Delta-Radiomics Biomarker in Colorectal Cancer Liver Metastases Treated with Cetuximab Plus Avelumab (CAVE Trial)
Abstract
1. Introduction
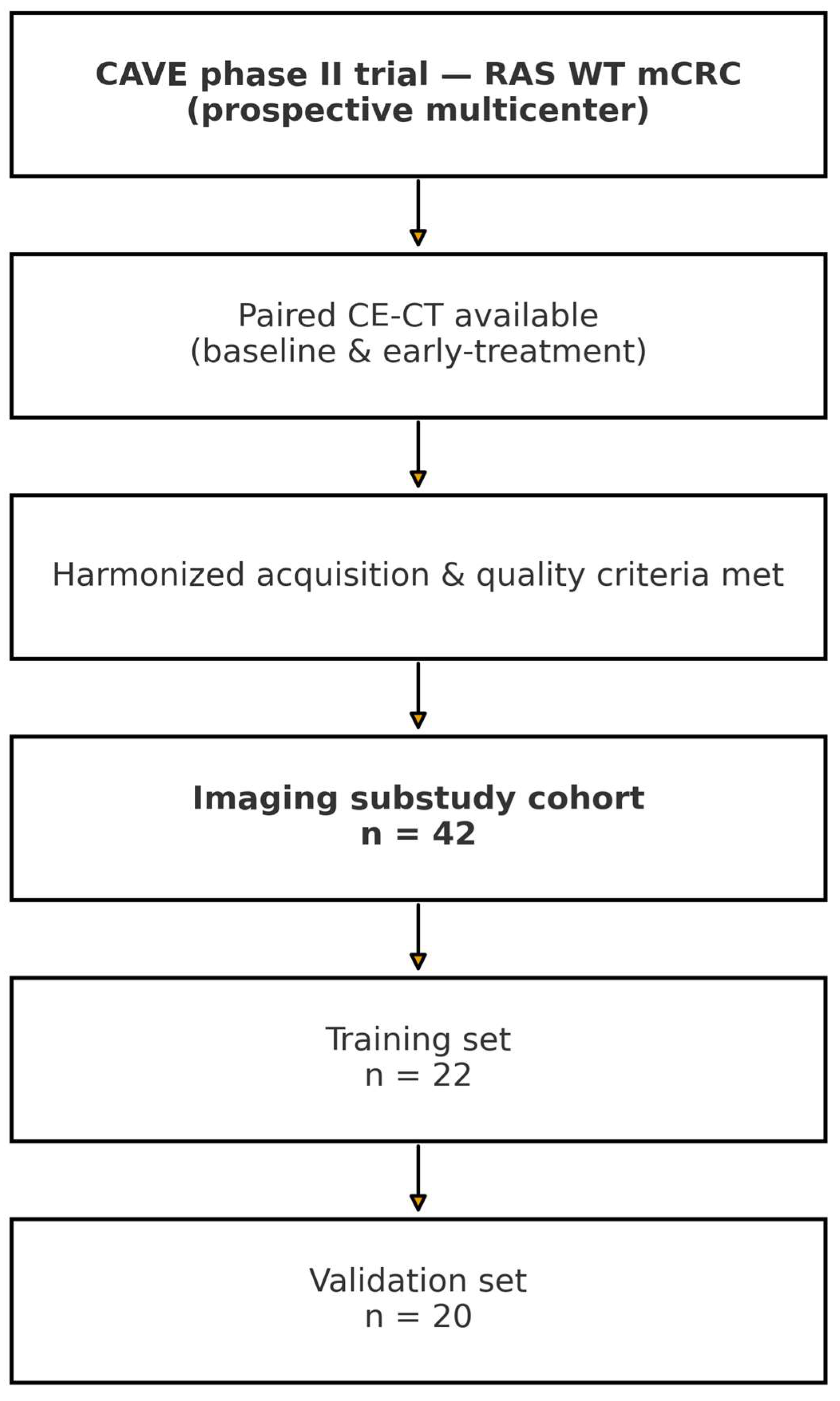
2. Methods
2.1. CT Imaging Protocol
2.2. Tumor Segmentation and Feature Extraction
2.3. Response Assessment
2.4. Feature Selection and Statistical Analysis
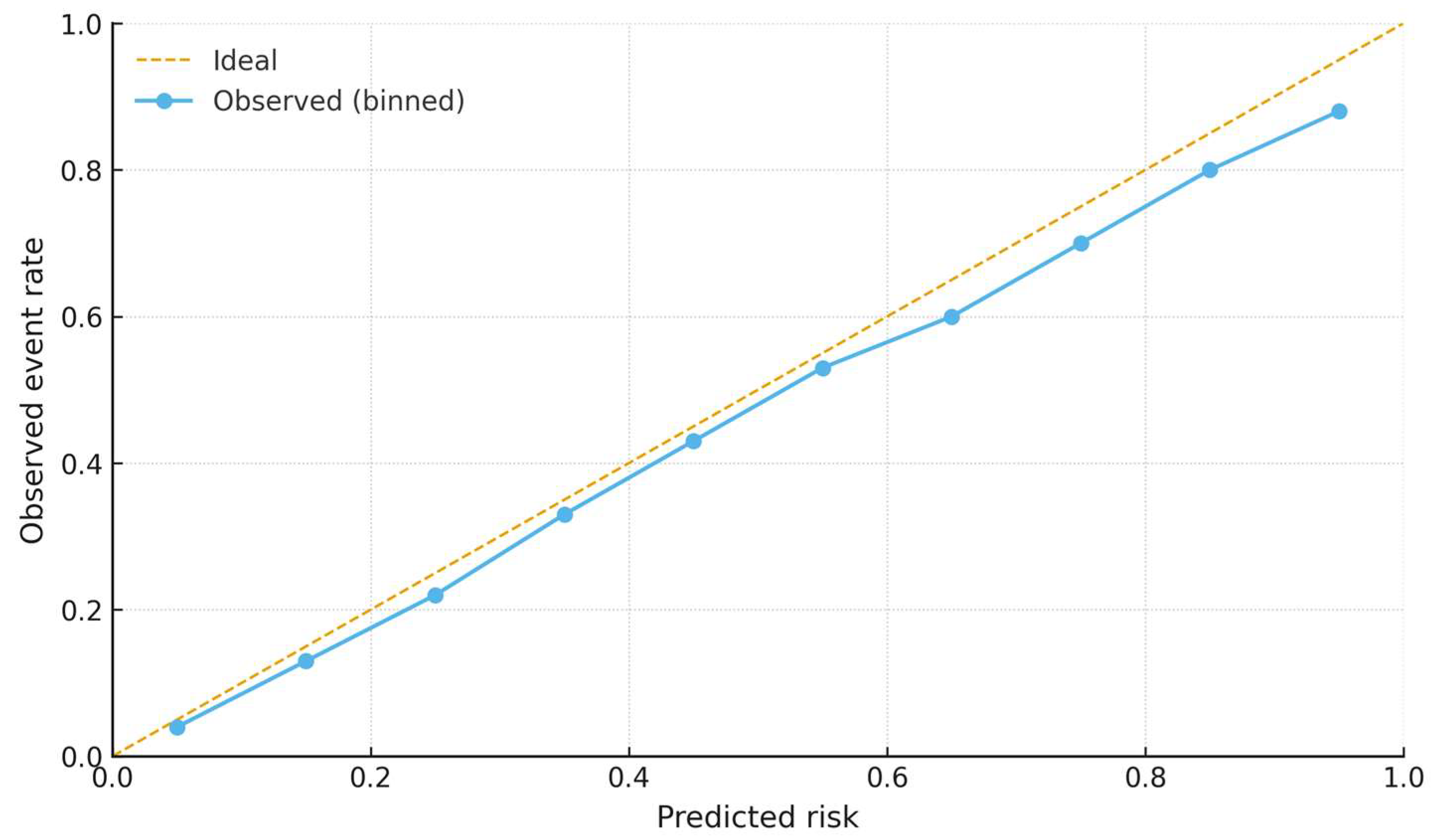
2.5. Penalized Sensitivity Analysis
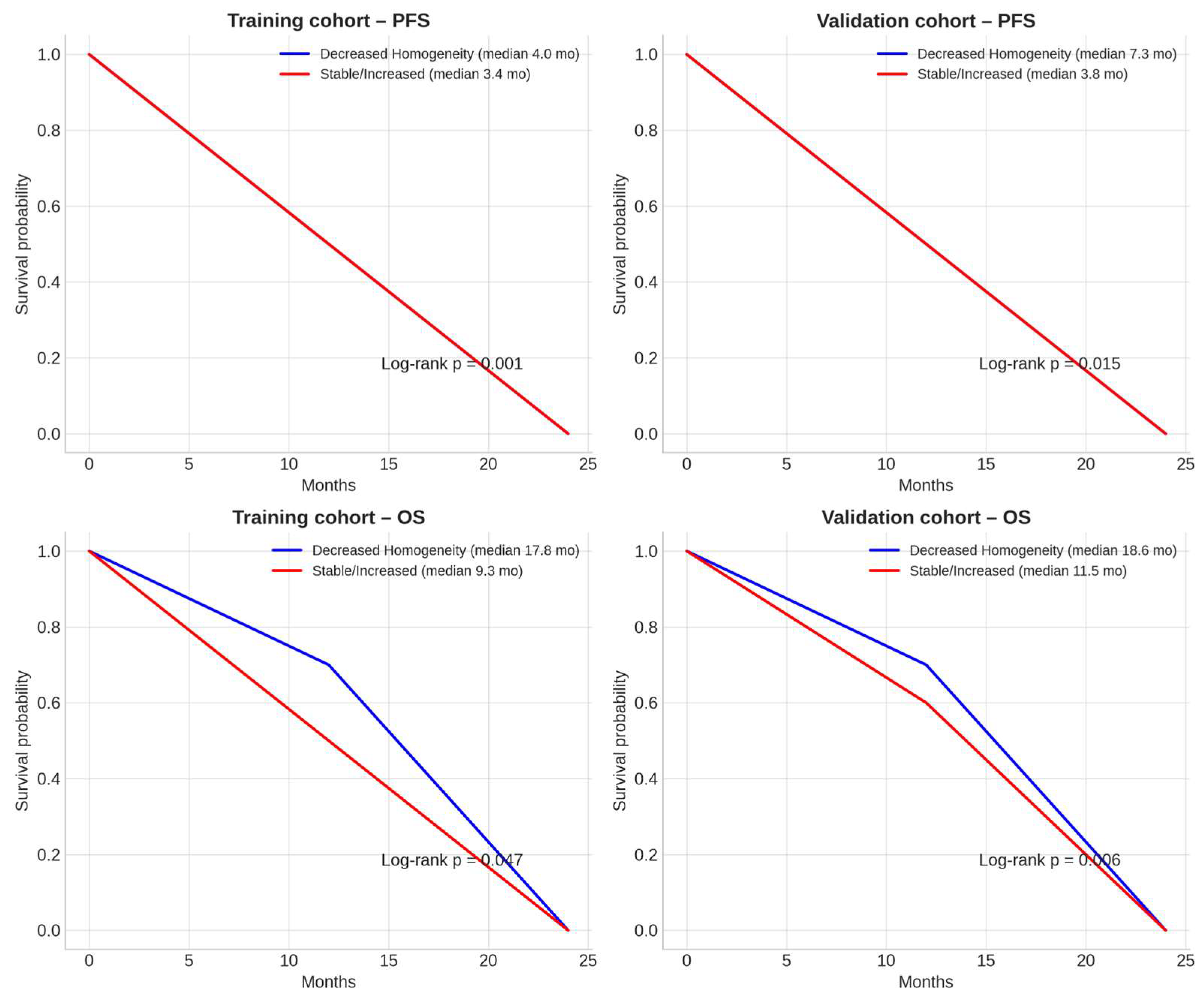
3. Results
Penalized Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reginelli, A.; Patanè, V.; Cappabianca, S. Colorectal cancer liver metastases: A radiologic point of view. World J. Gastrointest. Oncol. 2025, 17, 103473. [Google Scholar] [CrossRef]
- Campisi, M.C.; Lancellotta, V.; Fionda, B.; De Angeli, M.; Manfrida, S.; Cornacchione, P.; Macchia, G.; Morganti, A.G.; Mattiucci, G.C.; Gambacorta, M.A.; et al. A systematic review on the role of interventional radiotherapy for treatment of anal squamous cell cancer: Multimodal and multidisciplinary therapeutic approach. Radiol. Med. 2024, 129, 1739–1750. [Google Scholar] [CrossRef]
- Catona, G.M.; Marcu, L.G. Qualitative and quantitative evaluation of the role of CBCT in rectal cancer radiotherapy. Radiol. Med. 2025, 130, 150–160. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Nakaura, T.; Ohuchi, M.; Ogawa, K.; Kato, R.; Maeda, Y.; Eto, K.; Iwatsuki, M.; Baba, Y.; Hirai, T.; et al. Radiomics-based Machine Learning Approach to Predict Chemotherapy Responses in Colorectal Liver Metastases. J. Anus Rectum Colon. 2025, 9, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, Z.; Zhang, L.; Li, L.; Xu, W.; Su, Q. Prediction of pathological complete response to neoadjuvant chemoimmunotherapy in non–small cell lung cancer using 18F-FDG PET radiomics features of primary tumour and lymph nodes. BMC Cancer 2025, 25, 520. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Chen, C.; Cai, W.; Guo, Y.; Guo, K.; Chen, Y.; Shi, Y.; Chen, J.; Lin, X.; et al. Integration of Deep Learning and Sub-regional Radiomics Improves the Prediction of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer Patients. Acad. Radiol. 2025, 32, 3384–3396. [Google Scholar] [CrossRef]
- Qiu, L.; Fei, Y.; Zhu, Y.; Yuan, J.; Shi, K.; Wu, M.; Jiang, G.; Sun, X.; Luo, J.; Li, Y.; et al. Radiomic analysis based on machine learning of multi-sequences MR to assess early treatment response in locally advanced nasopharyngeal carcinoma. Sci. Prog. 2025, 108, 368504251338930. [Google Scholar] [CrossRef]
- Liao, Z.; Luo, D.; Tang, X.; Huang, F.; Zhang, X. MRI-based radiomics for predicting pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review and meta-analysis. Front. Oncol. 2025, 15, 1550838. [Google Scholar] [CrossRef]
- Borisov, A.; Karelidze, D.; Ivannikov, M.; Shakhvalieva, E.; Sultanova, P.; Arzamasov, K.; Nudnov, N.; Vasilev, Y. Application of Radiomics for Differentiating Lung Neuroendocrine Neoplasms. Diagnostics 2025, 15, 874. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Wang, H. The clinical implications and interpretability of computational medical imaging (radiomics) in brain tumors. Insights Imaging 2025, 16, 77. [Google Scholar] [CrossRef]
- Gennaro, N.; Soliman, M.; Borhani, A.A.; Kelahan, L.; Savas, H.; Avery, R.; Subedi, K.; Trabzonlu, T.A.; Krumpelman, C.; Yaghmai, V.; et al. Delta Radiomics and Tumor Size: A New Predictive Radiomics Model for Chemotherapy Response in Liver Metastases from Breast and Colorectal Cancer. Tomography 2025, 11, 20. [Google Scholar] [CrossRef]
- Alshuhri, M.S.; Al-Mubarak, H.F.; Qaisi, A.; Alhulail, A.A.; AlMansour, A.G.M.; Madkhali, Y.; Alotaibi, S.; Aljuhani, M.; Alomair, O.I.; Almudayni, A.; et al. MRI Delta Radiomics to Track Early Changes in Tumor Following Radiation: Application in Glioblastoma Mouse Model. Biomedicines 2025, 13, 815. [Google Scholar] [CrossRef]
- Wagner-Larsen, K.S.; Lura, N.; Gulati, A.; Ryste, S.; Hodneland, E.; Fasmer, K.E.; Woie, K.; Bertelsen, B.I.; Salvesen, Ø.; Halle, M.K.; et al. MRI delta radiomics during chemoradiotherapy for prognostication in locally advanced cervical cancer. BMC Cancer 2025, 25, 122. [Google Scholar] [CrossRef]
- Li, L.; Wei, W.; Yang, L.; Zhang, W.; Dong, J.; Liu, Y.; Huang, H.; Zhao, W. CT-Mamba: A hybrid convolutional State Space Model for low-dose CT denoising. Comput. Med. Imaging Graph. 2025, 124, 102595. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Sansone, M.; Ciani, G.; Patanè, V.; Genco, C.; Grassi, R.; Savarese, G.; Montella, M.; Monti, R.; Cappabianca, S.; et al. Radiomic Analysis and Liquid Biopsy in Preoperative CT of NSCLC: An Explorative Experience. Thorac. Cancer 2025, 16, e70115. [Google Scholar] [CrossRef] [PubMed]
- Hertel, A.; Kuru, M.; Tollens, F.; Tharmaseelan, H.; Nörenberg, D.; Rathmann, N.; Schoenberg, S.O.; Froelich, M.F. Comparison of diagnostic accuracy of radiomics parameter maps and standard reconstruction for the detection of liver lesions in computed tomography. Front. Oncol. 2024, 14, 1444115. [Google Scholar] [CrossRef]
- Nussinov, R.; Yavuz, B.R.; Jang, H. Molecular principles underlying aggressive cancers. Signal Transduct. Target. Ther. 2025, 10, 42. [Google Scholar] [CrossRef]
- Nussinov, R.; Yavuz, B.R.; Jang, H. Drug resistance and tumor heterogeneity: Cells and ensembles. Biophys. Rev. 2025, 17, 759–779. [Google Scholar] [CrossRef]
- Nussinov, R.; Yavuz, B.R.; Jang, H. Tumors and their microenvironments: Learning from pediatric brain pathologies. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2025, 1880, 189328. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Rausa, E.; Ferrari, D.; Rosa, R.; Maccauro, M.; Pusceddu, S.; Sabella, G.; Cantù, P.; Vitellaro, M.; Coppa, J.; et al. Pathological Characteristics, Management, and Prognosis of Rectal Neuroendocrine Tumors: A Retrospective Study from a Tertiary Hospital. Diagnostics 2024, 14, 1881. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Rubini, D.; Gagliardi, F.; Del Tufo, S.; Belfiore, M.P.; Boldrini, L.; Desideri, I.; Cappabianca, S. Delta radiomics: An updated systematic review. Radiol. Med. 2024, 129, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Cicalini, I.; Chiarelli, A.M.; Chiacchiaretta, P.; Perpetuini, D.; Rosa, C.; Mastrodicasa, D.; d’Annibale, M.; Trebeschi, S.; Serafini, F.L.; Cocco, G.; et al. Multi-omics staging of locally advanced rectal cancer predicts treatment response: A pilot study. Radiol. Med. 2024, 129, 712–726. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, J.D.; Chen, Y.K. Use of Laxative-Augmented Contrast Medium Increases the Accuracy in the Detection of Colorectal Neoplasms. Diagnostics 2024, 14, 1936. [Google Scholar] [CrossRef]
- Pagano, B.; Lauri, I.; De Tito, S.; Persico, G.; Chini, M.G.; Malmendal, A.; Novellino, E.; Randazzo, A. Use of NMR in profiling of cocaine seizures. Forensic Sci. Int. 2013, 231, 120–124. [Google Scholar] [CrossRef]
- Caraglia, M.; Correale, P.; Giannicola, R.; Staropoli, N.; Botta, C.; Pastina, P.; Nesci, A.; Caporlingua, N.; Francini, E.; Ridolfi, L.; et al. GOLFIG Chemo-Immunotherapy in Metastatic Colorectal Cancer Patients. A Critical Review on a Long-Lasting Follow-Up. Front. Oncol. 2019, 9, 1102. [Google Scholar] [CrossRef]
- Reginelli, A.; Del Canto, M.; Clemente, A.; Gragnano, E.; Cioce, F.; Urraro, F.; Martinelli, E.; Cappabianca, S. The Role of Dual-Energy CT for the Assessment of Liver Metastasis Response to Treatment: Above the RECIST 1.1 Criteria. J. Clin. Med. 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Boeken, T.; Pellerin, O.; Bourreau, C.; Palle, J.; Gallois, C.; Zaanan, A.; Taieb, J.; Lahlou, W.; Di Gaeta, A.; Al Ahmar, M.; et al. Clinical value of sequential circulating tumor DNA analysis using next-generation sequencing and epigenetic modifications for guiding thermal ablation for colorectal cancer metastases: A prospective study. Radiol. Med. 2024, 129, 1530–1542. [Google Scholar] [CrossRef]
- Patanè, V.; Atripaldi, U.; Sansone, M.; Marinelli, L.; Del Tufo, S.; Arrichiello, G.; Ciardiello, D.; Selvaggi, F.; Martinelli, E.; Reginelli, A. MRI-based radiomics for preoperative T-staging of rectal cancer: A retrospective analysis. Int. J. Color. Dis. 2025, 40, 174. [Google Scholar] [CrossRef] [PubMed]
- Pezzullo, F.; Comune, R.; D’Avino, R.; Mandato, Y.; Liguori, C.; Lassandro, G.; Tamburro, F.; Galluzzo, M.; Scaglione, M.; Tamburrini, S. CT prognostic signs of postoperative complications in emergency surgery for acute obstructive colonic cancer. Radiol. Med. 2024, 129, 525–535. [Google Scholar] [CrossRef]
- Salvà, F.; Saoudi, N.; Rodríguez, M.; Baraibar, I.; Ros, J.; García, A.; Tabernero, J.; Elez, E. Determinants of Metastatic Colorectal Cancer With Permanent Liver- Limited Disease. Clin. Color. Cancer 2024, 23, 207–214. [Google Scholar] [CrossRef]
- Patel, R.K.; Rahman, S.; Schwantes, I.R.; Bartlett, A.; Eil, R.; Farsad, K.; Fowler, K.; Goodyear, S.M.; Hansen, L.; Kardosh, A.; et al. Updated Management of Colorectal Cancer Liver Metastases: Scientific Advances Driving Modern Therapeutic Innovations. Cell Mol. Gastroenterol. Hepatol. 2023, 16, 881–894. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Patanè, V.; Sangiovanni, A.; Grassi, R.; Russo, A.; Correale, P.; Giordano, D.S.; Zaccaria, C.; Belfiore, M.P.; et al. Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy. Curr. Oncol. 2024, 31, 6673–6685. [Google Scholar] [CrossRef]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef]
- Curcean, S.; Hendea, R.M.; Buiga, R.; Tipcu, A.; Curcean, A.; Vlad, C.; Fekete, Z.; Muntean, A.S.; Martin, D.; Irimie, A. B7H3 Immune Checkpoint Overexpression Is Associated with Decreased Complete Response Rates to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Diagnostics 2024, 14, 2023. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Monzonís, X.; Vidal, J.; Linares, J.; Montagut, C. Unveiling acquired resistance to anti-EGFR therapies in colorectal cancer: A long and winding road. Front. Pharmacol. 2024, 15, 1398419. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Liu, G.; Fang, T.; Lu, N.; Bei, W.; Dong, S.; Li, W.; Xia, W.; Liang, H.; et al. Combination strategy exploration for prior treated recurrent or metastatic nasopharyngeal carcinoma in the era of immunotherapy. Sci. Rep. 2024, 14, 1768. [Google Scholar] [CrossRef]
- Leite, L.F.; Noronha, M.M.; de Menezes, J.S.A.; da Conceição, L.D.; Almeida, L.F.C.; Cappellaro, A.P.; Belotto, M.; Biachi de Castria, T.; Peixoto, R.D.A.; Megid, T.B.C. Anti-EGFR Therapy in Metastatic Colorectal Cancer: Identifying, Tracking, and Overcoming Resistance. Cancers 2025, 17, 2804. [Google Scholar] [CrossRef]
- Cremolini, C.; Montagut, C.; Ronga, P.; Venturini, F.; Yamaguchi, K.; Stintzing, S.; Sobrero, A. Rechallenge with anti-EGFR therapy to extend the continuum of care in patients with metastatic colorectal cancer. Front. Oncol. 2022, 12, 946850. [Google Scholar] [CrossRef]
- Shen, H.; Jin, Z.; Chen, Q.; Zhang, L.; You, J.; Zhang, S.; Zhang, B. Image-based artificial intelligence for the prediction of pathological complete response to neoadjuvant chemoradiotherapy in patients with rectal cancer: A systematic review and meta-analysis. Radiol. Med. 2024, 129, 598–614. [Google Scholar] [CrossRef]
- Reginelli, A.; Patanè, V.; Urraro, F.; Russo, A.; De Chiara, M.; Clemente, A.; Atripaldi, U.; Balestrucci, G.; Buono, M.; D’Ippolito, E.; et al. Magnetic Resonance Imaging Evaluation of Bone Metastases Treated with Radiotherapy in Palliative Intent: A Multicenter Prospective Study on Clinical and Instrumental Evaluation Assessment Concordance (MARTE Study). Diagnostics 2023, 13, 2334. [Google Scholar] [CrossRef]
- Nardone, V.; Belfiore, M.P.; De Chiara, M.; De Marco, G.; Patanè, V.; Balestrucci, G.; Buono, M.; Salvarezza, M.; Di Guida, G.; D’Angiolella, D.; et al. CARdioimaging in Lung Cancer PatiEnts Undergoing Radical RadioTherapy: CARE-RT Trial. Diagnostics 2023, 13, 1717. [Google Scholar] [CrossRef]
- Boldrini, L.; Chiloiro, G.; Cusumano, D.; Yadav, P.; Yu, G.; Romano, A.; Piras, A.; Votta, C.; Placidi, L.; Broggi, S.; et al. Radiomics-enhanced early regression index for predicting treatment response in rectal cancer: A multi-institutional 0.35 T MRI-guided radiotherapy study. Radiol. Med. 2024, 129, 615–622. [Google Scholar] [CrossRef]
- Smesseim, I.; Groot Lipman, K.B.W.; Lalezari, F.; Burgers, J.A.; Trebeschi, S. The legend of the response evaluation criteria in solid tumors: A historical overview. Cancer 2025, 131, e70064. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; De Marco, G.; Natale, G.; Patanè, V.; De Chiara, M.; Buono, M.; Russo, G.M.; Monti, R.; Balestrucci, G.; et al. Role of Cardiac Biomarkers in Non-Small Cell Lung Cancer Patients. Diagnostics 2023, 13, 400. [Google Scholar] [CrossRef]
- Zelenova, E.; Belysheva, T.; Sofronov, D.; Semenova, V.; Radjabova, G.; Vishnevskaya, Y.; Kletskaya, I.; Sharapova, E.; Karasev, I.; Romanov, D.; et al. Cutaneous Metastasis of Rectal Cancer as a Diagnostic Challenge: A Clinical Case and Literature Review. Diagnostics 2024, 14, 2420. [Google Scholar] [CrossRef]
- Ren, S.; Qin, B.; Daniels, M.J.; Zeng, L.; Tian, Y.; Wang, Z.Q. Developing and validating a computed tomography radiomics strategy to predict lymph node metastasis in pancreatic cancer. World J. Radiol. 2025, 17, 109373. [Google Scholar] [CrossRef]
- Lișcu, H.-D.; Verga, N.; Atasiei, D.-I.; Ilie, A.-T.; Vrabie, M.; Roșu, L.; Poștaru, A.; Glăvan, S.; Lucaș, A.; Dinulescu, M.; et al. Therapeutic Management of Locally Advanced Rectal Cancer: Existing and Prospective Approaches. J. Clin. Med. 2025, 14, 912. [Google Scholar] [CrossRef]
- Barbera, F.; Frassine, F.; Volpi, G.; Ghedi, B.; Pasinetti, N. Locally advanced cervical cancer: How the improvement in techniques in external beam radiotherapy and brachytherapy impacts on survival outcomes and long-term toxicities. Radiol. Med. 2023, 128, 1542–1552. [Google Scholar] [CrossRef]
- Inoue, A.; Tanabe, M.; Ihara, K.; Hideura, K.; Higashi, M.; Benkert, T.; Imai, H.; Yamane, M.; Yamaguchi, T.; Ueda, T.; et al. Evaluation of diffusion-weighted magnetic resonance imaging of the rectal cancers: Comparison between modified reduced field-of-view single-shot echo-planar imaging with tilted two-dimensional radiofrequency excitation pulses and conventional full field-of-view readout-segmented echo-planar imaging. Radiol. Med. 2023, 128, 1192–1198. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, S.; Liu, J.; Yin, Y.; Zhang, L.; Han, Y.; Yan, J.; Li, S. Clinical evaluation of deep learning-based automatic clinical target volume segmentation: A single-institution multi-site tumor experience. Radiol. Med. 2023, 128, 1250–1261. [Google Scholar] [CrossRef]
- Akkaya, H.; Dilek, O.; Özdemir, S.; Öztürkçü, T.; Gürbüz, M.; Tas, Z.A.; Çetinkünar, S.; Gülek, B. Rectal Cancer and Lateral Lymph Node Staging: Interobserver Agreement and Success in Predicting Locoregional Recurrence. Diagnostics 2024, 14, 2570. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef]
- Cohen, R.; Raeisi, M.; Chibaudel, B.; Shi, Q.; Yoshino, T.; Zalcberg, J.R.; Adams, R.; Cremolini, C.; Van Cutsem, E.; Heinemann, V.; et al. Prognostic value of liver metastases in colorectal cancer treated by systemic therapy: An ARCAD pooled analysis. Eur. J. Cancer 2024, 207, 114160. [Google Scholar] [CrossRef]
- Luo, Z.; Bi, X. Surgical treatment of colorectal cancer liver metastases: Individualized comprehensive treatment makes a difference. Hepatobiliary Surg. Nutr. 2021, 10, 899–901. [Google Scholar] [CrossRef]
- Martelli, V.; Vidal, J.; Gibert, J.; Fernández-Rodríguez, M.C.; Linares, J.; García-Alfonso, P.; Páez, D.; Alonso, V.; Gomez-España, M.A.; Guix, M.; et al. Clinical validation of liquid biopsy-RECIST (LB-RECIST) in metastatic colorectal cancer (mCRC) patients: Findings from the PLATFORM-B study. ESMO Open 2025, 10, 105760. [Google Scholar] [CrossRef]
- Steup, C.; Kennel, K.B.; Neurath, M.F.; Fichtner-Feigl, S.; Greten, F.R. Current and emerging concepts for systemic treatment of metastatic colorectal cancer. Gut 2025, 74, 2070–2095. [Google Scholar] [CrossRef]
- Medici, B.; Benatti, S.; Dominici, M.; Gelsomino, F. New Frontiers of Biomarkers in Metastatic Colorectal Cancer: Potential and Critical Issues. Int. J. Mol. Sci. 2025, 26, 5268. [Google Scholar] [CrossRef]
- Abdel Hamid, M.; Pammer, L.M.; Oberparleiter, S.; Günther, M.; Amann, A.; Gruber, R.A.; Mair, A.; Nocera, F.I.; Ormanns, S.; Zimmer, K.; et al. Multidimensional differences of right- and left-sided colorectal cancer and their impact on targeted therapies. npj Precis. Oncol. 2025, 9, 116. [Google Scholar] [CrossRef]
- Pagano, D.; Barresi, V.; Tropea, A.; Galvano, A.; Bazan, V.; Caldarella, A.; Sani, C.; Pompeo, G.; Russo, V.; Liotta, R.; et al. Clinical Validation of a Machine Learning-Based Biomarker Signature to Predict Response to Cytotoxic Chemotherapy Alone or Combined with Targeted Therapy in Metastatic Colorectal Cancer Patients: A Study Protocol and Review. Life 2025, 15, 320. [Google Scholar] [CrossRef]
- Papargyriou, A.; Najajreh, M.; Cook, D.P.; Maurer, C.H.; Bärthel, S.; Messal, H.A.; Ravichandran, S.K.; Richter, T.; Knolle, M.; Metzler, T.; et al. Heterogeneity-driven phenotypic plasticity and treatment response in branched-organoid models of pancreatic ductal adenocarcinoma. Nat. Biomed. Eng. 2025, 9, 836–864. [Google Scholar] [CrossRef]
- Sharma, N.; Rájová, J.; Mermelekas, G.; Thrane, K.; Lundeberg, J.; Shamikh, A.; Vikström, S.; Babačić, H.; Jensdottir, M.; Lehtiö, J.; et al. In-depth patient-specific analysis of tumor heterogeneity in melanoma brain metastasis: Insights from spatial transcriptomics and multi-region bulk sequencing. Transl. Oncol. 2025, 59, 102468. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Yang, Z.; Yu, C.K.W.; Hu, Y.; Qin, J. Tumour heterogeneity and personalized treatment screening based on single-cell transcriptomics. Comput. Struct. Biotechnol. J. 2025, 27, 307–320. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, J.; He, L.; Zhao, M.; Si, Y.; Chang, S.; Zhang, G.; Cheng, S.; Ding, W. Reconstruction of cancer marker analysis with holistic anatomical precision implicates heterogeneity development during breast tumor progression. Discov. Oncol. 2024, 15, 564. [Google Scholar] [CrossRef]
- D’Ambola, M.; Fiengo, L.; Chini, M.G.; Cotugno, R.; Bader, A.; Bifulco, G.; Braca, A.; De Tommasi, N.; Dal Piaz, F. Fusicoccane Diterpenes from Hypoestes forsskaolii as Heat Shock Protein 90 (Hsp90) Modulators. J. Nat. Prod. 2019, 82, 539–549. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, K.; Yuan, W.; Jia, Z.; Wu, Y.; Duan, X.; Yang, W.; Wen, Z.; Zhong, L.; Liu, X. A CT-based deep learning for segmenting tumors and predicting microsatellite instability in patients with colorectal cancers: A multicenter cohort study. Radiol. Med. 2025, 130, 214–225. [Google Scholar] [CrossRef]
- Ottaiano, A.; Grassi, F.; Sirica, R.; Genito, E.; Ciani, G.; Patanè, V.; Monti, R.; Belfiore, M.P.; Urraro, F.; Santorsola, M.; et al. Associations between Radiomics and Genomics in Non-Small Cell Lung Cancer Utilizing Computed Tomography and Next-Generation Sequencing: An Exploratory Study. Genes 2024, 15, 803. [Google Scholar] [CrossRef]
- Reginelli, A.; Belfiore, M.P.; Monti, R.; Cozzolino, I.; Costa, M.; Vicidomini, G.; Grassi, R.; Morgillo, F.; Urraro, F.; Nardone, V.; et al. The texture analysis as a predictive method in the assessment of the cytological specimen of CT-guided FNAC of the lung cancer. Med. Oncol. 2020, 37, 54. [Google Scholar] [CrossRef]








| Clinical Parameter | Training Dataset | Validation Dataset |
|---|---|---|
| Males | 14 | 11 |
| Females | 8 | 9 |
| <50 | 3 | 3 |
| 51–65 | 11 | 10 |
| >65 | 8 | 7 |
| Left Colon | 10 | 16 |
| Right Colon | 1 | 1 |
| Rectal Cancer | 11 | 3 |
| Synchronous Metastases | 17 | 13 |
| Metachronous Metastases | 5 | 7 |
| ECOG 0 | 15 | 16 |
| ECOG 1 | 7 | 4 |
| PFS median | 4.01 months | 3.88 months |
| OS median | 12.5 months | 14.6 months |
| Radiomic Feature | ICC | Odds Ratio (95% CI) | p-Value | Adjusted p (Bonferroni) | Significance |
|---|---|---|---|---|---|
| Δ GLCM Homogeneity | 0.91 | 0.28 (0.14–0.55) | < 0.001 | < 0.001 | Significant |
| Δ GLCM Dissimilarity | 0.88 | 2.43 (1.36–4.33) | 0.001 | 0.002 | Significant |
| Δ Entropy | 0.82 | 1.97 (1.12–3.45) | 0.009 | 0.021 | Significant |
| Δ GLCM Contrast | 0.79 | 1.42 (0.88–2.29) | 0.12 | 0.28 | n.s. |
| Δ GLCM Correlation | 0.76 | 0.91 (0.53–1.57) | 0.72 | 0.91 | n.s. |
| Δ Skewness | 0.84 | 1.05 (0.61–1.80) | 0.86 | 0.98 | n.s. |
| Δ Kurtosis | 0.73 | 0.97 (0.55–1.69) | 0.90 | 0.99 | n.s. |
| Δ Volume | 0.86 | 1.12 (0.65–1.92) | 0.67 | 0.89 | n.s. |
| Δ Sphericity | 0.80 | 0.88 (0.48–1.61) | 0.64 | 0.85 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, V.; Patanè, V.; Marinelli, L.; D’Ambrosio, L.; Del Tufo, S.; De Chiara, M.; Brunese, M.C.; Rubini, D.; Grassi, R.; Russo, A.; et al. Delta-Radiomics Biomarker in Colorectal Cancer Liver Metastases Treated with Cetuximab Plus Avelumab (CAVE Trial). Diagnostics 2025, 15, 2914. https://doi.org/10.3390/diagnostics15222914
Nardone V, Patanè V, Marinelli L, D’Ambrosio L, Del Tufo S, De Chiara M, Brunese MC, Rubini D, Grassi R, Russo A, et al. Delta-Radiomics Biomarker in Colorectal Cancer Liver Metastases Treated with Cetuximab Plus Avelumab (CAVE Trial). Diagnostics. 2025; 15(22):2914. https://doi.org/10.3390/diagnostics15222914
Chicago/Turabian StyleNardone, Valerio, Vittorio Patanè, Luca Marinelli, Luca D’Ambrosio, Sara Del Tufo, Marco De Chiara, Maria Chiara Brunese, Dino Rubini, Roberta Grassi, Anna Russo, and et al. 2025. "Delta-Radiomics Biomarker in Colorectal Cancer Liver Metastases Treated with Cetuximab Plus Avelumab (CAVE Trial)" Diagnostics 15, no. 22: 2914. https://doi.org/10.3390/diagnostics15222914
APA StyleNardone, V., Patanè, V., Marinelli, L., D’Ambrosio, L., Del Tufo, S., De Chiara, M., Brunese, M. C., Rubini, D., Grassi, R., Russo, A., Belfiore, M. P., Ciardiello, F., Cappabianca, S., Martinelli, E., & Reginelli, A. (2025). Delta-Radiomics Biomarker in Colorectal Cancer Liver Metastases Treated with Cetuximab Plus Avelumab (CAVE Trial). Diagnostics, 15(22), 2914. https://doi.org/10.3390/diagnostics15222914







