Fetal Speckle Tracking Technology for Critical Aortic Stenosis: Advancing Through Innovation
Abstract
1. Background
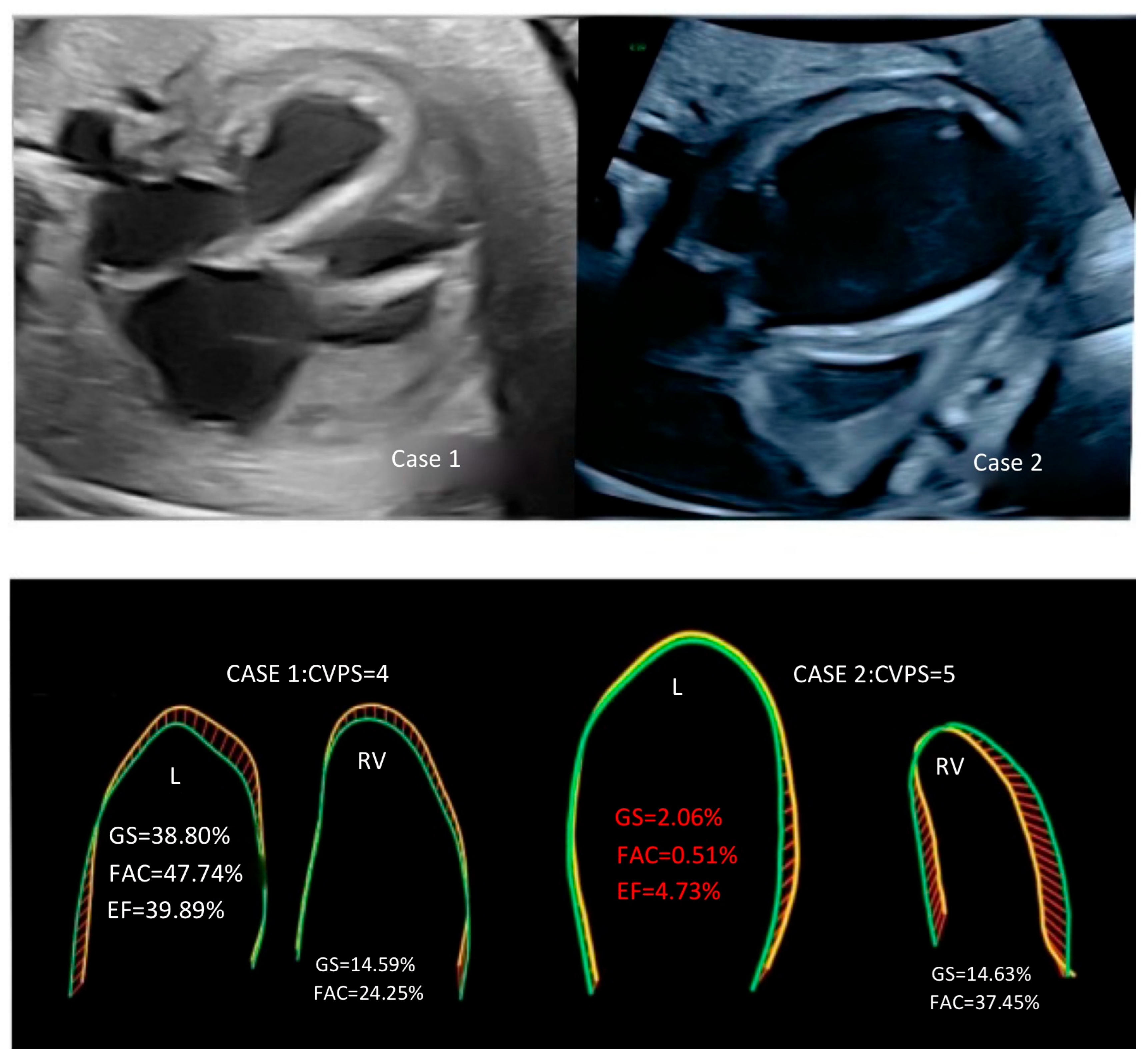
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovalchin, J.P.; Brook, M.M.; Rosenthal, G.L.; Suda, K.; Hoffman, J.I.; Silverman, N.H. Echocardiographic hemodynamic and morphometric predictors of survival after two-ventricle repair in infants with critical aortic stenosis. J. Am. Coll. Cardiol. 1998, 32, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, C.K.; Hanseus, K.; Ramgren, J.J.; Synnergren, M.J.; Sunnegårdh, J. Outcomes in neonatal critical and non-critical aortic stenosis: A retrospective cohort study. Arch. Dis. Child. 2023, 108, 398–404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parsons, M.K.; Moreau, G.A.; Graham, T.P., Jr.; Johns, J.A.; Boucek, R.J., Jr. Echocardiographic estimation of critical left ventricular size in infants with isolated aortic valve stenosis. J. Am. Coll. Cardiol. 1991, 18, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- DeVore, G.R.; Klas, B.; Cuneo, B.; Satou, G.; Sklansky, M. Review of speckle tracking analysis to measure the size, shape, and contractility of the fetal heart in fetuses with congenital heart defects. Echocardiography 2024, 41, e15870. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Ren, M.; Wiputra, H.; Mojumder, J.; Chan, W.X.; Tulzer, A.; Tulzer, G.; Buist, M.L.; Mattar, C.N.Z.; Lee, L.C.; et al. Biomechanics of Human Fetal Hearts with Critical Aortic Stenosis. Ann. Biomed. Eng. 2021, 49, 1364–1379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, H.S.; Wiputra, H.; Tulzer, A.; Tulzer, G.; Yap, C.H. Fluid Mechanics of Fetal Left Ventricle During Aortic Stenosis with Evolving Hypoplastic Left Heart Syndrome. Ann. Biomed. Eng. 2022, 50, 1158–1172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freud, L.R.; Moon-Grady, A.; Escobar-Diaz, M.C.; Gotteiner, N.L.; Young, L.T.; McElhinney, D.B.; Tworetzky, W. Low rate of prenatal diagnosis among neonates with critical aortic stenosis: Insight into the natural history in utero. Ultrasound Obstet. Gynecol. 2015, 45, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Murlewska, J.; Sylwestrzak, O.; Respondek-Liberska, M.; Sklansky, M.; Devore, G. Longitudinal Surveillance of Fetal Heart Failure Using Speckle Tracking Analysis. J. Clin. Med. 2022, 11, 7102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tei, C.; Ling, L.H.; Hodge, D.O.; Bailey, K.R.; Oh, J.K.; Rodeheffer, R.J.; Tajik, A.J.; Seward, J.B. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J. Cardiol. 1995, 26, 357–366. [Google Scholar] [PubMed]
- Nishitani, S.; Torii, N.; Imai, H.; Haraguchi, R.; Yamada, S.; Takakuwa, T. Development of Helical Myofiber Tracts in the Human Fetal Heart: Analysis of Myocardial Fiber Formation in the Left Ventricle From the Late Human Embryonic Period Using Diffusion Tensor Magnetic Resonance Imaging. J. Am. Heart Assoc. 2020, 9, e016422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeVore, G.R.; Klas, B.; Satou, G.; Sklansky, M. Quantitative evaluation of fetal right and left ventricular fractional area change using speckle-tracking technology. Ultrasound Obstet. Gynecol. 2019, 53, 219–228. [Google Scholar] [CrossRef] [PubMed]
| Critical Aortic Stenosis, FHF, Restricted FO, Prenatal Oxygen Therapy, Steroid Therapy, Antibiotic Therapy, and Glycosides for Cases 1 + 2 | Case 1 | Case 2 |
|---|---|---|
| Gestational age at prenatal diagnosis, established at last menstrual period | 35 w 4 d | 35 w 3 d |
| EFW [g] established at Hadlock [5] | 4125 | 2430 |
| CVPS points | 4 | 5 |
| HA/CA | 0.6 | 0.6 |
| AFI [cm] | 23 | 20 |
| UMB PI—umbilical cord pulsatility index | 0.52 | 2.37 |
| MCA PI—middle cerebral artery pulsatility index | 0.92 | 1.4 |
| DV—ductus venosus reversal flow | present | absent |
| MCA PSV [cm/s]—middle cerebral artery peak systolic velocity | 50 | 48 |
| Tei LV—Tei index for left ventricle | 0.7 | 0.77 |
| Tei RV—Tei index for right ventricle | 0.7 | 0.7 |
| AoV diameter [mm]— aortic valve diameter | 3 | 4.8 |
| AoV PSV [cm/s]— aortic valve peak systolic velocity | 300 | 260 |
| PAV diameter [mm]—pulmonary artery valve diameter | 9.7 | 11 |
| PAV PSV [cm/s]—pulmonary artery valve peak systolic velocity | 98 | 65.3 |
| PV PSV [cm/s]—pulmonary vein peak systolic velocity | 53 | 50 |
| M—mode EF [%] | 40 | 5 |
| MV/TV annular ratio | 0.9 | 0.5 |
| AoV/PAV annular ratio | 0.8 | 0.4 |
| LV/RV mid-cavity ratio | 1.0 | 0.4 |
| LV/RA long-axis ratio | 1.1 | 0.6 |
| Global strain LV [%] | −38.80 | −2.06 |
| Frac. area change LV [%]—fractional area change | 47.74 | −0.51 |
| EF LV [%]—ejection fraction for left ventricle | 39.89 | 4.73 |
| Global strain RV [%] | −14.59 | −14.63 |
| Frac. area change RV [%]—fractional area change | 24.25 | 37.45 |
| Global strain RA [%] | −17.98 | −32.62 |
| Frac. area change RA [%]—fractional area change | 26.09 | 44.28 |
| Global strain LA [%] | −3.14 | −2.01 |
| Frac. area change LA [%]—fractional area change | 3.12 | 2.12 |
| Time of delivery | 35 w 7 d | 36 w 1 d |
| Newborn weight | 3300 g | 2750 g |
| Newborn Apgar scores | 7,7,7 | 7,8,8 |
| Newborn gender | boy | girl |
| Type of delivery | CS | CS |
| Balloon atrial septostomy | 8th day of life | 6th day of life |
| BAV—balloon aortic valvuloplasty | 8th day of life | 6th day of life |
| Norwood procedure | 12th day of life | 6th day of life |
| Follow-up | 231 days of hospitalization: NEC—necrotizing enterocolitis, ascites | ECMO, death on the 28th day of life |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murlewska, J.; Witkowski, S.; Strzelecka, I.; Respondek-Liberska, M. Fetal Speckle Tracking Technology for Critical Aortic Stenosis: Advancing Through Innovation. Diagnostics 2025, 15, 2591. https://doi.org/10.3390/diagnostics15202591
Murlewska J, Witkowski S, Strzelecka I, Respondek-Liberska M. Fetal Speckle Tracking Technology for Critical Aortic Stenosis: Advancing Through Innovation. Diagnostics. 2025; 15(20):2591. https://doi.org/10.3390/diagnostics15202591
Chicago/Turabian StyleMurlewska, Julia, Sławomir Witkowski, Iwona Strzelecka, and Maria Respondek-Liberska. 2025. "Fetal Speckle Tracking Technology for Critical Aortic Stenosis: Advancing Through Innovation" Diagnostics 15, no. 20: 2591. https://doi.org/10.3390/diagnostics15202591
APA StyleMurlewska, J., Witkowski, S., Strzelecka, I., & Respondek-Liberska, M. (2025). Fetal Speckle Tracking Technology for Critical Aortic Stenosis: Advancing Through Innovation. Diagnostics, 15(20), 2591. https://doi.org/10.3390/diagnostics15202591





