Pattern of Mandibular Bone Invasion as a Prognostic Factor
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Histopathological Examination Protocol
2.3. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
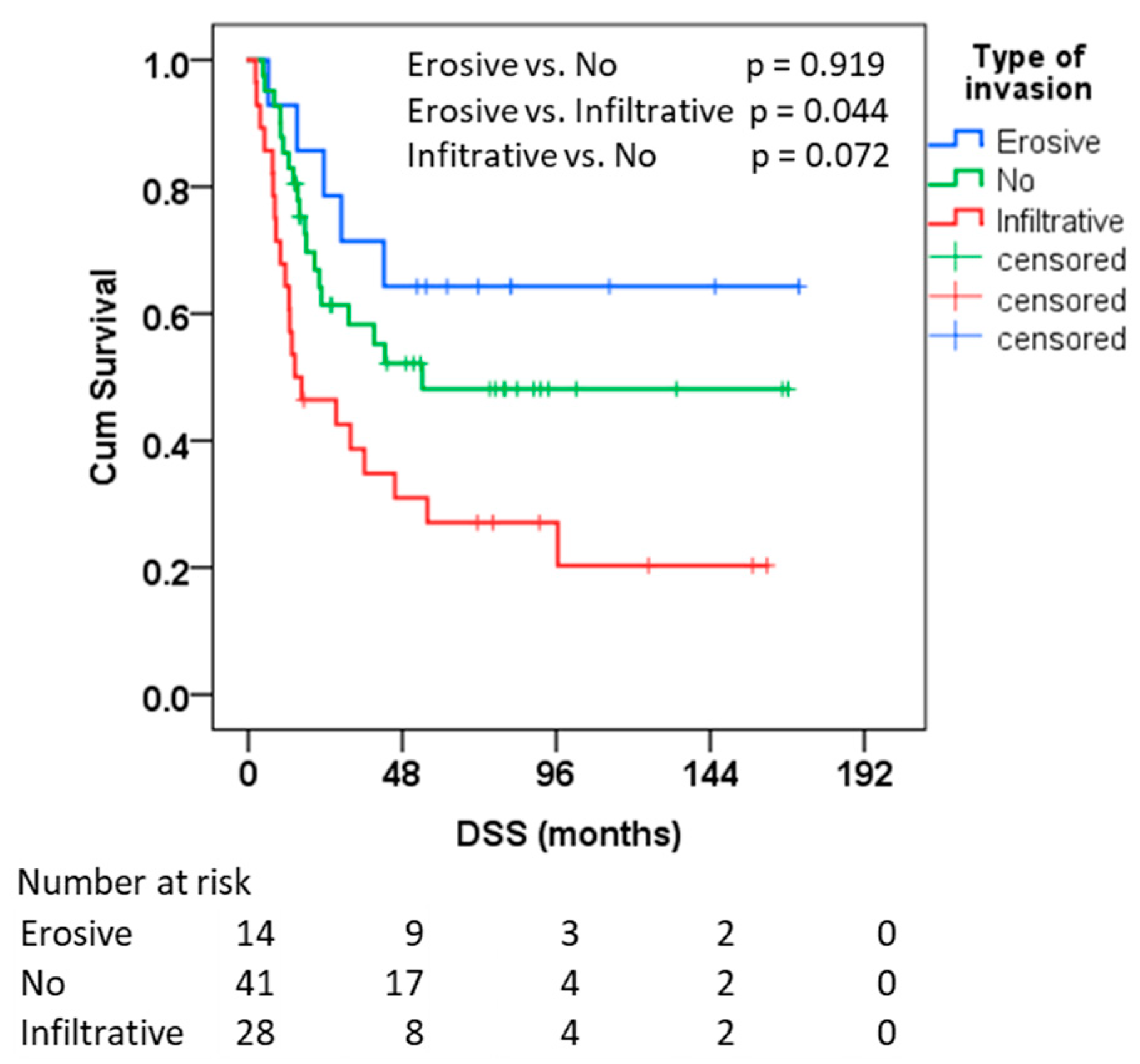
3.2. Survival Outcomes
3.3. Radiologic–Pathologic Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Warnakulasuriya, S. Causes of oral cancer—An appraisal of controversies. Br. Dent. J. 2009, 207, 471–475. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: Rationale and implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Almangush, A.; Bello, I.O.; Keski–Säntti, H.; Mäkinen, L.K.; Kauppila, J.H.; Pukkila, M.; Hagström, J.; Laranne, J.; Tommola, S.; Nieminen, O.; et al. Depth of invasion, tumor budding, and worst pattern of invasion: Prognostic indicators in early-stage oral tongue cancer. Head Neck 2014, 36, 811–818. [Google Scholar] [CrossRef]
- Almangush, A.; Pirinen, M.; Heikkinen, I.; A Mäkitie, A.; Salo, T.; Leivo, I. Tumour budding in oral squamous cell carcinoma: A meta-analysis. Br. J. Cancer 2018, 118, 577–586. [Google Scholar] [CrossRef]
- Binmadi, N.; Alsharif, M.; Almazrooa, S.; Aljohani, S.; Akeel, S.; Osailan, S.; Shahzad, M.; Elias, W.; Mair, Y. Perineural invasion is a significant prognostic factor in OSCC: Systematic review/meta-analysis. Diagnostics 2023, 13, 3339. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, Y.; Cai, H.; Zhang, Y.; Hou, J. Impact of lymphovascular invasion in OSCC: Meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 319–328.e1. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Troiano, G.; Togni, L.; Zhurakivska, K.; Santarelli, A.; Laino, L.; Rubini, C.; Muzio, L.L.; Mascitti, M. Pattern/localization of perineural invasion predicts poor survival in oral tongue carcinoma. Oral Dis. 2023, 29, 411–422. [Google Scholar] [CrossRef]
- Chen, T.-C.; Wang, C.-P.; Ko, J.-Y.; Yang, T.-L.; Hsu, C.-W.; Yeh, K.-A.; Chang, Y.-L.; Lou, P.-J. Impact of PNI and/or LVI on survival of early-stage OSCC. Ann. Surg. Oncol. 2013, 20, 2388–2395. [Google Scholar] [CrossRef]
- Quintana, D.M.V.O.; Dedivitis, R.A.; Kowalski, L.P. Prognostic impact of perineural invasion in oral cancer: Systematic review. Acta Otorhinolaryngol. Ital. 2022, 42, 17–25. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Li, Z.; Han, X.; Que, L. Perineural invasion and its role in survival stratification of OSCC patients. Front. Oncol. 2021, 11, 683825. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Murali, R.; Gao, K.; Elliott, M.S.; Clark, J.R. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011, 117, 1309–1317. [Google Scholar] [CrossRef]
- Brown, J.S.; Lowe, D.; Kalavrezos, N.; D’Souza, J.; Magennis, P.; Woolgar, J. Patterns of invasion and routes of tumor entry into the mandible by oral squamous cell carcinoma. Head Neck 2002, 24, 370–383. [Google Scholar] [CrossRef]
- Ash, C.S.; Nason, R.W.; Abdoh, A.A.; Cohen, M.A. Prognostic implications of mandibular invasion in oral cancer. Head Neck 2000, 22, 794–798. [Google Scholar] [CrossRef]
- Wong, R.J.; Keel, S.B.; Glynn, R.J.; Varvares, M.A. Histological pattern of mandibular invasion by oral squamous cell carcinoma. Laryngoscope 2000, 110, 65–72. [Google Scholar] [CrossRef]
- Okura, M.; Yanamoto, S.; Umeda, M.; Otsuru, M.; Ota, Y.; Kurita, H.; Kamata, T.; Kirita, T.; Yamakawa, N.; Yamashita, T.; et al. Prognostic and staging implications of mandibular canal invasion in lower gingival squamous cell carcinoma. Cancer Med. 2016, 5, 3378–3385. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, J.; Men, Y.; Yang, W.; Mi, F.; Li, L. Does Medullary Versus Cortical Invasion of the Mandible Affect Prognosis in Patients With Oral Squamous Cell Carcinoma? J. Oral Maxillofac. Surg. 2017, 75, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Vaassen, L.A.; Speel, E.-J.M.; Kessler, P.A.W.H. Bone invasion by oral squamous cell carcinoma: Molecular alterations leading to osteoclastogenesis—A review literature. J. Cranio-Maxillofac. Surg. 2017, 45, 1464–1471. [Google Scholar] [CrossRef]
- Vidiri, A.; Guerrisi, A.; Pellini, R.; Manciocco, V.; Covello, R.; Mattioni, O.; Guerrisi, I.; Di Giovanni, S.; Spriano, G.; Crecco, M. MDCT and MRI in the evaluation of mandibular invasion by oral cavity SCC: Correlation with pathology. J. Exp. Clin. Cancer Res. 2010, 29, 73. [Google Scholar] [CrossRef]
- Imaizumi, A.; Yoshino, N.; Yamada, I.; Nagumo, K.; Amagasa, T.; Omura, K.; Okada, N.; Kurabayashi, T. A potential pitfall of MR imaging for assessing mandibular invasion of squamous cell carcinoma in the oral cavity. AJNR Am. J. Neuroradiol. 2006, 27, 114–122. [Google Scholar] [PubMed]
- Nae, A.; O’LEary, G.; Feeley, L.; Fives, C.; Fitzgerald, B.; Chiriac, E.; Sheahan, P. Utility of CT and MRI in assessment of mandibular involvement in oral cavity cancer. World J. Otorhinolaryngol. Head. Neck Surg. 2019, 5, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.H.; Yoon, D.Y.; Park, C.H.; Chang, S.K.; Lim, K.J.; Seo, Y.L.; Yun, E.J.; Choi, C.S.; Bae, S.H. CT, MR, 18F-FDG PET/CT, and their combined use for assessment of mandibular invasion by oral cavity SCC. Acta Radiol. 2010, 51, 1111–1119. [Google Scholar] [CrossRef]
- Timmer, V.C.M.L.; Crombag, G.A.J.C.; van Kuijk, S.M.J.; Vaassen, L.A.A.; Kessler, P.A.W.H.; Postma, A.A. The accuracy of dual energy CT on evaluation of bone invasion caused by oral squamous cell carcinoma—A comparison to MRI. J. Craniomaxillofac. Surg. 2025, 53, 1731–1737. [Google Scholar] [CrossRef]
- Jo, G.-D.; Oh, K.-Y.; Kim, J.-E.; Yi, W.-J.; Heo, M.-S.; Lee, S.-S.; Huh, K.-H. Underlying bone change in oral squamous cell carcinoma observed from MRI and CT: Implications for aggressiveness and prognosis. J Dent Sci. 2024, 19, 2082–2089. [Google Scholar] [CrossRef]
- College of American Pathologists (CAP). Protocol for the Examination of Specimens from Patients with Cancers of Oral Cavity. June 2023. Available online: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (accessed on 22 September 2025).
- Slootweg, P.J.; Müller, H. Mandibular invasion by oral squamous cell carcinoma. J. Cranio-Maxillofac. Surg. 1989, 17, 69–74. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Mahajan, A.; Dhone, N.; Vaish, R.; Singhania, A.; Malik, A.; Prabhash, K.; Ahuja, A.; Sable, N.; Chaturvedi, P.; Noronha, V.; et al. Prognostic impact of pattern of mandibular involvement in GBC-SCC: Marrow/mandibular-canal staging. Front. Oncol. 2022, 11, 752018. [Google Scholar] [CrossRef]
- Kang, C.-J.; Wen, Y.-W.; Lee, S.-R.; Lee, L.-Y.; Hsueh, C.; Lin, C.-Y.; Fan, K.-H.; Wang, H.-M.; Hsieh, C.-H.; Ng, S.-H.; et al. Surgical margins status and prognosis after resection of oral cavity SCC: Nationwide registry study. Cancers 2021, 14, 15. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Lee, T.-L.; Chang, C.-W.; Wang, C.-P.; Lin, M.-C.; Lou, P.-J.; Chen, T.-C. Margin to depth-of-invasion ratio as an indicator for stratifying close margins in early-stage OSCC. Oral Oncol. 2024, 151, 106726. [Google Scholar] [CrossRef] [PubMed]
- Sultania, M.; Chaudhary, I.; Jain, P.; Ghalige, H.; Rajan, D.; Sudhakar, G.; Raghuram, K.; Muduly, D.; Barik, S.; Pathak, M.; et al. Margin to Depth of Invasion Ratio: Predictor of survival in oral cancer. JCO Glob. Oncol. 2023, 9, e2300144. [Google Scholar] [CrossRef]
- Liu, T.; Clark, J.; David, M.; David, M.; Schache, A.; Bajwa, M.S.; Low, T.H.; Gupta, R.; Batstone, M.D. The Impact of Stratified Surgical Margins on Survival Outcomes in Oral Cavity Squamous Cell Carcinoma: A Multicenter Analysis. Head Neck 2025. online ahead of print. [Google Scholar] [CrossRef]
- Klibngern, H.; Kang, C.-J.; Lee, L.-Y.; Ng, S.-H.; Lin, C.-Y.; Fan, K.-H.; Chen, W.-C.; Lin, J.-C.; Tsai, Y.-T.; Lee, S.-R.; et al. Margin-to-depth ratio as an independent prognostic factor in resected OSCC: Nationwide cohort. Oral Oncol. 2024, 159, 107102. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-J.; Lu, C.-L.; Cheng, Y.-P.; Cheng, P.-C.; Chen, Y.-C.; Chiang, C.-J.; Lee, W.-C.; You, S.-L.; Hsu, W.-L. Cervical lymph-node positive probability predicts survival in OSCC: Nationwide study. Cancers 2025, 17, 2704. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Iandelli, A.; Marchi, F.; Huang, Y.; Tai, S.; Hung, S.; Kao, H.; Chang, K. Prognostic value of lymph-node burden in oral cavity cancer: Meta-analysis. Laryngoscope 2022, 132, 88–95. [Google Scholar] [CrossRef]
- Huang, T.H.; Li, K.Y.; Choi, W.S. Lymph node ratio as a prognostic variable in OSCC: Meta-analysis. Oral Oncol. 2019, 89, 133–143. [Google Scholar] [CrossRef]
- Mermod, M.; Tolstonog, G.; Simon, C.; Monnier, Y. Extracapsular spread in HNSCC: Systematic review and meta-analysis. Oral Oncol. 2016, 62, 60–71. [Google Scholar] [CrossRef]
- Henson, C.E.; Abou-Foul, A.K.; Morton, D.J.; McDowell, L.; Baliga, S.; Bates, J.; Lee, A.; Bonomo, P.; Szturz, P.; Nankivell, P.; et al. Extranodal extension in HNC: State-of-the-art review and gap analysis. Front. Oncol. 2023, 13, 1263347. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lowe, D.; Woolgar, J.A.; Brown, J.S.; Vaughan, E.D.; Evans, C.; Lewis-Jones, H.; Hanlon, R.; Hall, G.L.; Rogers, S.N. Extracapsular spread in OSCC. Head Neck 2010, 32, 714–722. [Google Scholar] [CrossRef]
- Ferlito, A.; Rinaldo, A.; Devaney, K.O.; MacLennan, K.; Myers, J.N.; Petruzzelli, G.J.; Shaha, A.R.; Genden, E.M.; Johnson, J.T.; de Carvalho, M.B.; et al. Prognostic significance of microscopic/macroscopic extracapsular spread in cervical nodes. Oral Oncol. 2002, 38, 747–751. [Google Scholar] [CrossRef]
- Mascitti, M.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Muzio, L.L.; Rubini, C.; Santarelli, A.; Troiano, G. Prognostic significance of tumor budding thresholds in oral tongue SCC. Oral Dis. 2023, 29, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Naegeli-Pullankavumkal, C.; Ferrari, R.; Gander, T.; Lanzer, M. Staging and treatment implications in small oral SCC with bone infiltration. Biomedicines 2025, 13, 628. [Google Scholar] [CrossRef] [PubMed]
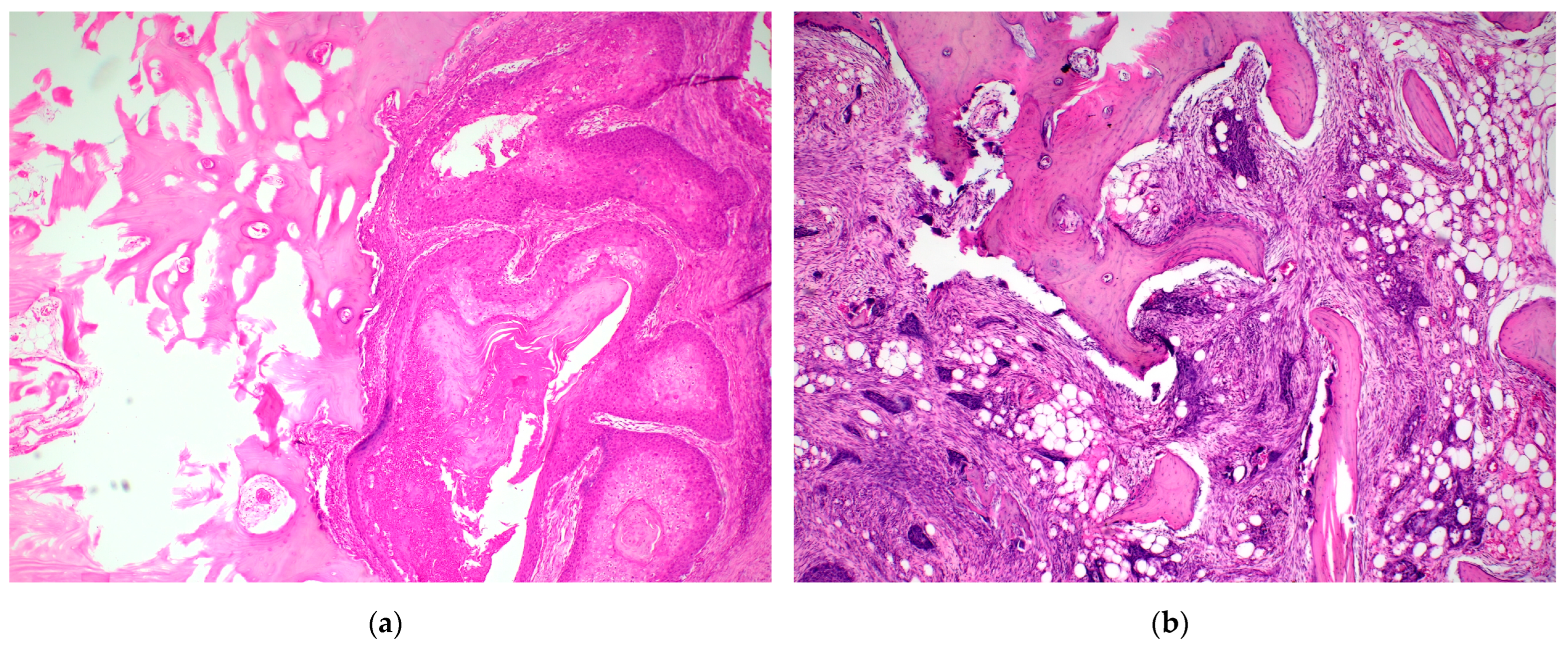
| Mean | 95% CI for Mean | Median | 95% CI for Median | |
|---|---|---|---|---|
| DSS (months) | 83.0 | 65.8–100.3 | 42.3 | 18.7–65.8 |
| DFS (months) | 66.8 | 50.0–83.5 | 15.4 | 0.0–35.7 |
| OS (months) | 62.7 | 48.5–77.0 | 29.0 | 12.8–45.2 |
| DSS (Months) | Mean | 95% CI for Mean | Median | 95% CI for Median |
|---|---|---|---|---|
| No invasion | 92.2 | 67.7–116.6 | 54.2 | - |
| Infiltrative invasion | 51.9 | 28.7–75.1 | 14.5 | 0.0–32.8 |
| Erosive invasion | 118.6 | 81.2–156.1 | - |
| DSS | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% Pro HR | p-Value | HR | 95% Pro HR | p-Value | HR | 95% Pro HR | p-Value | |
| Type of invasion 0 | 1 | 0.017 | 1 | 0.163 | 1 | 0.021 | |||
| infiltrative | 1.930 | 1.023–3.640 | 0.042 | 1.577 | 0.871–2.855 | 0.133 | 1.416 | 0.822–2.436 | 0.210 |
| erosive | 0.520 | 0.191–1.421 | 0.202 | 0.786 | 0.345–1.790 | 0.567 | 0.394 | 0.161–0.962 | 0.041 |
| Positive surgical margin | 3.295 | 1.744–6.224 | 0.0002 | 2.541 | 1.441–4.479 | 0.001 | 2.484 | 1.471–4.195 | 0.001 |
| Grade G3 (vs. Grade 1–2) | 2.081 | 1.123–3.856 | 0.020 | 1.715 | 0.978–3.006 | 0.060 | 1.742 | 1.031–2.941 | 0.038 |
| ENE (present) | 1.681 | 0.908–3.113 | 0.098 | 1.281 | 0.716–2.292 | 0.404 | 1.561 | 0.909–2.680 | 0.106 |
| Cohen’s Kappa (95% CI) Classification | % of Agreement (95% CI) | McNemar’s Test | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| CT invasion vs. Type of invasion (0, infitrative+erosive) | 0.449 (0.250–0.649) moderate agreement | 72.7% (61.9–81.4%) | 0.664 | 78.57% | 68.29% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pink, R.; Michálek, J.; Dvořák, Z.; Tvrdý, P.; Šašková, L.; Herman, M.; Heinz, P.; Hermanová, M.; Zapletalová, J.; Mozoľa, M. Pattern of Mandibular Bone Invasion as a Prognostic Factor. Diagnostics 2025, 15, 2989. https://doi.org/10.3390/diagnostics15232989
Pink R, Michálek J, Dvořák Z, Tvrdý P, Šašková L, Herman M, Heinz P, Hermanová M, Zapletalová J, Mozoľa M. Pattern of Mandibular Bone Invasion as a Prognostic Factor. Diagnostics. 2025; 15(23):2989. https://doi.org/10.3390/diagnostics15232989
Chicago/Turabian StylePink, Richard, Jaroslav Michálek, Zdeněk Dvořák, Peter Tvrdý, Lenka Šašková, Michal Herman, Petr Heinz, Markéta Hermanová, Jana Zapletalová, and Michal Mozoľa. 2025. "Pattern of Mandibular Bone Invasion as a Prognostic Factor" Diagnostics 15, no. 23: 2989. https://doi.org/10.3390/diagnostics15232989
APA StylePink, R., Michálek, J., Dvořák, Z., Tvrdý, P., Šašková, L., Herman, M., Heinz, P., Hermanová, M., Zapletalová, J., & Mozoľa, M. (2025). Pattern of Mandibular Bone Invasion as a Prognostic Factor. Diagnostics, 15(23), 2989. https://doi.org/10.3390/diagnostics15232989






