Advances in Point-of-Care Infectious Disease Diagnostics: Integration of Technologies, Validation, Artificial Intelligence, and Regulatory Oversight
Abstract
1. Introduction
2. Market Analysis
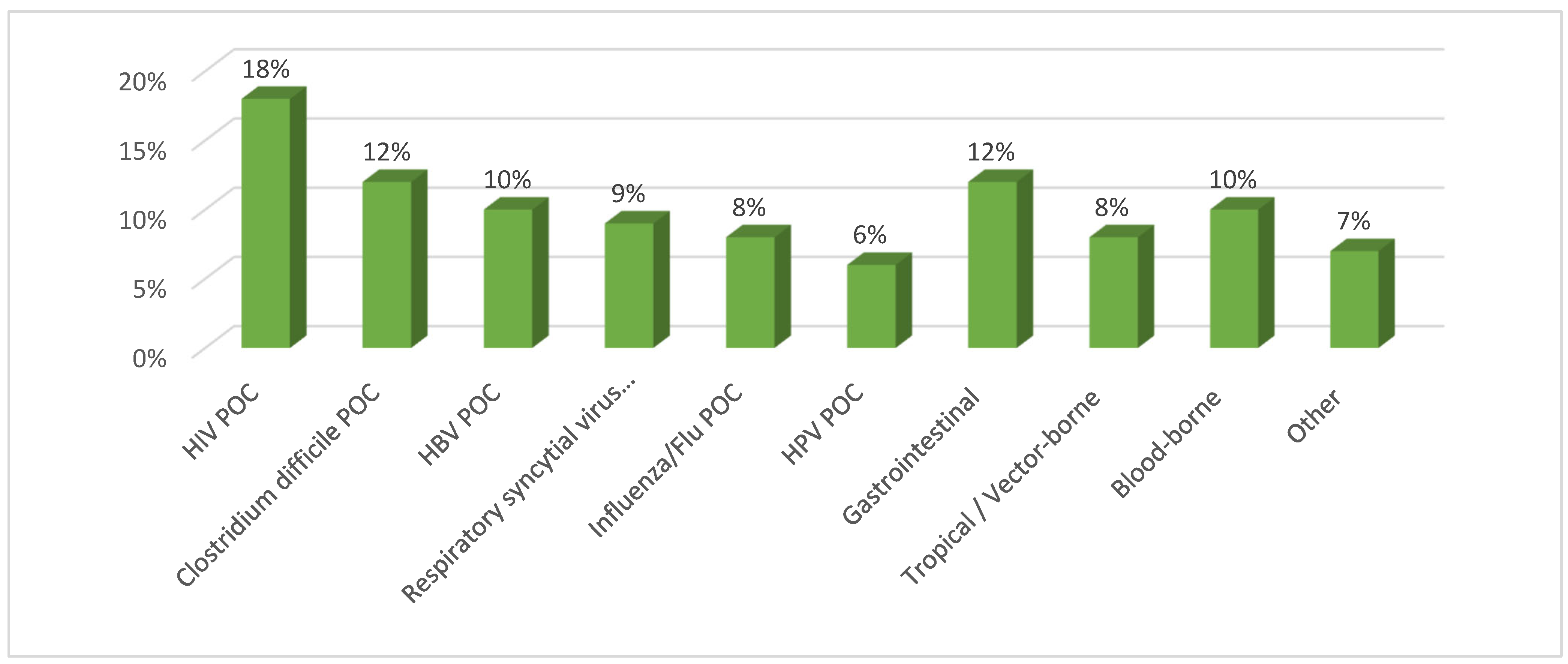
2.1. Segmentation by Disease
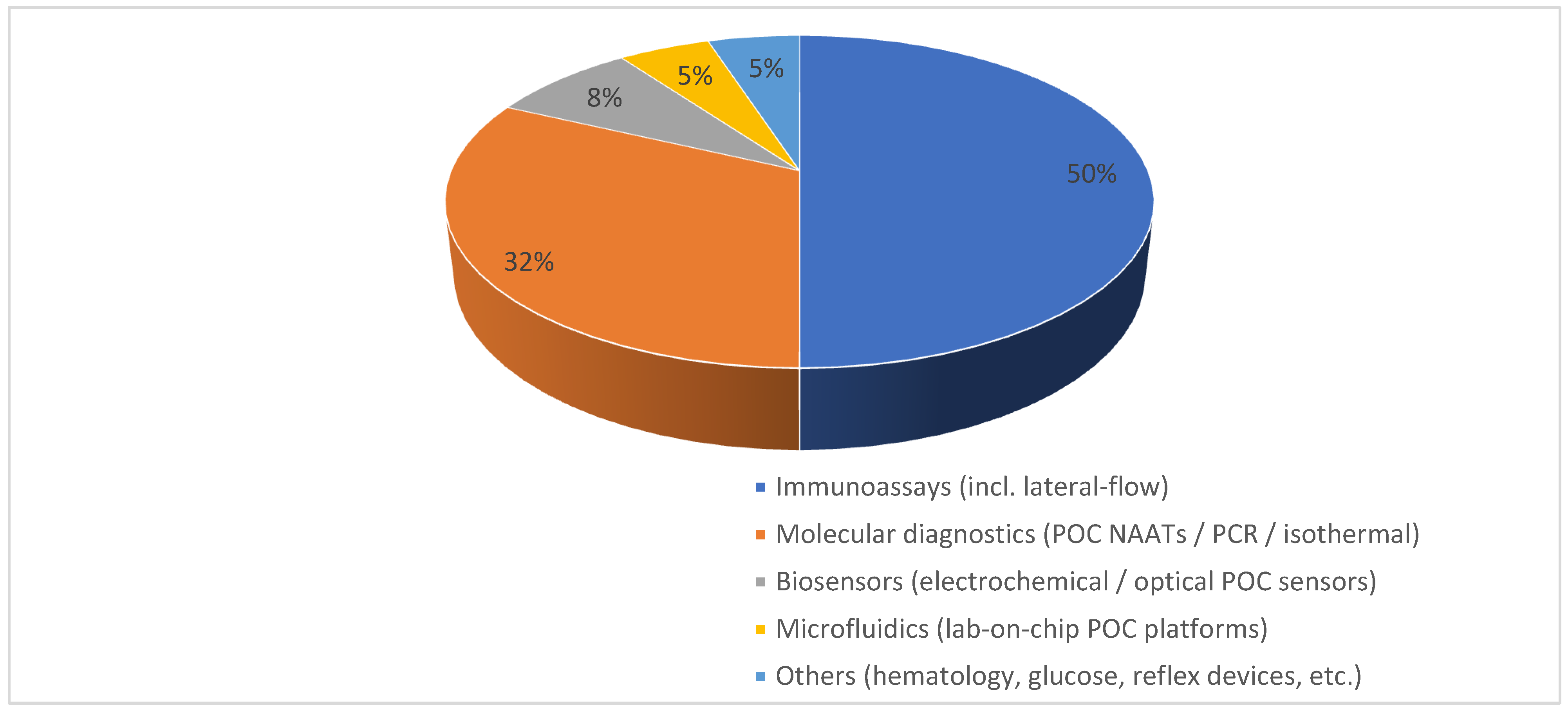
2.2. Segmentation by Technology
3. AI Integration in POC Infectious Disease Diagnostics
4. Point-of-Care Infectious Disease Diagnostic Technologies
4.1. Immunoassays
4.2. Molecular Diagnostics
4.3. Biosensors
4.4. Microfluidics
4.5. Other Emerging Technologies
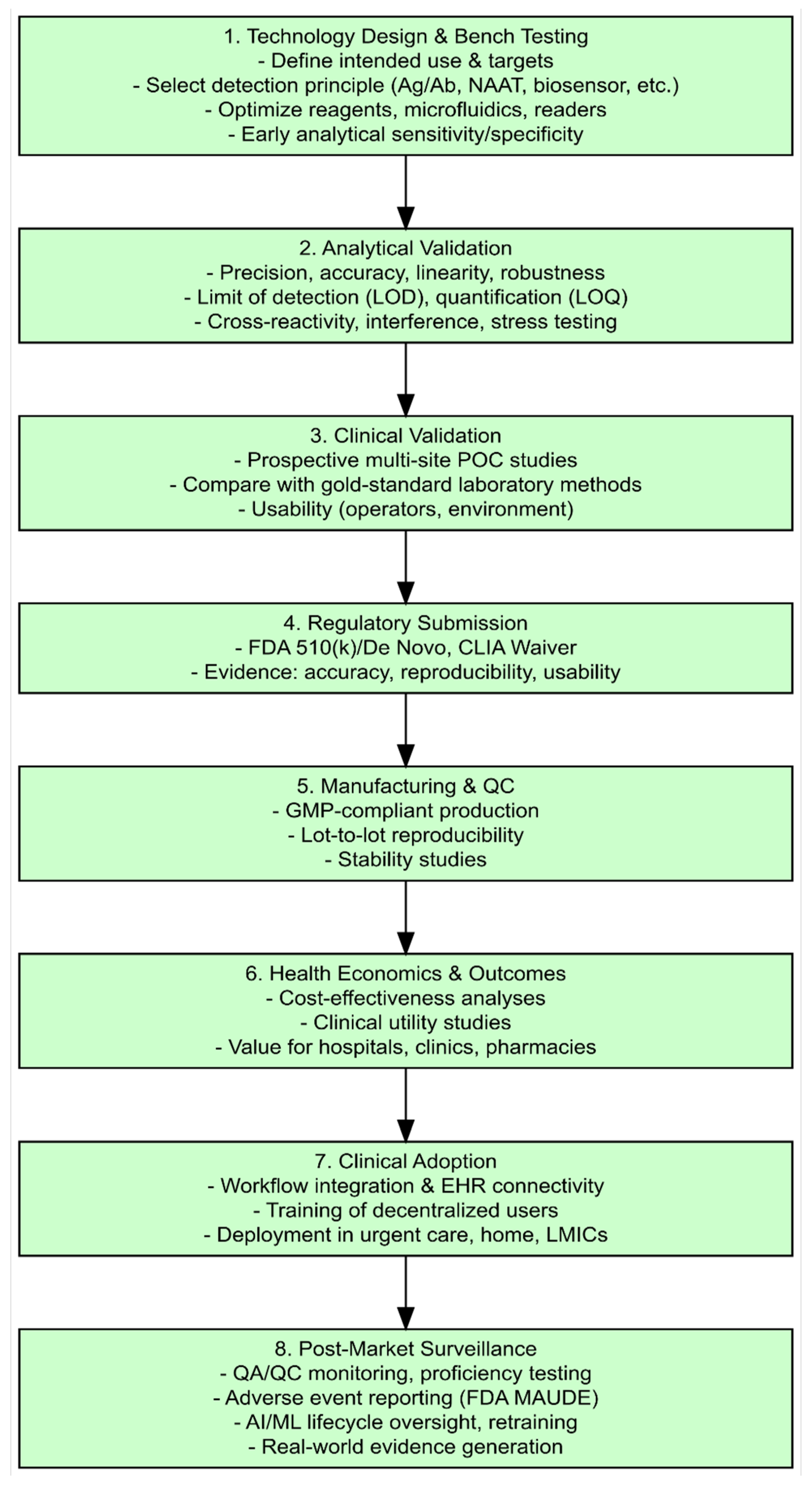
5. Validation of POC Infectious Disease Diagnostics
6. Regulatory and Implementation Considerations
6.1. Regulatory Pathways
6.2. AI-Enabled Diagnostics
6.3. Implementation and Adoption
7. Public Health Considerations
8. Future Directions and Gaps
8.1. Artificial Intelligence and Digital Integration
8.2. Global Harmonization and Standards
8.3. Post-Market Surveillance
8.4. Novel Technologies and Platforms
8.5. Economic and Equity Considerations
9. Discussion
9.1. Market–Technology Integration
9.2. Validation–Regulation Pipeline
9.3. Implementation and Adoption
9.4. AI Integration in the Pipeline
9.5. Public Health
9.6. Synthesis and Gaps
- Heterogeneous validation across populations (LMICs, pediatric, and immunocompromised) limits generalizability.
- Incomplete AI regulatory harmonization across jurisdictions complicates multinational deployment.
- Unclear reimbursement pathways impede clinical adoption despite demonstrable system value.
- Fragmented integration with surveillance systems reduces the population-level utility of POC data streams.
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization. Global Health Estimates 2023: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2022; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82 (Suppl. 5), v1–v6. [Google Scholar] [CrossRef]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef]
- Drain, P.K.; Garrett, N.J. The arrival of a true point-of-care molecular assay ready for global implementation? Lancet Glob. Health 2015, 3, e663–e664. [Google Scholar] [CrossRef] [PubMed]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems, and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 test sensitivity—A strategy for containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef]
- Chua, A.C.; Cunningham, J.; Moussy, F.; Perkins, M.D.; Formenty, P. The case for improved diagnostic tools to control Ebola virus disease in West Africa and how to get there. PLoS Negl. Trop. Dis. 2015, 9, e0003734. [Google Scholar] [CrossRef]
- Lingervelder, D.; Koffijberg, H.; Kusters, R.; IJzerman, M.J. Health Economic Evidence of Point-of-Care Testing: A Systematic Review. Pharmacoecon Open 2021, 5, 157–173. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. In Vitro Diagnostics EUAs; FDA: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas (accessed on 18 September 2025).
- European Commission. Regulation (EU) 2017/746 of the European Parliament and of the Council on In Vitro Diagnostic Medical Devices (IVDR); EU: Brussels, Belgium, 2017. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning-Based Software as a Medical Device (AI/ML SaMD); FDA: Silver Spring, MD, USA, 2019.
- Brendish, N.J.; Poole, S.; Naidu, V.V.; Mansbridge, C.T.; Norton, N.J.; Wheeler, H.; Presland, L.; Kidd, S.; Cortes, N.J.; Borca, F.; et al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): A prospective, interventional, non-randomised, controlled study. Lancet Respir. Med. 2020, 8, 1192–1200. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Applying for CLIA Waiver by Application. Guidance for Industry and Food and Drug Administration Staff; FDA: Silver Spring, MD, USA, 2017.
- World Health Organization. Consolidated Guidelines on HIV Testing Services; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Department of Health and Human Services. Clinical Laboratory Improvement Amendments (CLIA). Fed. Regist. 2020, 85, 77438–77467. [Google Scholar]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Johnson, C.C.; Kennedy, C.; Fonner, V.; Siegfried, N.; Figueroa, C.; Dalal, S.; Sands, A.; Baggaley, R. Examining the effects of HIV self-testing compared to standard HIV testing services: A systematic review and meta-analysis. J. Int. AIDS Soc. 2017, 20, 21594. [Google Scholar] [CrossRef]
- Saweri, O.P.M.; Batura, N.; Al Adawiyah, R.; Causer, L.M.; Pomat, W.S.; Vallely, A.J.; Wiseman, V. Economic evaluation of point-of-care testing and treatment for sexually transmitted and genital infections in pregnancy in low- and middle-income countries: A systematic review. PloS ONE 2021, 16, e0253135. [Google Scholar] [CrossRef]
- IMARC Group. Point-Of-Care Diagnostics Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024–2033; IMARC Group: Brooklyn, NY, USA, 2024. [Google Scholar]
- Allied Market Research. Asia-Pacific Point-of-Care Diagnostics Market Outlook 2024–2033; Allied Market Research: Portland, OR, USA, 2024. [Google Scholar]
- Peeling, R.W.; Heymann, D.L. Innovations in COVID-19 testing: The road from pandemic response to control. Lancet Infect. Dis. 2021, 21, 1334–1335. [Google Scholar] [CrossRef]
- Tolley, A.; Bansal, A.; Murerwa, R.; Howard Dicks, J. Cost-effectiveness of point-of-care diagnostics for AMR: A systematic review. J. Antimicrob. Chemother. 2024, 79, 1248–1269. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. CLIA Waiver by Application Decision Summaries; FDA: Silver Spring, MD, USA, 2025.
- Tan, C.; Chan, C.K.; Ofner, M.; O’Brien, J.; Thomas, N.R.; Callahan, J.; Pascual, B.; Palmer, S.J.; Serapion, V.; Fabro, H.; et al. Implementation of point-of-care molecular testing for respiratory viruses in congregate living settings. Infect. Control. Hosp. Epidemiol. 2024, 45, 1085–1089. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Moschopoulos, C.D.; Dimopoulou, D.; Ong, D.S.Y.; Dimopoulou, K.; Nelson, P.P.; Schweitzer, V.A.; Janocha, H.; Karofylakis, E.; Papathanasiou, K.A.; et al. Performance of point-of care molecular and antigen-based tests for SARS-CoV-2: A living systematic review and meta-analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2023, 29, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab. Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Khan, M.; Khan, R. Artificial Intelligence in Point-of-Care Testing. Ann. Lab Med. 2023, 43, 401–407. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine learning for medical imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef]
- Zayed, B.A.; Ali, A.N.; Elgebaly, A.A.; Talaia, N.M.; Hamed, M.; Mansour, F.R. Smartphone-based point-of-care testing of the SARS-CoV-2: A systematic review. Sci. Afr. 2023, 21, e01757. [Google Scholar] [CrossRef]
- Grand View Research. Artificial Intelligence in Diagnostics Market Size, Share & Trends Analysis Report, 2024–2034; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- O’Kennedy, R.; O’Fágáin, C. Antibody-based diagnostics: From immunoassays to LFIAs. Biochem. Soc. Trans. 2019, 47, 513–522. [Google Scholar]
- Miglietta, L.; Rawson, T.M.; Galiwango, R.; Tasker, A.; Ming, D.K.; Akogo, D.; Ferreyra, C.; Aboagye, E.O.; Gordon, N.C.; Garcia-Vidal, C.; et al. Artificial intelligence and infectious disease diagnostics: State of the art and future perspectives. Lancet Infect. Dis. Advance online publication. 2025. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Bender, A.T.; Boyle, D.S.; Drain, P.K.; Posner, J.D. Current state of commercial point-of-care nucleic acid tests for infectious diseases. Anal. 2021, 146, 2449–2462. [Google Scholar] [CrossRef]
- RaoCarter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Shen, M.; Zhou, Y.; Ye, J.; Al-Maskri, A.A.A.; Kang, Y.; Zeng, S.; Cai, S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020, 10, 97–101. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H.T. Handbook of Immunoassay Technologies; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Morales-Narváez, E.; Merkoçi, A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Zhao, W.; Ali, M.M.; Brook, M.A.; Li, Y. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab. Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Quick, J.; Loman, N.J.; Duraffour, S.; Simpson, J.T.; Severi, E.; Cowley, L.; Bore, J.A.; Koundouno, R.; Dudas, G.; Mikhail, A.; et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 2016, 530, 228–232. [Google Scholar] [CrossRef]
- Elangovan, D.; Long, C.S.; Bakrin, F.S.; Tan, C.S.; Goh, K.W.; Yeoh, S.F.; Loy, M.J.; Hussain, Z.; Lee, K.S.; Idris, A.C.; et al. The Use of Blockchain Technology in the Health Care Sector: Systematic Review. JMIR Med. Inform. 2022, 10, e17278. [Google Scholar] [CrossRef]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Kozel, T.R.; Burnham-Marusich, A.R. Point-of-care testing for infectious diseases: Past, present, and future. J. Clin. Microbiol. 2017, 55, 2313–2320. [Google Scholar] [CrossRef]
- Linnet, K.; Bossuyt, P.M.; Moons, K.G.; Reitsma, J.B. Quantifying the accuracy of a diagnostic test or marker. Clin. Chem. 2012, 58, 1292–1301. [Google Scholar] [CrossRef]
- Jani, I.V.; Peter, T.F. How point-of-care testing could drive innovation in global health. N. Engl. J. Med. 2013, 368, 2319–2324. [Google Scholar] [CrossRef]
- Donato, L.J.; Trivedi, V.A.; Stransky, A.M.; Misra, A.; Pritt, B.S.; Binnicker, M.J.; Karon, B.S. Evaluation of the Cue Health point-of-care COVID-19 (SARS-CoV-2 nucleic acid amplification) test at a community drive through collection center. Diagn. Microbiol. Infect. Dis. 2021, 100, 115307. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). CLIA Waiver by Application Guidance for Industry and FDA Staff; FDA: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clia-waiver-application (accessed on 18 September 2025).
- World Health Organization. Consolidated guidelines for Malaria; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Reitsma, J.B.; Moons, K.G.; Bossuyt, P.M.; Linnet, K. Systematic reviews of studies quantifying the accuracy of diagnostic tests and markers. Clinical chemistry 2012, 58, 1534–1545. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. In Vitro Diagnostics (IVD) Regulatory Assistance; FDA: Silver Spring, MD, USA, 2022.
- U.S. Food and Drug Administration (FDA). Recommendations for Dual 510(k) and CLIA Waiver by Application Studies, Guidance for Industry and FDA Staff; FDA: Silver Spring, MD, USA, 2021.
- Centers for Medicare; Medicaid Services. Clinical Laboratory Improvement Amendments (CLIA) Program; CMS: Baltimore, MD, USA, 2023.
- Health Canada. Guidance for Industry: Management of Applications for Medical Device Licences; Health Canada: Ottawa, ON, Canada, 2022. [Google Scholar]
- World Health Organization. Global Model Regulatory Framework for Medical Devices Including IVDs; WHO: Geneva, Switzerland, 2022; Available online: https://cdn.who.int/media/docs/default-source/biologicals/bs-2022.2425_global-model-regulatory-framework-for-medical-devices-including-ivds_11-july-2022-dl_14-july.pdf?sfvrsn=28d107c5_1 (accessed on 21 September 2025).
- World Health Organization. WHO Prequalification of In Vitro Diagnostics Programme (PQDx): Guidance Document; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- International Medical Device Regulators Forum. Global Harmonization Task Force/IMDRF Regulatory Frameworks; IMDRF: Woden, ACT, Australia, 2022. [Google Scholar]
- Clinical and Laboratory Standards Institute. POCT Quality Management: Approved Guideline; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Kardjadj, M. Regulatory Approved Point-of-Care Diagnostics (FDA & Health Canada): A Comprehensive Framework for Analytical Validity, Clinical Validity, and Clinical Utility in Medical Devices. J. Appl. Lab. Med. 2025, 10, 1622–1637. [Google Scholar] [CrossRef]
- Korte, B.J.; Rompalo, A.; Manabe, Y.C.; Gaydos, C.A. Overcoming Challenges with the Adoption of Point-of-Care Testing: From Technology Push and Clinical Needs to Value Propositions. Point Care 2020, 19, 77–83. [Google Scholar] [CrossRef]
- Mashamba-Thompson, T.P. Implementation Science: Bridging the Gap between Point-of-Care Diagnostics Research and Practice. Diagnostics 2022, 12, 1648. [Google Scholar] [CrossRef]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N.-G.; Woolley, A.E.; Segel, M.; Barretto, R.P.; Ranu, A.; Macrae, R.K.; Faure, G.; et al. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N. Engl. J. Med. 2020, 383, 1492–1494. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Neumann, P.J.; Cohen, J.T.; Ollendorf, D.A. Economic evaluation in health care: Is it really useful for decision makers? Health Aff. 2024, 43, 225–233. [Google Scholar] [CrossRef]
- Shuren, J.; Patel, B.; Gottlieb, S. FDA regulation of artificial intelligence and machine learning in medical devices. JAMA 2025, 333, 635–637. [Google Scholar]
- Patel, V.; Holmes, J.; Chertow, D. Postmarket performance evaluation of AI-enabled diagnostic devices: Emerging frameworks and challenges. Nat. Med. 2024, 30, 2241–2248. [Google Scholar]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- O’Neil, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. London: Review on Antimicrobial Resistance. 2024. Available online: https://amr-review.org (accessed on 21 September 2025).
- Plebani, M.; Nichols, J.H.; Luppa, P.B.; Greene, D.; Sciacovelli, L.; Shaw, J.; Khan, A.I.; Carraro, P.; Freckmann, G.; Dimech, W.; et al. Point-of-care testing: State of the art and perspectives. Clin. Chem. Lab. Med. 2024, 63, 35–51. [Google Scholar] [CrossRef]
- Hayden, O.T.; Luppa, P.B.; Min, J. Point-of-care testing—New horizons for technologies and applications. Anal. Bioanal. Chem. 2022, 414, 3161–3173. [Google Scholar] [CrossRef]
- Kost, G.J. The Impact of Repeating COVID-19 Rapid Antigen Tests on Prevalence Boundary Performance and Missed Diagnoses. Diagnostics 2023, 13, 3223. [Google Scholar] [CrossRef]
| Technology | Estimated % AI-Enabled in 2024 | Projected % AI-Enabled in 2034 | Rationale |
|---|---|---|---|
| Immunoassays (LFAs) | 3% | 15% | AI mainly via smartphone readers/image analysis and QC; adoption is slower for simple strips but rising with digital readers. |
| Molecular (POC NAAT/PCR) | 7% | 35% | Molecular platforms already have software; AI can assist interpretation, QC, and multiplex deconvolution: faster uptake expected. |
| Biosensors | 8% | 40% | Biosensors often produce signals that benefit from ML denoising and pattern detection: strong AI synergy. |
| Microfluidics | 10% | 45% | Microfluidic lab-on-chip platforms with integrated sensors and data streams are prime targets for embedded AI. |
| Others | 2% | 10% | Miscellaneous devices may incorporate niche AI features (e.g., algorithm-assisted interpretation in niche platforms). |
| Technology | Detection Principle | Typical Sample Types | Common Targets/Biomarkers | Representative Commercial Examples * | Time-to-Result | Strengths | Limitations |
|---|---|---|---|---|---|---|---|
| Immunoassay (Lateral flow; reader-assisted FIA) | Antigen–antibody binding; colorimetric or fluorescent readout | Nasal/nasopharyngeal swab, oral fluid, fingerstick blood/serum/plasma | Viral antigens (SARS-CoV-2, Influenza, RSV), bacterial antigens (Strep A), host antibodies (HIV, HCV), parasite antigens (malaria) | Abbott BinaxNOW (COVID-19, Influenza), BD Veritor (Flu/RSV/COVID), Quidel Sofia 2 (Flu/RSV/COVID/Strep), OraQuick HIV-1/2, OraQuick HCV | ~10–30 min | Low cost; simple workflow; CLIA-waived/OTC options; huge installed base | Lower analytical sensitivity vs. NAAT; performance varies with viral load and collection; limited multiplexing (except some reader-based FIAs) |
| Molecular (NAAT: PCR/RT-PCR; isothermal) | Nucleic-acid amplification and detection (optical/fluorescent) | Swabs, urine, whole blood/plasma, vaginal/cervical swabs | Pathogen genomes (SARS-CoV-2, Flu/RSV, CT/NG, TB, HIV RNA) | Cepheid Xpert Xpress (Flu/RSV/COVID), Roche cobas Liat (Flu/RSV/COVID/Strep), Abbott ID NOW (Flu/COVID/Strep), Visby Medical Sexual Health (CT/NG/TV), binx io CT/NG | ~13–60 min | High clinical sensitivity/specificity; closed-cartridge “sample-in/answer-out”; multiplex panels | Higher cost; some systems moderate complexity; supply/logistics for single-use cartridges |
| Biosensors (electrochemical/optical) | Biorecognition (antibody/aptamer/NA) transduced to electrical/optical signal | Fingerstick whole blood/serum/plasma, saliva, swabs | Antigens, antibodies, short nucleic-acid targets | Reader-based immunosensors (e.g., handheld fluorimeters/electrochemical meters powering branded rapid tests), smartphone-coupled readers | ~5–30 min | Quantitation possible; small readers; connectivity/AI-friendly | Fewer FDA-cleared infectious examples than LFA/NAAT; calibration & drift; often single-analyte |
| Microfluidics (lab-on-chip/cartridge) | Integrated sample prep, metering, reactions, detection in sealed cartridge | Whole blood, swabs, urine, saliva | Multiplex antigen/NA panels; host biomarkers | Many NAAT cartridges and some immunoassay cassettes are microfluidic under the hood (e.g., Liat, Visby, Xpert) | ~10–60 min | Automates multi-step workflows; reduces contamination; supports multiplexing | Device/cartridge cost; waste; thermal/valving complexity |
| Others (emerging/adjacent) | CRISPR readouts; portable sequencing; phone-based optics | Swabs, blood, saliva | Genetic targets; broad metagenomics | CRISPR strip/reader prototypes; Oxford Nanopore MinION (surveillance/near-POC) | ~20–60+ min (var.) | Ultra-sensitive or broad target scope; future-proof | Limited FDA-cleared POC use to date; workflow standardization maturing |
| Disease/Syndrome | POC Method | Representative U.S. Products (FDA Cleared; Many CLIA-Waived) ** | Typical Sample | Time-to-Result | Accuracy (Qualitative, per IFU/Comparator) |
|---|---|---|---|---|---|
| COVID-19 | Antigen (LFA/FIA) | Abbott BinaxNOW COVID-19 Ag Card; Quidel Sofia 2 SARS Antigen FIA; BD Veritor SARS-CoV-2 | Nasal swab | 10–20 min | Moderate–High (load-dependent) |
| NAAT (PCR/Isothermal) | Cepheid Xpert Xpress SARS-CoV-2; Roche cobas Liat SARS-CoV-2; Abbott ID NOW COVID-19 | Nasal/NPS swab | 13–45 min | High | |
| Influenza A/B (± RSV) | Antigen (FIA) | Quidel Sofia 2 Flu + SARS Antigen; BD Veritor Flu A+B; Abbott BinaxNOW Influenza | Nasal/NPS swab | 10–20 min | Moderate–High |
| NAAT (PCR) | Roche cobas Liat Influenza A/B (± RSV); Cepheid Xpert Xpress Flu/RSV | Nasal/NPS swab | 20–45 min | High | |
| Group A Streptococcus | Antigen (LFA/FIA) | Quidel QuickVue Strep A; BD Veritor Strep A; Abbott Strep A tests | Throat swab | 5–15 min | Moderate–High (culture confirm often recommended if negative) |
| NAAT (isothermal/PCR) | Abbott ID NOW Strep A; Roche cobas Liat Strep A | Throat swab | 6–20 min | High | |
| HIV-1/2 (antibody/Ag-Ab) | Rapid immunoassay | OraQuick ADVANCE Rapid HIV-1/2 Ab; Determine HIV-1/2 Ag/Ab Combo *** | Fingerstick whole blood, oral fluid (OraQuick) | 20–30 min | High (oral-fluid sensitivity slightly lower vs. serum) |
| Hepatitis C (HCV) | Rapid antibody test | OraQuick HCV Rapid Antibody Test | Fingerstick whole blood/serum/plasma | ~20 min | High (antibody only; RNA confirm required) |
| CT/NG (Chlamydia/ Gonorrhea) | NAAT (POC) | binx io CT/NG; Visby Medical Sexual Health (CT/NG/TV) | Vaginal/urethral swab; urine (per IFU) | ~30 min (binx)/~30–40 min (Visby) | High |
| RSV | Antigen (FIA) | Quidel Sofia 2 RSV; BD Veritor RSV | Nasal/NPS swab | 10–15 min | Moderate–High |
| Malaria | Rapid antigen test | BinaxNOW Malaria | Whole blood (fingerstick) | ~15 min | High for P. falciparum HRP-2; lower for non-falciparum antigens |
| Feature | FDA (U.S.) | Health Canada | EU (IVDR) | WHO Prequalification (PQDx) |
|---|---|---|---|---|
| Regulatory authority/legal basis | FDA; Center for Devices & Radiological Health (CDRH). IVDs cleared via 510(k), De Novo, or PMA; CLIA categorization determines “waived” status. | Health Canada; Medical Device Directorate; devices licensed via Medical Device Licence (MDL) for Class II–IV. Guidance documents for near-patient devices. | EU Regulation 2017/746 (IVDR); conformity assessment by Notified Bodies; stricter clinical evidence and post-market requirements vs. IVDD. | WHO PQDx: voluntary prequalification for priority IVDs (HIV, malaria, viral load, etc.) focused on suitability for low-resource settings; used for UN procurement. |
| Risk classification (typical for POC IVDs) | IVDs range Class I–III by risk; many POC infectious tests treated as moderate-risk (Class II). CLIA complexity categories (waived/moderate/high) differ from FDA device class. | Risk-based rules (Schedule I) classify IVDs I–IV; near-patient often Class II or higher depending on intended use. | IVDR risk classes A–D (D highest). Many clinically actionable POC infectious IVDs fall into Class B or C under IVDR (increased clinical evidence required). | PQ focuses on priority disease targets (not a national regulatory approval). Devices must meet WHO technical specifications/TPPs for intended use. |
| Analytical evidence required | LoD, precision, linearity, interfering substances, cross-reactivity, stability studies; documented per FDA guidance. | Similar analytical dossier; Health Canada requests validation data proportional to classification. | IVDR requires performance evaluation report (analytical performance) and supporting documentation to Notified Body. | PQ requires analytical validation demonstrating fitness for intended use in target contexts (stability, matrix effects). |
| Clinical evidence required | Clinical performance compared to reference methods (sensitivity/specificity, PPA/NPA) in intended-use populations; CLIA-waiver submissions often require usability/clinical studies. | Clinical evidence proportional to risk class; Health Canada expects data or justification with equivalent evidence. | IVDR requires clinical performance studies and evidence in intended populations. | PQ expects clinical/field evaluation evidence in intended settings, often prospective field studies. |
| Human factors/usability studies | Required for CLIA-waiver and home-use claims; FDA provides guidance for usability and labeling. | Requested where relevant to demonstrate safe near-patient use. | IVDR/MDCG emphasize usability and labeling; may form part of clinical evidence. | PQ evaluates ease-of-use and suitability for LMICs (training needs, storage, stability). |
| POC-specific criteria (e.g., CLIA waiver) | CLIA-waiver requires demonstration of simplicity and low error risk; OTC/home-use tests automatically waived. | No CLIA-equivalent; Health Canada may authorize near-patient use with labeling and risk mitigation. | CE-marking under IVDR permits POC use as per IFU; no waiver scheme equivalent to CLIA. | PQDx evaluates POC suitability via TPPs and field performance; used for procurement. |
| Post-market surveillance & vigilance | PMS under 21 CFR 820, MDR reporting, MAUDE adverse event reporting. FDA also piloting AI/ML lifecycle oversight. | Vigilance reporting and MDL maintenance; proportional to device risk. | IVDR mandates PMS, UDI, periodic safety update reports. | PQ requires ongoing monitoring and periodic reassessment for continued eligibility. |
| Typical timelines/practical notes | 510(k): months; CLIA-waiver adds human-factors/robustness review. De Novo/PMA longer. EUA faster. Early FDA interaction recommended. | Timelines vary by class and dossier completeness; pre-submission meetings are recommended. | Timelines depend on class and Notified Body capacity; IVDR increases evidence requirements since 2022. |
| Infectious Disease | POC Diagnostic Type | Demonstrated Public Health Impact |
|---|---|---|
| HIV | Rapid antibody/Ag-Ab tests (e.g., OraQuick, Determine Combo) | Increased testing uptake in community and home settings; earlier ART initiation; reduced loss-to-follow-up in LMICs |
| Tuberculosis (TB) | Molecular NAAT (e.g., GeneXpert, Truenat) | Faster diagnosis and treatment initiation; reduced transmission; improved case detection in peripheral clinics |
| Malaria | Rapid antigen tests (HRP2-based RDTs) | Expanded access in remote areas; improved case management; major role in WHO test-and-treat strategies |
| COVID-19 | Antigen LFAs and rapid NAAT | Enabled mass decentralized screening; informed isolation/quarantine decisions; supported outbreak containment |
| Influenza/RSV | Rapid antigen and molecular assays (e.g., Sofia, Liat) | Improved antimicrobial stewardship; reduced unnecessary antibiotic prescriptions; faster triage in emergency settings |
| HCV | Rapid antibody test (e.g., OraQuick HCV) | Increased community screening; improved linkage-to-care in marginalized populations |
| Emerging pathogens (e.g., Ebola, Zika) | Prototype LFAs, molecular assays | Field-deployed tools during outbreaks; enabled surveillance and case finding in epidemic hotspots |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardjadj, M. Advances in Point-of-Care Infectious Disease Diagnostics: Integration of Technologies, Validation, Artificial Intelligence, and Regulatory Oversight. Diagnostics 2025, 15, 2845. https://doi.org/10.3390/diagnostics15222845
Kardjadj M. Advances in Point-of-Care Infectious Disease Diagnostics: Integration of Technologies, Validation, Artificial Intelligence, and Regulatory Oversight. Diagnostics. 2025; 15(22):2845. https://doi.org/10.3390/diagnostics15222845
Chicago/Turabian StyleKardjadj, Moustafa. 2025. "Advances in Point-of-Care Infectious Disease Diagnostics: Integration of Technologies, Validation, Artificial Intelligence, and Regulatory Oversight" Diagnostics 15, no. 22: 2845. https://doi.org/10.3390/diagnostics15222845
APA StyleKardjadj, M. (2025). Advances in Point-of-Care Infectious Disease Diagnostics: Integration of Technologies, Validation, Artificial Intelligence, and Regulatory Oversight. Diagnostics, 15(22), 2845. https://doi.org/10.3390/diagnostics15222845






