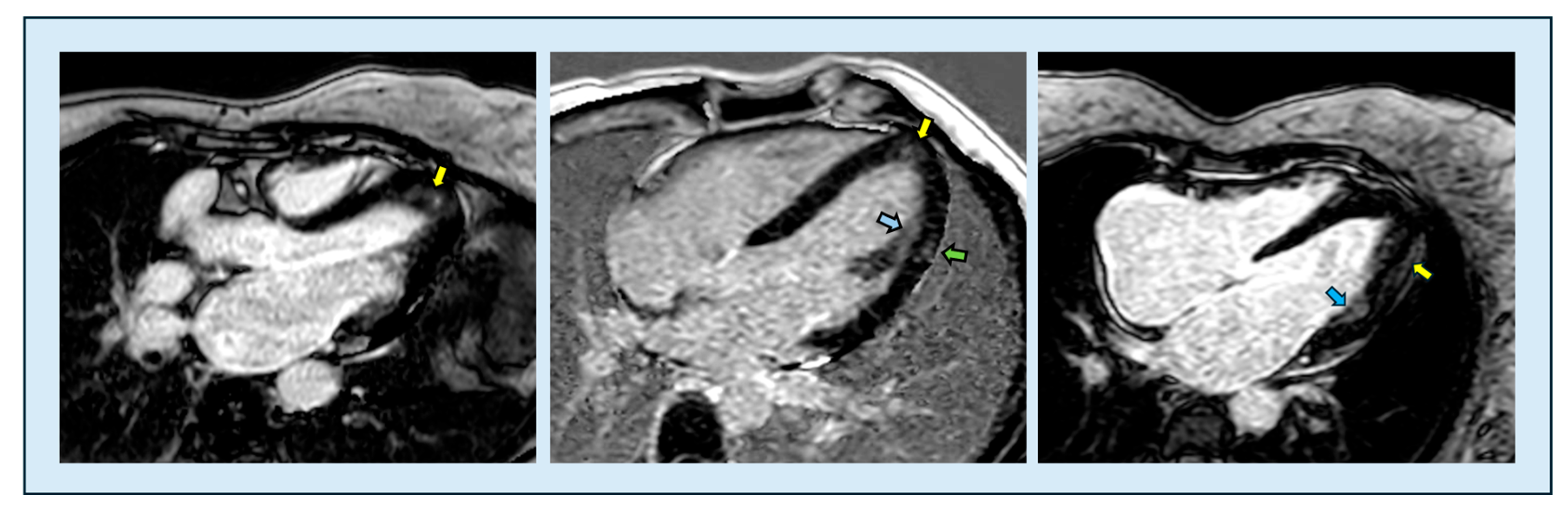
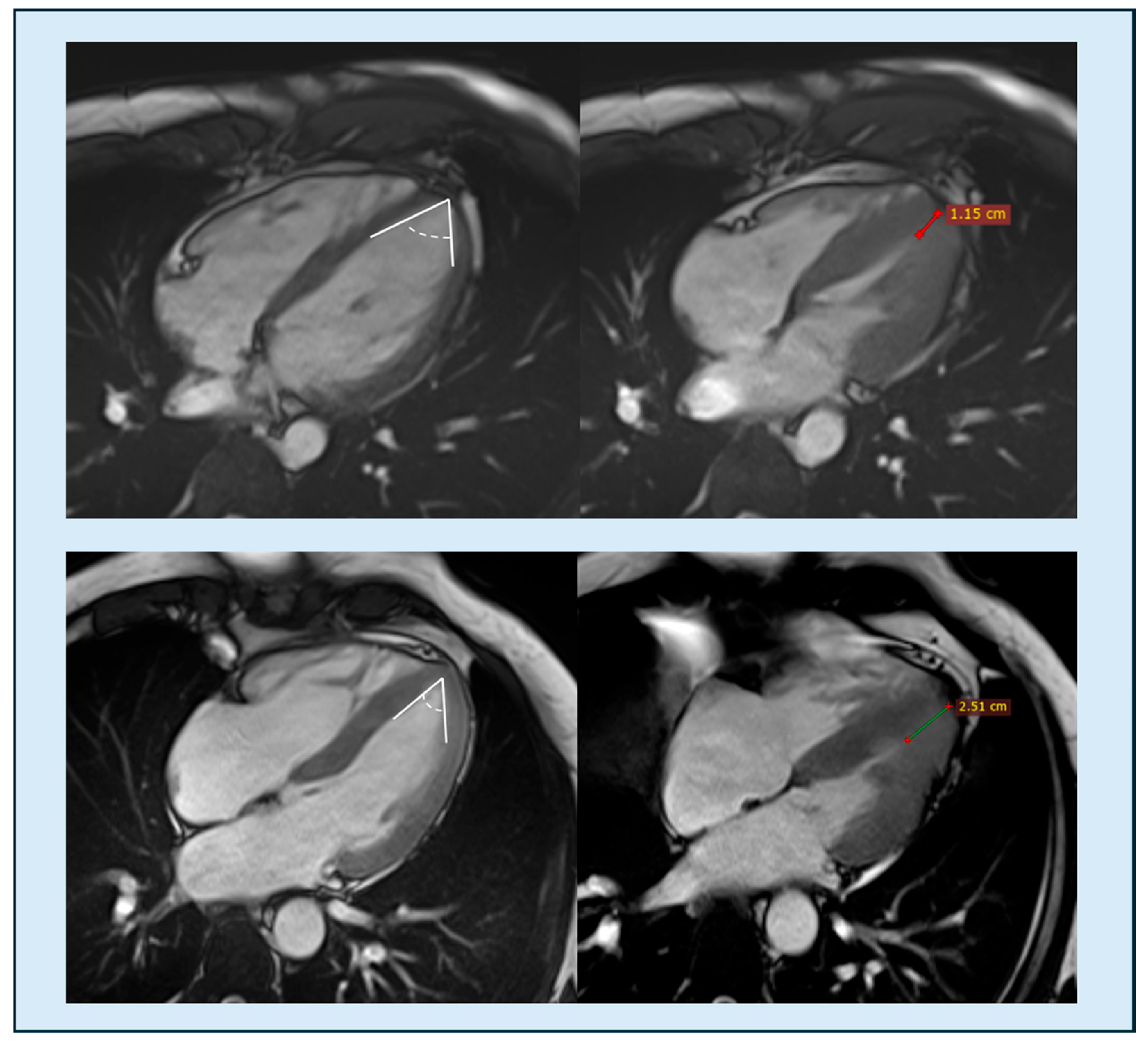
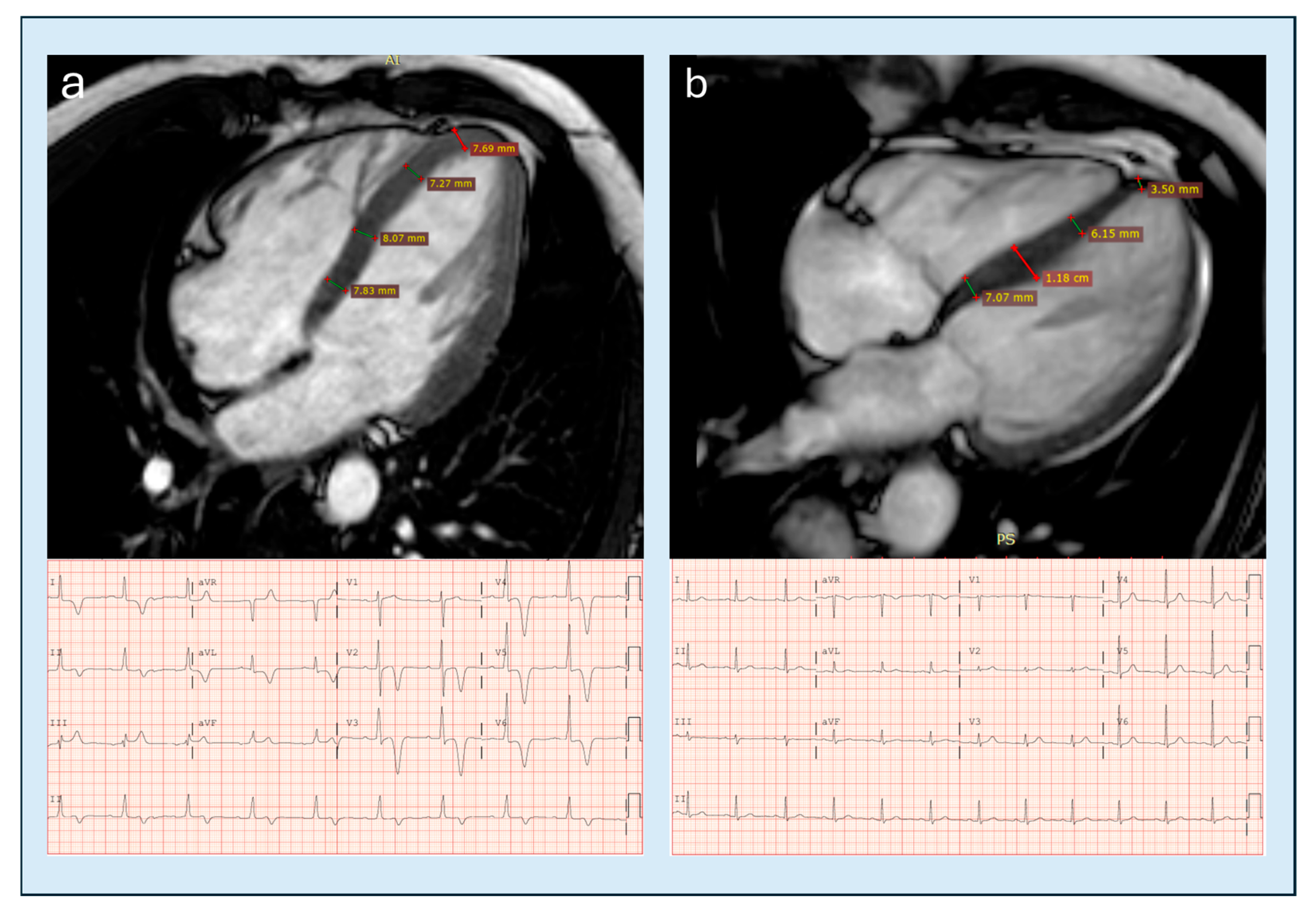
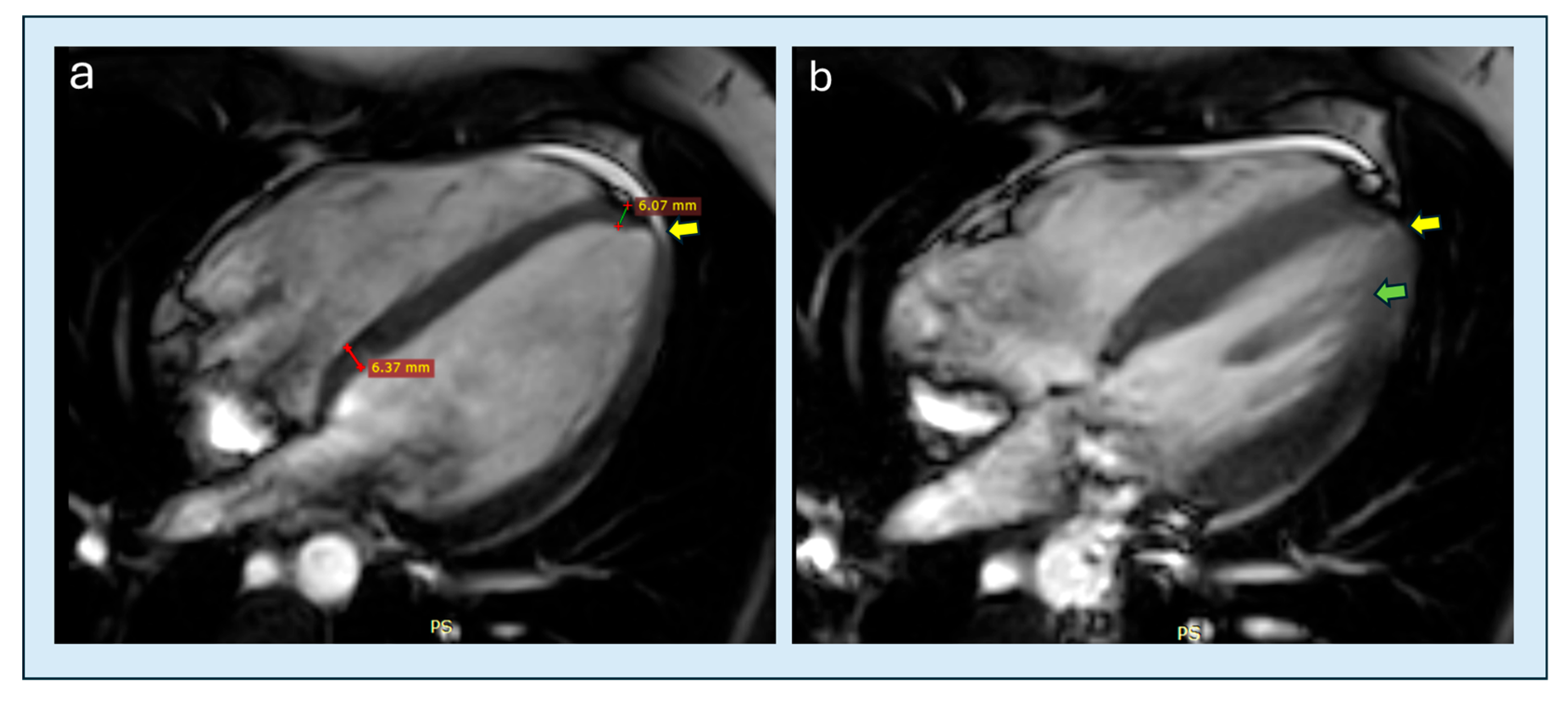
| Feature | Classic HCM | Apical HCM |
|---|---|---|
| Epidemiology | Prevalence: 1/500–1/200 | 1–10% in Europe/North America; up to 25% in Asian cohorts |
| Prognosis | Overall annual mortality ≈ 1% | Overall annual mortality 0.5–3.5% |
| Electrocardiographic features | LV hypertrophy criteria, nonspecific T-wave inversion or repolarization abnormalities | Deep or “giant” negative T waves (10–15 mm) |
| Echocardiographic Detection | Good sensitivity, especially for septal hypertrophy; color-Doppler useful for LVOT obstruction | Often underestimated without contrast; up to 43% of apical aneurysms may be missed by TTE |
| Advanced Imaging (CMRi) | Confirms diagnosis; differentiates non-sarcomeric causes; quantifies fibrosis (LGE) | Gold standard for apical wall thickness and aneurysm; differentiates non-sarcomeric etiologies; quantifies fibrosis (LGE) |
| Criterion | Modality | Cut-Off/Definition | Validation Cohort | Diagnostic Accuracy | Evidence | TTE Feasibility | Reference |
|---|---|---|---|---|---|---|---|
| Indexed apical maximum wall thickness | CMRi | ≥5.2–5.6 mm/m2 | >4000 controls +104 ApHCM pts | AUC 0.94; Sens 99/78%; FP 3% | High | ++ | Hughes 2024 [25] |
| Loss of basal–apical tapering | CMRi | Apical ≥ basal | 22 relative ApHCM vs. controls | Supportive; no AUC | Moderate | +++ | Flett 2015 [74] |
| Apical–basal thickness ratio | CMRi | >1 (prev ≥1.3) | Mixed CMR cohorts | Not available | Moderate | +++ | Flett 2015 [74] |
| Apical angle | CMRi | ≤75–76° | 71 pts | AUC 0.77 | Low–mod | ++ | Li 2021 [64] |
| Apical thickness progression | CMRi | Mean ≥7.6 mm; Max ≥9.5 mm | 71 pts | AUC 0.87–0.898 | Moderate | ++ | Li 2021 [64] |
| Indexed apical obliteration length | TTE | >0.5 | ~180–190 ApHCM | Prognostic | Moderate | +++ | Kim 2016 [75] |
| Absolute apical obliteration | TTE | >20 mm | ~188 ApHCM | Specific | Moderate | +++ | Kim 2016 [75] |
| Apical papillary displacement | CMRi | Apical beyond mid-LV | >150 HCM + ctrls | Supportive | Moderate | + | Filomena 2023 [76] |
| Apical cavity obliteration severity | TTE/CMRi | End-systolic closure | Echo/CMR cohorts | Prognostic | Moderate | +++ | Hamza 2025 [20] |
| Apical aneurysm detection | CMRi→TTE | Dyskinesis + scar | CMR aneurysm cohorts | Echo misses 43% | High | + | Hamza 2025 [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangini, F.; Grimaldi, M.; Spinelli, F.; Dellegrottaglie, S.; Di Monaco, A.; Quarta, S.; Casavecchia, G.; Gravina, M.; Bellomo, V.; Sgarra, L.; et al. Multimodality Imaging in Apical Hypertrophic Cardiomyopathy: Can Echocardiography Learn from Cardiac Magnetic Resonance? Diagnostics 2025, 15, 3013. https://doi.org/10.3390/diagnostics15233013
Mangini F, Grimaldi M, Spinelli F, Dellegrottaglie S, Di Monaco A, Quarta S, Casavecchia G, Gravina M, Bellomo V, Sgarra L, et al. Multimodality Imaging in Apical Hypertrophic Cardiomyopathy: Can Echocardiography Learn from Cardiac Magnetic Resonance? Diagnostics. 2025; 15(23):3013. https://doi.org/10.3390/diagnostics15233013
Chicago/Turabian StyleMangini, Francesco, Massimo Grimaldi, Francesco Spinelli, Santo Dellegrottaglie, Antonio Di Monaco, Simona Quarta, Grazia Casavecchia, Matteo Gravina, Vincenzo Bellomo, Luca Sgarra, and et al. 2025. "Multimodality Imaging in Apical Hypertrophic Cardiomyopathy: Can Echocardiography Learn from Cardiac Magnetic Resonance?" Diagnostics 15, no. 23: 3013. https://doi.org/10.3390/diagnostics15233013
APA StyleMangini, F., Grimaldi, M., Spinelli, F., Dellegrottaglie, S., Di Monaco, A., Quarta, S., Casavecchia, G., Gravina, M., Bellomo, V., Sgarra, L., Suma, S., Citarelli, G., Filograna, E., Biederman, R. W. W., & Calbi, R. (2025). Multimodality Imaging in Apical Hypertrophic Cardiomyopathy: Can Echocardiography Learn from Cardiac Magnetic Resonance? Diagnostics, 15(23), 3013. https://doi.org/10.3390/diagnostics15233013






