Quantitative Ultrasound for Hepatic Steatosis: A Systematic Review Highlighting the Diagnostic Performance of Ultrasound-Derived Fat Fraction
Abstract
1. Introduction
2. Materials and Methods
- ✓
- Adult population.
- ✓
- UDFF assessment of hepatic steatosis.
- ✓
- MRI-PDFF as a reference standard.
- ✓
- Quantification of liver fat content.
- ○
- Studies involving a pediatric population.
- ○
- Review articles without explicit UDFF evaluation.
- ⮚
- Study characteristics (authors, year, study design).
- ⮚
- Sample size and population demographics.
- ⮚
- Imaging techniques used (UDFF and MRI-PDFF acquisition details).
- ⮚
- Main findings, including AUC, ICC values, and diagnostic performance metrics.
- ⮚
- ⮚
- ⮚
- ⮚
- ⮚
- ⮚
- ⮚
- AUC: Measure of diagnostic accuracy. Values range from 0.5 (no discrimination) to 1.0 (perfect discrimination).
- ⮚
- ICC: Reliability statistics describing inter- or intra-observer agreement. Values >0.90 indicate excellent agreement.
- ⮚
- UDFF cut-off values: Thresholds (expressed as %) used to define mild, moderate, and severe hepatic steatosis.
- ⮚
- Sensitivity and specificity: The true positives and true negatives correctly identified by UDFF at a given threshold.
- ⮚
- r Pearson’s correlation: Between UDFF and MRI-PDFF values. Values close to +1 indicate strong positive correlation.
Statistical Analysis
3. Results
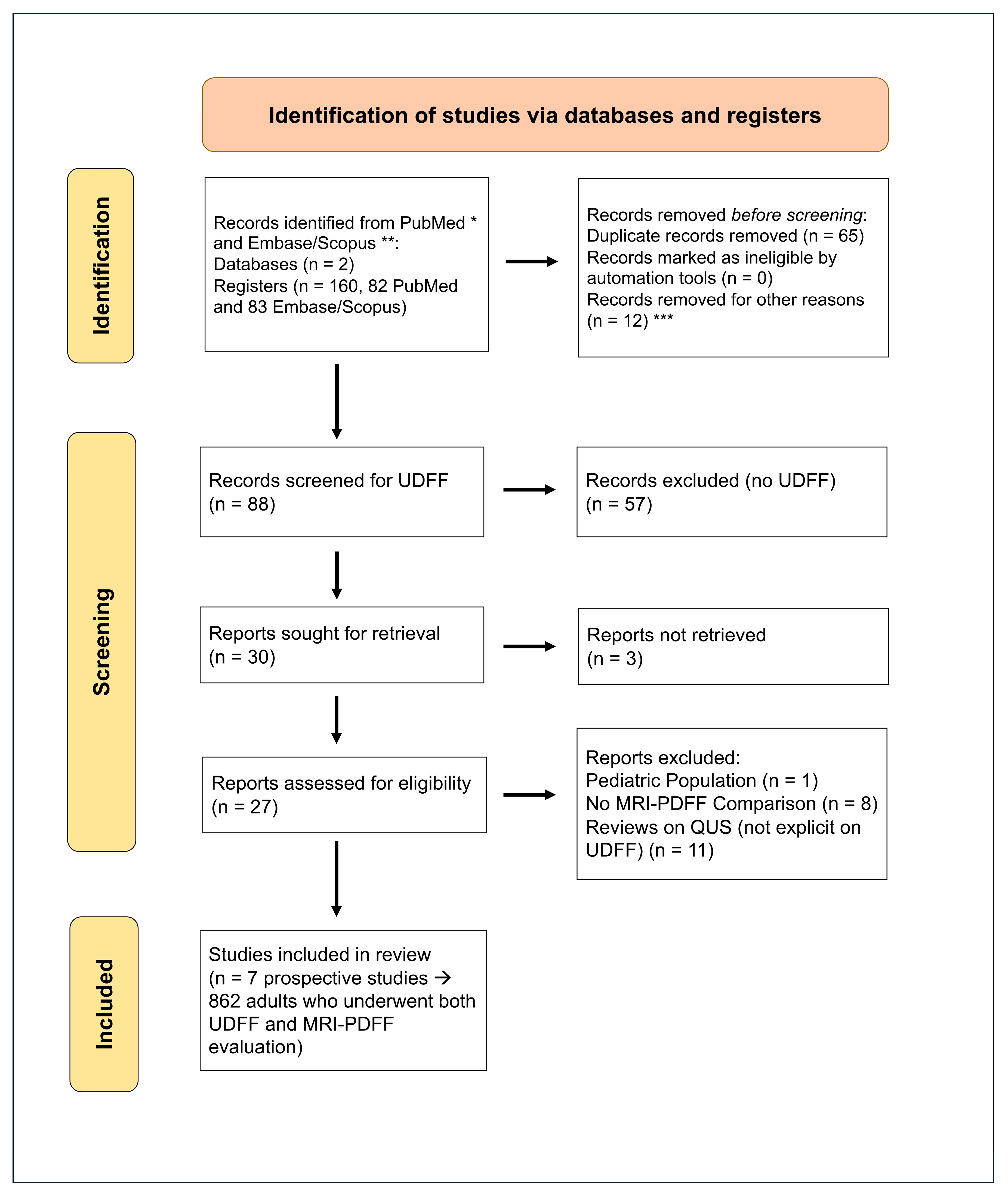
3.1. Study Selection and Characteristics
3.2. Diagnostic Performance of UDFF vs. MRI-PDFF
- S1 (mild): 0.747–0.99 (heterogeneous at MRI-PDFF threshold of 5.0% and in healthy/low-fat cohorts).
- S2 (moderate): 0.95–0.96 (consistently excellent).
- S3 (severe): 0.95–0.97 (consistently excellent).
Correlation Analysis of UDFF vs. MRI-PDFF
- ⮚
- Five studies (n = 570: Labyed 2020 [11]; Dillman 2022 [13]; De Robertis 2023 [3]; Qi 2024 [6]; Wang 2024 [15]) were pooled using a random effects model on the Fisher-z scale. The pooled correlation was r = 0.85 (95% CI 0.81–0.89), with I2 = 67%, Q = 12.19, and τ2 = 0.019; the prediction interval was 0.74–0.92. This supports a strong association between UDFF and MRI-PDFF while indicating moderate between-study heterogeneity.
- ⮚
- ⮚
- ⮚
- Thresholds were provided based on descriptive statistics:
- ○
- S1: Median 0.90, range 0.747–0.99 (n-weighted mean ~0.874).
- ○
- S2: Median 0.95, range 0.950–0.960 (n-weighted mean ~0.952).
- ○
- S3: Median 0.95, range 0.950–0.970 (n-weighted mean ~0.955).
3.3. Diagnostic Performance of UDFF vs. Other Modalities
3.4. Proposed Cut-Off Values for UDFF in Steatosis Grading
3.5. Factors Affecting UDFF Performance
- ○
- ○
- Patient Positioning: Supine or slightly (30°) left lateral decubitus position and right arm raised above their head to optimize intercostal access [4,16].
- A study also suggests dorsal decubitus position with maximal right arm abduction [25].
- ○
- ○
- Selecting Region of Interest (ROI): Artifact-free area within the right lobe of the liver (no vessels, no large hepatic ducts, no rib shadows, etc.) with the ROI at 1.5 to 2 cm below the liver capsule and the liver capsule marker parallel with the echogenic interface of the liver capsule [4,16].
- A study suggests investigating how the depth at which measurements are made (1.5, 2, 3, 4, and 5 cm below the liver capsule) affects the accuracy and reliability of the UDFF values [30].
- ○
- Patient Breathing Instructions: Before measurement acquisition, ask the patient to hold their breath for 10–15 s until acquisition is complete [4,11,16].
- Song et al. preferred the end-expiratory breath-hold for more consistent readings [22].
- ○
- Ten ROIs were suggested to be placed at different levels in the right hepatic lobe, and the median was calculated for analysis [3].
4. Discussion
4.1. Economic and Environmental Considerations
4.2. Future Research
4.3. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Attenuation coefficient |
| AUC | Area Under the Curve |
| BSC | Backscatter coefficient |
| BSC-D | Backscatter coefficient—derived |
| CAP | Controlled Attenuation Parameter |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| iATT | Integrated Attenuation |
| MAFLD | Metabolic-dysfunction-associated fatty liver disease |
| MASLD | Metabolic-dysfunction-associated steatotic liver disease |
| MRI | Magnetic resonance imaging |
| MRI-PDFF | Magnetic Resonance Imaging Proton Density Fat Fraction |
| NAFLD | Nonalcoholic fatty liver disease |
| pSWE | point SWE |
| QUS | Quantitative ultrasound |
| ROI | Region of Interest |
| StC | Skin-to-Capsule distance |
| SWE | Shear Wave Elastography |
| SWV | Shear Wave Velocity |
| TAI | Tissue Attenuation Imaging |
| TSI | Tissue Scatter Distribution Imaging |
| UDFF | Ultrasound-Derived Fat Fraction |
| UGAP | Ultrasound-Guided Attenuation Parameter |
| USFF | Ultrasound fat fraction |
Appendix A
| Study (Author, Year) | Patient Selection | Index Test (UDFF) | Reference Standard (MRI-PDFF) | Flow and Timing | Overall Risk of Bias |
|---|---|---|---|---|---|
| Labyed et al., 2020 [11] | Low (adults with NAFLD, clear inclusion) | Low (UDFF applied consistently, blinding not specified → could be unclear) | Low (3T MRI-PDFF, robust method) | Low | Low–moderate |
| Dillman et al., 2022 [13] | Low (overweight/obese adults prospectively enrolled) | Low (prespecified UDFF measurements, reproducibility reported) | Low (3T MRI-PDFF, reference standard) | Low | Low |
| De Robertis et al., 2023 [3] | Low (healthy adults, clear criteria) | Unclear (thresholds less well-defined, reproducibility not fully reported) | Low | Low | Low–moderate |
| Kubale et al., 2024 [14] | Low (broad patient population) | Unclear (variability with dietary state, thresholds not prespecified) | Low (3T MRI-PDFF) | Low | Low–moderate |
| Qi et al., 2024 [6] | Low (well-defined adult cohort) | Low (multiple cofactors assessed, thresholds prespecified) | Low (3T MRI-PDFF, Siemens) | Low | Low |
| Song et al., 2024 [22] | Low (MASLD patients) | Low (careful analysis of body position/respiration, reproducibility tested) | Low (MRI-PDFF, subset at 1.5T) | Low | Low |
| Wang et al., 2024 [15] | Low (MASLD patients, risk-stratified) | Low (reproducibility high, thresholds prespecified) | Low (3T MRI-PDFF) | Low | Low |
References
- Chen, L.; Tao, X.; Zeng, M.; Mi, Y.; Xu, L. Clinical and Histological Features under Different Nomenclatures of Fatty Liver Disease: NAFLD, MAFLD, MASLD and MetALD. J. Hepatol. 2024, 80, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lee, R.; Trujillo, M. Reliability of Performing Multiparametric Ultrasound in Adult Livers. J. Ultrasound Med. 2022, 41, 699–711. [Google Scholar] [CrossRef]
- De Robertis, R.; Spoto, F.; Autelitano, D.; Guagenti, D.; Olivieri, A.; Zanutto, P.; Incarbone, G.; D’Onofrio, M. Ultrasound-Derived Fat Fraction for Detection of Hepatic Steatosis and Quantification of Liver Fat Content. La Radiol. Medica 2023, 128, 1174–1180. [Google Scholar] [CrossRef]
- Ferraioli, G.; Berzigotti, A.; Barr, R.G.; Choi, B.I.; Cui, X.W.; Dong, Y.; Gilja, O.H.; Lee, J.Y.; Lee, D.H.; Moriyasu, F.; et al. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Raimondi, A.; Silvestri, A.D.; Filice, C.; Barr, R.G. Toward Acquisition Protocol Standardization for Estimating Liver Fat Content Using Ultrasound Attenuation Coefficient Imaging. Ultrasonography 2023, 42, 446. [Google Scholar] [CrossRef]
- Qi, R.; Lu, L.; He, T.; Zhang, L.; Lin, Y.; Bao, L. Comparing Ultrasound-Derived Fat Fraction and MRI-PDFF for Quantifying Hepatic Steatosis: A Real-World Prospective Study. Eur. Radiol. 2024, 35, 2580–2588. [Google Scholar] [CrossRef]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Non-Invasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef]
- Bozic, D.; Podrug, K.; Mikolasevic, I.; Grgurevic, I. Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics 2022, 12, 2287. [Google Scholar] [CrossRef]
- Jamalinia, M.; Zare, F.; Noorizadeh, K.; Bagheri Lankarani, K. Systematic Review with Meta-Analysis: Steatosis Severity and Subclinical Atherosclerosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Aliment. Pharmacol. Ther. 2024, 59, 445–458. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Zhang, Y.; Zhan, X.; Tian, X.; Li, J.; Wang, R.; He, Y.; Wang, A.; Wu, S. Severity and Remission of Metabolic Dysfunction-Associated Fatty/Steatotic Liver Disease With Chronic Kidney Disease Occurrence. J. Am. Heart Assoc. 2024, 13, e032604. [Google Scholar] [CrossRef] [PubMed]
- Labyed, Y.; Milkowski, A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J. Ultrasound Med. 2020, 39, 2427–2438. [Google Scholar] [CrossRef]
- De Rosa, L.; Salvati, A.; Martini, N.; Chiappino, D.; Cappelli, S.; Mancini, M.; Demi, L.; Ghiadoni, L.; Bonino, F.; Brunetto, M.R.; et al. An Ultrasound Multiparametric Method to Quantify Liver Fat Using Magnetic Resonance as Standard Reference. Liver Int. 2024, 44, 3008–3019. [Google Scholar] [CrossRef]
- Dillman, J.R.; Thapaliya, S.; Tkach, J.A.; Trout, A.T. Quantification of Hepatic Steatosis by Ultrasound: Prospective Comparison With MRI Proton Density Fat Fraction as Reference Standard. Am. J. Roentgenol. 2022, 219, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Kubale, R.; Schneider, G.; Lessenich, C.P.N.; Buecker, A.; Wassenberg, S.; Torres, G.; Gurung, A.; Hall, T.; Labyed, Y. Ultrasound-Derived Fat Fraction for Hepatic Steatosis Assessment: Prospective Study of Agreement With MRI PDFF and Sources of Variability in a Heterogeneous Population. Am. J. Roentgenol. 2024, 222, e2330775. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, D.; Han, J.; Zhang, J.; Chen, H.; Gao, R.; Shen, H.; Li, J. Comparing Three Ultrasound-Based Techniques for Diagnosing and Grading Hepatic Steatosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Acad. Radiol. 2024, 32, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Siemens Healthineers. White Paper: Ultrasound Derived Fat Fraction (UDFF). 2024. Available online: https://www.siemens-healthineers.com/gr/ultrasound/new-era-ultrasound/acuson-sequoia/udff-liver-whitepaper (accessed on 1 September 2025).
- Wear, K.A.; Han, A.; Rubin, J.M.; Gao, J.; Lavarello, R.; Cloutier, G.; Bamber, J.; Tuthill, T. US Backscatter for Liver Fat Quantification: An AIUM-RSNA QIBA Pulse-Echo Quantitative Ultrasound Initiative. Radiology 2022, 305, 526–537. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M. Inter-Platform Reproducibility of Ultrasound-Based Fat Fraction for Evaluating Hepatic Steatosis in Nonalcoholic Fatty Liver Disease. Insights Imaging 2024, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kavvadas, D.; Rafailidis, V.; Liakos, A.; Sinakos, E.; Partovi, S.; Papamitsou, T.; Prassopoulos, P. Quantitative Ultrasound for Hepatic Steatosis: A Systematic Review Highlighting the Diagnostic Performance of Ultrasound-Derived Fat Fraction; INPLASY—International Platform of Registered Systematic Review and Meta-analysis Protocols: Brasília, Brazil, 2025. [Google Scholar]
- Nakamura, Y.; Hirooka, M.; Koizumi, Y.; Yano, R.; Imai, Y.; Watanabe, T.; Yoshida, O.; Tokumoto, Y.; Abe, M.; Hiasa, Y. Diagnostic Accuracy of Ultrasound-Derived Fat Fraction for the Detection and Quantification of Hepatic Steatosis in Patients with Liver Biopsy. J. Med. Ultrason. 2025, 52, 85–94. [Google Scholar] [CrossRef]
- Song, D.; Wang, P.; Han, J.; Chen, H.; Gao, R.; Li, L.; Li, J. Reproducibility of Ultrasound-Derived Fat Fraction in Measuring Hepatic Steatosis. Insights Imaging 2024, 15, 254. [Google Scholar] [CrossRef]
- Gao, J.; Wong, C.; Maar, M.; Park, D. Reliability of Performing Ultrasound Derived SWE and Fat Fraction in Adult Livers. Clin. Imaging 2021, 80, 424–429. [Google Scholar] [CrossRef]
- Sporea, I.; Lupusoru, R.; Sirli, R.; Bende, F.; Cotrau, R.; Foncea, C.; Popescu, A. Fatty liver quantification using ultrasound derived fat fraction (UDFF) as compared to controlled attenuation parameter (cap) in a mixed cohort of patients. Ultrasound Med. Biol. 2022, 48, S16. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Cheng, J.; Wang, Y.; Xu, X.-L.; Wang, S.-W.; Wei, L.; Dong, Y. Hepatic Steatosis Using Ultrasound-Derived Fat Fraction: First Technical and Clinical Evaluation. Clin. Hemorheol. Microcirc. 2024, 86, 51–61. [Google Scholar] [CrossRef]
- Tavaglione, F.; Flagiello, V.; Terracciani, F.; Gallo, P.; Capparelli, E.; Spiezia, C.; De Vincentis, A.; Palermo, A.; Scriccia, S.; Galati, G.; et al. Non-Invasive Assessment of Hepatic Steatosis by Ultrasound-Derived Fat Fraction (UDFF) in Individuals at High-Risk for Metabolic Dysfunction-Associated Steatotic Liver Disease. Dig. Liver Dis. 2024, 56, S51. [Google Scholar] [CrossRef]
- Meng, L.; Yang, H.; Hu, Y.; Jiang, Y.; Yang, Z. Evaluation of Ultrasound Derived Fat Fraction for Metabolic Associated Fatty Liver Disease in Obese Patients with Polycystic Ovary Syndrome. J. Ultrasound 2025, 28, 379–387. [Google Scholar] [CrossRef]
- Ferraioli, G. Editorial Comment: A Step Toward the Clinical Application of Quantitative Ultrasound in the Diagnosis of Steatotic Liver Disease. Am. J. Roentgenol. 2024, 222, e2431215. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Raimondi, A.; Maiocchi, L.; De Silvestri, A.; Poma, G.; Kumar, V.; Barr, R.G. Liver Fat Quantification With Ultrasound: Depth Dependence of Attenuation Coefficient. J. Ultrasound Med. 2023, 42, 2247–2255. [Google Scholar] [CrossRef]
- Ferraioli, G.; Roccarina, D.; Barr, R.G. Intersystem and Interoperator Agreement of US Attenuation Coefficient for Quantifying Liver Steatosis. Radiology 2024, 313, e240162. [Google Scholar] [CrossRef] [PubMed]
- Brancato, V.; Della Pepa, G.; Bozzetto, L.; Vitale, M.; Annuzzi, G.; Basso, L.; Cavaliere, C.; Salvatore, M.; Rivellese, A.A.; Monti, S. Evaluation of a Whole-Liver Dixon-Based MRI Approach for Quantification of Liver Fat in Patients with Type 2 Diabetes Treated with Two Isocaloric Different Diets. Diagnostics 2022, 12, 514. [Google Scholar] [CrossRef]
- Jesper, D.; Klett, D.; Schellhaas, B.; Pfeifer, L.; Leppkes, M.; Waldner, M.; Neurath, M.F.; Strobel, D. Ultrasound-Based Attenuation Imaging for the Non-Invasive Quantification of Liver Fat—A Pilot Study on Feasibility and Inter-Observer Variability. IEEE J. Transl. Eng. Health Med. 2020, 8, 1800409. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Yasuda, S.; Sone, Y.; Hashinokuchi, S.; Ogawa, S.; Oguri, T.; Kamiyama, N.; Chuma, M.; et al. Liver Stiffness Does Not Affect Ultrasound-Guided Attenuation Coefficient Measurement in the Evaluation of Hepatic Steatosis. Hepatol. Res. 2020, 50, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Jacq, T.; Anselme, S.; Ursic-Bedoya, J.; Blanc, P.; Faure, S.; Belgour, A.; Guiu, B. Diagnostic Performance of Attenuation to Stage Liver Steatosis with MRI Proton Density Fat Fraction as Reference: A Prospective Comparison of Three US Machines. Radiology 2022, 305, 353–361. [Google Scholar] [CrossRef]
- De Rosa, L.; Salvati, A.; Bonino, F.; Brunetto, M.R.; Demi, L.; Faita, F. Non-Invasive Quantification of Steatosis: A New Ultrasound Based Model to Predict Fatty Liver Content. In Proceedings of the 2022 IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022. [Google Scholar]
- Kuroda, H.; Oguri, T.; Kamiyama, N.; Toyoda, H.; Yasuda, S.; Imajo, K.; Suzuki, Y.; Sugimoto, K.; Akita, T.; Tanaka, J.; et al. Multivariable Quantitative US Parameters for Assessing Hepatic Steatosis. Radiology 2023, 309, e230341. [Google Scholar] [CrossRef]
- Byenfeldt, M.; Kihlberg, J.; Nasr, P.; Grönlund, C.; Lindam, A.; Bartholomä, W.C.; Lundberg, P.; Ekstedt, M. Altered Probe Pressure and Body Position Increase Diagnostic Accuracy for Men and Women in Detecting Hepatic Steatosis Using Quantitative Ultrasound. Eur. Radiol. 2024, 34, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.H.; Cho, S.J.; Choi, S.C.; Jee, J.H.; Yun, J.; Hwang, J.A.; Park, G.; Kim, K.; Kang, W.; Kang, M.; et al. Diagnostic Performance of Quantitative Ultrasonography for Hepatic Steatosis in a Health Screening Program: A Prospective Single-Center Study. Ultrasonography 2024, 43, 250–262. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, J.; Huang, C.; Lok, U.-W.; Tang, S.; Liu, H.; DeRuiter, R.; Petersen, K.; Knoll, K.; Robinson, K.; et al. Novel Quantitative Liver Steatosis Assessment Method With Ultrasound Harmonic Imaging. J. Ultrasound Med. 2025, 44, 77–85. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Park, S.J. Quantitative Ultrasound Radiofrequency Data Analysis for the Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Using Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Korean J. Radiol. 2021, 22, 1077–1086. [Google Scholar] [CrossRef]
- Rónaszéki, A.D.; Budai, B.K.; Csongrády, B.; Stollmayer, R.; Hagymási, K.; Werling, K.; Fodor, T.; Folhoffer, A.; Kalina, I.; Győri, G.; et al. Tissue Attenuation Imaging and Tissue Scatter Imaging for Quantitative Ultrasound Evaluation of Hepatic Steatosis. Medicine 2022, 101, e29708. [Google Scholar] [CrossRef]
- Zsombor, Z.; Rónaszéki, A.D.; Csongrády, B.; Stollmayer, R.; Budai, B.K.; Folhoffer, A.; Kalina, I.; Győri, G.; Bérczi, V.; Maurovich-Horvat, P.; et al. Evaluation of Artificial Intelligence-Calculated Hepatorenal Index for Diagnosing Mild and Moderate Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease. Medicina 2023, 59, 469. [Google Scholar] [CrossRef]
- Boglárka, Z.; Zsombor, Z.; Rónaszéki, A.D.; Egresi, A.; Stollmayer, R.; Himsel, M.; Bérczi, V.; Kalina, I.; Werling, K.; Győri, G.; et al. Construction of a Compound Model to Enhance the Accuracy of Hepatic Fat Fraction Estimation with Quantitative Ultrasound. Diagnostics 2025, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Yoon, J.H.; Lee, G. Two-Dimensional Convolutional Neural Network Using Quantitative US for Noninvasive Assessment of Hepatic Steatosis in NAFLD. Radiology 2023, 307, e221510. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Kim, Y.R.; Kang, D.M.; Yoon, K.H.; Lee, Y.H. Usefulness of US Attenuation Imaging for the Detection and Severity Grading of Hepatic Steatosis in Routine Abdominal Ultrasonography. Clin. Imaging 2021, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zapata, I.; Chen, J.; Erpelding, T.N.; Adamson, C.; Park, D. Quantitative Ultrasound Biomarkers to Assess Nonalcoholic Fatty Liver Disease. J. Ultrasound Med. 2023, 42, 1675–1688. [Google Scholar] [CrossRef]
- Ogawa, S.; Kumada, T.; Gotoh, T.; Niwa, F.; Toyoda, H.; Tanaka, J.; Shimizu, M. A Comparative Study of Hepatic Steatosis Using Two Different Qualitative Ultrasound Techniques Measured Based on Magnetic Resonance Imaging-Derived Proton Density Fat Fraction. Hepatol. Res. 2024, 54, 638–654. [Google Scholar] [CrossRef]
- Gotoh, T.; Kumada, T.; Ogawa, S.; Niwa, F.; Toyoda, H.; Hirooka, M.; Koizumi, Y.; Hiasa, Y.; Akita, T.; Tanaka, J.; et al. Comparison Between Attenuation Measurement and the Controlled Attenuation Parameter for the Assessment of Hepatic Steatosis Based on MRI Images. Liver Int. 2025, 45, e16210. [Google Scholar] [CrossRef] [PubMed]
- Kaposi, P.N.; Zsombor, Z.; Rónaszéki, A.D.; Budai, B.K.; Csongrády, B.; Stollmayer, R.; Kalina, I.; Győri, G.; Bérczi, V.; Werling, K.; et al. The Calculation and Evaluation of an Ultrasound-Estimated Fat Fraction in Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease. Diagnostics 2023, 13, 3353. [Google Scholar] [CrossRef]
- Verdan, S.; Torri, G.B.; Marcos, V.N.; Moreira, M.H.S.; Defante, M.L.R.; Fagundes, M.D.C.; De Barros, E.M.J.; Dias, A.B.; Shen, L.; Altmayer, S. Ultrasound-Derived Fat Fraction for Diagnosing Hepatic Steatosis: A Systematic Review and Meta-Analysis. Eur. Radiol. 2025. [Google Scholar] [CrossRef]
- Smajerova, M.; Petrasova, H.; Little, J.; Ovesna, P.; Andrasina, T.; Valek, V.; Nemcova, E.; Miklosova, B. Contrast-Enhanced Ultrasonography in the Evaluation of Incidental Focal Liver Lesions: A Cost-Effectiveness Analysis. World J. Gastroenterol. 2016, 22, 8605. [Google Scholar] [CrossRef] [PubMed]
- Burak, K.W.; Douglas, L.; Congly, S.E. Comparing Magnetic Resonance Imaging and Contrast-Enhanced Ultrasound (CEUS) for the Characterization of Nodules Found on Hepatocellular Carcinoma Surveillance: CEUS Is Our Clear Choice. J. Ultrasound Med. 2023, 42, 1175–1180. [Google Scholar] [CrossRef]
- UK Parliament. Community Diagnostic Centres: Costs. 2025. Available online: https://questions-statements.parliament.uk/written-questions/detail/2025-06-23/61878/ (accessed on 1 September 2025).
- Centers for Medicare & Medicaid Services Calendar Year (CY) 2025 Medicare Physician Fee Schedule Final Rule. Medicare Parts A & B 2024. Available online: https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule (accessed on 1 September 2025).
- McAlister, S.; McGain, F.; Breth-Petersen, M.; Story, D.; Charlesworth, K.; Ison, G.; Barratt, A. The Carbon Footprint of Hospital Diagnostic Imaging in Australia. Lancet Reg. Health—West. Pac. 2022, 24, 100459. [Google Scholar] [CrossRef]
- Mariampillai, J.; Rockall, A.; Manuellian, C.; Cartwright, S.; Taylor, S.; Deng, M.; Sheard, S. The Green and Sustainable Radiology Department. Radiologie 2023, 63, 21–26. [Google Scholar] [CrossRef]
- Roletto, A.; Zanardo, M.; Bonfitto, G.R.; Catania, D.; Sardanelli, F.; Zanoni, S. The Environmental Impact of Energy Consumption and Carbon Emissions in Radiology Departments: A Systematic Review. Eur. Radiol. Exp. 2024, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.V.; Vosshenrich, J.; McKee, H.; Gunasekaran, S.; Brown, M.J.; Atalay, M.K.; Heye, T.; Markl, M.; Woolen, S.A.; Simonetti, O.P.; et al. Environmental Sustainability and MRI: Challenges, Opportunities, and a Call for Action. Magn. Reson. Imaging 2024, 59, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Woolen, S.A.; Becker, A.E.; Martin, A.J.; Knoerl, R.; Lam, V.; Folsom, J.; Eusemann, C.; Hess, C.P.; Deshpande, V. Ecodesign and Operational Strategies to Reduce the Carbon Footprint of MRI for Energy Cost Savings. Radiology 2023, 307, e230441. [Google Scholar] [CrossRef] [PubMed]
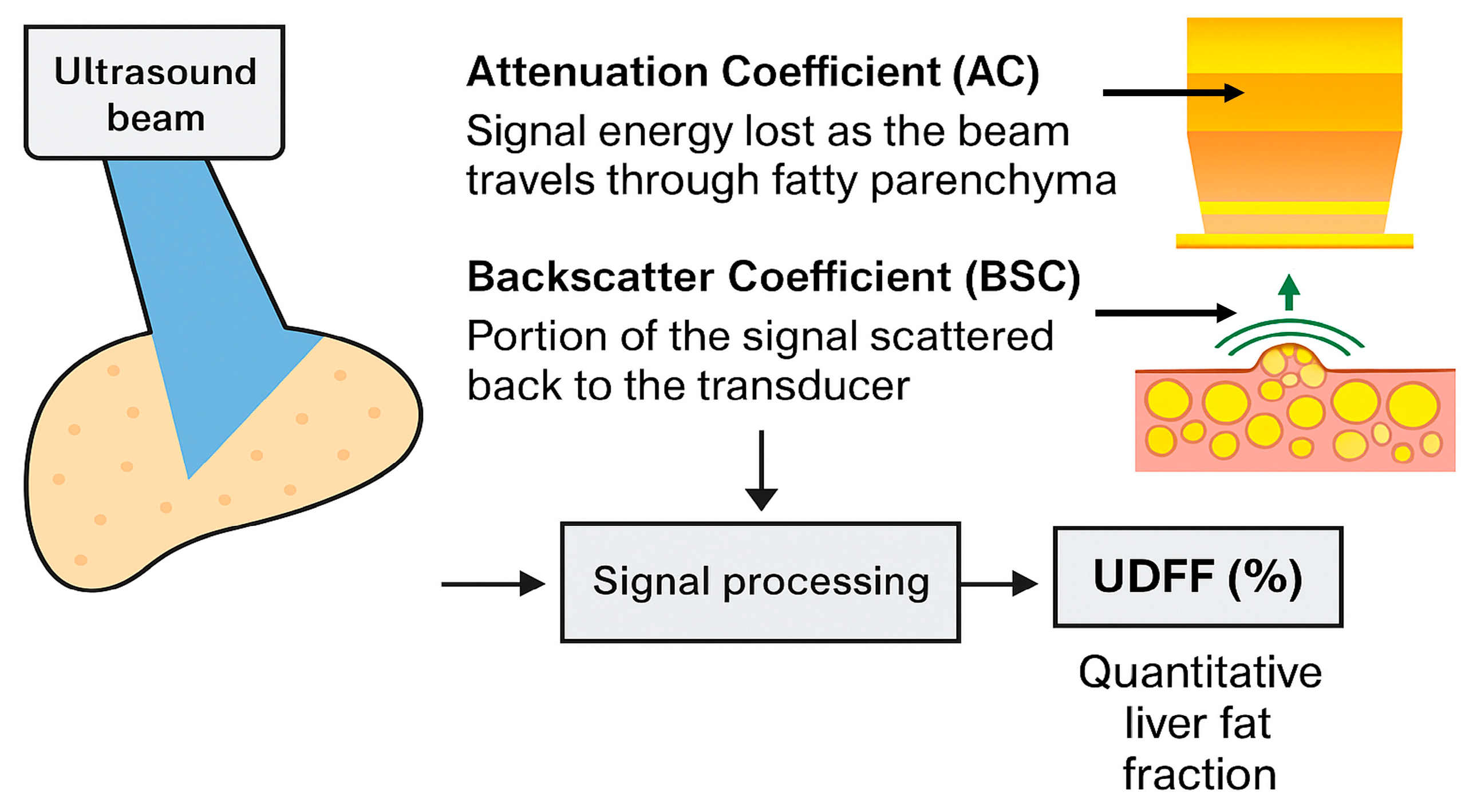
| Authors (Year) | Sample Size | MRI–PDFF Vendor/ Threshold (%) | Cofactors Evaluated | UDFF/ MRI-PDFF Correlation | Intra-Observer Agreement UDFF | Inter-Observer Agreement UDFF | UDFF AUC (Diagnostic Cut-Off > 5%) |
|---|---|---|---|---|---|---|---|
| Labyed et al. (2020) [11] | 101 adults with suspected or known NAFLD | 3T MRI (Signa Excite HD, GE Healthcare)/≥5.0% | BMI | r: 0.870 | NR | NR | 0.97 |
| Dillman et al. (2022) [13] | 56 overweight and obese adults | 3T MRI (GE SIGNA Architect)/≥5.5% | BMI, waist circumference, body positioning | ICC: 0.840 r: 0.820 | 0.98 | NR | 0.90 for ≥S1 |
| De Robertis et al. (2023) [3] | 122 healthy adults (steatosis grade < 2) | 3T MRI (Philips Ingenia Elition S)/≥5.0% | NR | r: 0.808 | NR | NR | 0.747 for ≥S1 |
| Kubale et al. (2024) [14] | 187 patients undergoing liver MRI for various indications | 3T MRI/≥5.0% | Dietary state | ICC: 0.790 | 0.985 | 0.935 | 0.90 for ≥S1, 0.95 for ≥S2, 0.95 for ≥S3 |
| Qi et al. (2024) [6] | 176 patients undergoing liver evaluation | 3T MRI (Siemens Magnetom Verio)/≥5.5% | ΒΜΙ | ICC: 0.899 r: 0.831 | 0.992 | 0.951 | 0.85 for ≥S1, 0.95 for ≥S2, 0.95 for ≥S3 |
| Song et al. (2024) [22] | 105 MASLD patients | 1.5T MRI (Siemens Magnetom Aera) on 25 patients (25/105)/≥5.0% | BMI, Skin-to-Capsule (StC) distance, body position, respiration, dietary state | NR | 0.960 | 0.940 | NR |
| Wang et al. (2024) [15] | 115 MASLD patients | 3T MRI-PDFF | Age, BMI, waist-to-hip ratio/ ≥5.0% | r: 0.910 | NR | 0.960 | 0.99 for ≥S1, 0.96 for ≥S2, 0.97 for ≥S3 |
| Total of 7 prospective studies on UDFF | 862 patients with MASLD or risk factors for developing MASLD | Various MRI systems (mostly 3T) | Almost the same in each study | ICC (average) = 0.843 (std. 0.04) r (average) = 0.848 (std. 0.04) | ICC (average) = 0.98 | ICC (average) = 0.978 | AUC (average) for ≥S1: 0.887) |
| Authors (Year) | Sample Size | Comparisons with Other Modalities | Cofactors Evaluated | UDFF Correlations | Intra-Observer Agreement | Inter-Observer Agreement | UDFF AUC (Diagnostic Cut-Off > 5%) |
|---|---|---|---|---|---|---|---|
| Gao et al. (2021) [23] | 21 adult volunteers | Auto-pSWE, pSWE | BMI, StC distance | NR | 0.97–0.99 | 0.87–0.96 | NR |
| Sporea et al. (2022) [24] | 271 patients, with or without chronic liver disease | CAP | BMI | UDFF/CAP r: 0.750 | NR | NR | 0.92 for ≥S1, 0.95 for ≥S2, 0.93 for ≥S3 |
| Huang et al. (2024) [25] | 38 adults with suspected MASLD | B-mode ultrasound | Age, gender, hepatic segment, StC distance | NR | 0.882 | NR | NR |
| Tavaglione et al. (2024) [26] | 302 obese individuals at high risk for MASLD | CAP and Hamaguchi scores | BMI, ALT, triglycerides, visceral adipose tissue | UDFF/CAP r: 0.730 UDFF/Hamaguchi score r: 0.790 | NR | NR | 0.92 for ≥S1 |
| Chen et al. (2024) [1] | 6 Bama minipigs | Histopathological biopsy | BMI, triglycerides, total cholesterol, HDL, LDL | UDFF/NAFLD Activity Score r: 0.800 | NR | NR | 0.95 for ≥S1 |
| Jeon et al. (2024) [18] | 41 adults with suspected MASLD | USFF | Visual hepatic steatosis grade, BMI, StC distance | UDFF/USFF r: 0.748 ICC: 0.842 | NR | 0.963 | NR |
| Nakamura et al. (2025) [21] | 73 MASLD patients | Liver biopsy | BMI, StC distance | NR | NR | NR | 0.956 for ≥S1, 0.926 for ≥S2, 0.971 for ≥S3 |
| Meng et al. (2025) [27] | 124 obese PCOS patients | Shear Wave Velocity (SWV), MAFLD stage | BMI, insulin resistance (HOMA-IR), testosterone, lipid profile | UDFF/MAFLD r: 0.603 | NR | NR | 0.935 for ≥S1 |
| Authors (Year) | UDFF Mild Steatosis Cut-Off (%) (Se/Sp, AUC) | UDFF Moderate Steatosis Cut-Off (%) (Se/Sp, AUC) | UDFF Severe Steatosis Cut-Off (%) (Se/Sp, AUC) |
|---|---|---|---|
| Labyed et al. (2020) [11] | 5.0 (NR/NR, 0.95) | 10.0 (NR/NR, 0.95) | NR |
| Sporea et al. (2022) [24] | 5.0 | 10.0 | 15.0 |
| Dillman et al. (2022) [13] | 5.5 (0.94/0.64, 0.90) | NR | NR |
| De Robertis et al. (2023) [3] | 5.0 (0.80/0.66, 0.75) | NR | NR |
| Chen et al. (2024) [1] | 5.5 (0.80/0.96, 0.95) | NR | NR |
| Qi et al. (2024) [6] | 5.5 (0.79/0.82, 0.85) | 15.5 (0.86/0.91, 0.95) | 17.5 (0.89/0.90, 0.95) |
| Kubale et al. (2024) [14] | 6.5 (NR/NR, 0.90) | 17.4 (NR/NR, 0.95) | 22.1 (NR/NR, 0.95) |
| Wang et al. (2024) [15] | 6.0 (NR/NR, 0.99) | 15.0 (NR/NR, 0.96) | 23.0 (NR/NR, 0.97) |
| Meng et al. (2025) [27] | 4.5 (0.92/0.85, 0.94) | NR | NR |
| Nakamura et al. (2025) [21] | 6.0 (0.95/0.82, 0.96) | 13.0 (0.77/0.88, 0.93) | 23.0 (1.00/0.94, 0.97) |
| Recommendation for further study | 5–6% UDFF for initial MASLD detection/mild steatosis | 10–15% for mild to moderate and 15–17.5% for moderate steatosis | 17.5–22% for moderate to severe steatosis and 23% for severe steatosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavvadas, D.; Rafailidis, V.; Liakos, A.; Sinakos, E.; Partovi, S.; Papamitsou, T.; Prassopoulos, P. Quantitative Ultrasound for Hepatic Steatosis: A Systematic Review Highlighting the Diagnostic Performance of Ultrasound-Derived Fat Fraction. Diagnostics 2025, 15, 2640. https://doi.org/10.3390/diagnostics15202640
Kavvadas D, Rafailidis V, Liakos A, Sinakos E, Partovi S, Papamitsou T, Prassopoulos P. Quantitative Ultrasound for Hepatic Steatosis: A Systematic Review Highlighting the Diagnostic Performance of Ultrasound-Derived Fat Fraction. Diagnostics. 2025; 15(20):2640. https://doi.org/10.3390/diagnostics15202640
Chicago/Turabian StyleKavvadas, Dimitrios, Vasileios Rafailidis, Aris Liakos, Emmanouil Sinakos, Sasan Partovi, Theodora Papamitsou, and Panos Prassopoulos. 2025. "Quantitative Ultrasound for Hepatic Steatosis: A Systematic Review Highlighting the Diagnostic Performance of Ultrasound-Derived Fat Fraction" Diagnostics 15, no. 20: 2640. https://doi.org/10.3390/diagnostics15202640
APA StyleKavvadas, D., Rafailidis, V., Liakos, A., Sinakos, E., Partovi, S., Papamitsou, T., & Prassopoulos, P. (2025). Quantitative Ultrasound for Hepatic Steatosis: A Systematic Review Highlighting the Diagnostic Performance of Ultrasound-Derived Fat Fraction. Diagnostics, 15(20), 2640. https://doi.org/10.3390/diagnostics15202640









