Fetal Cerebral Blood Flow (Dys)autoregulation
Abstract
1. Introduction
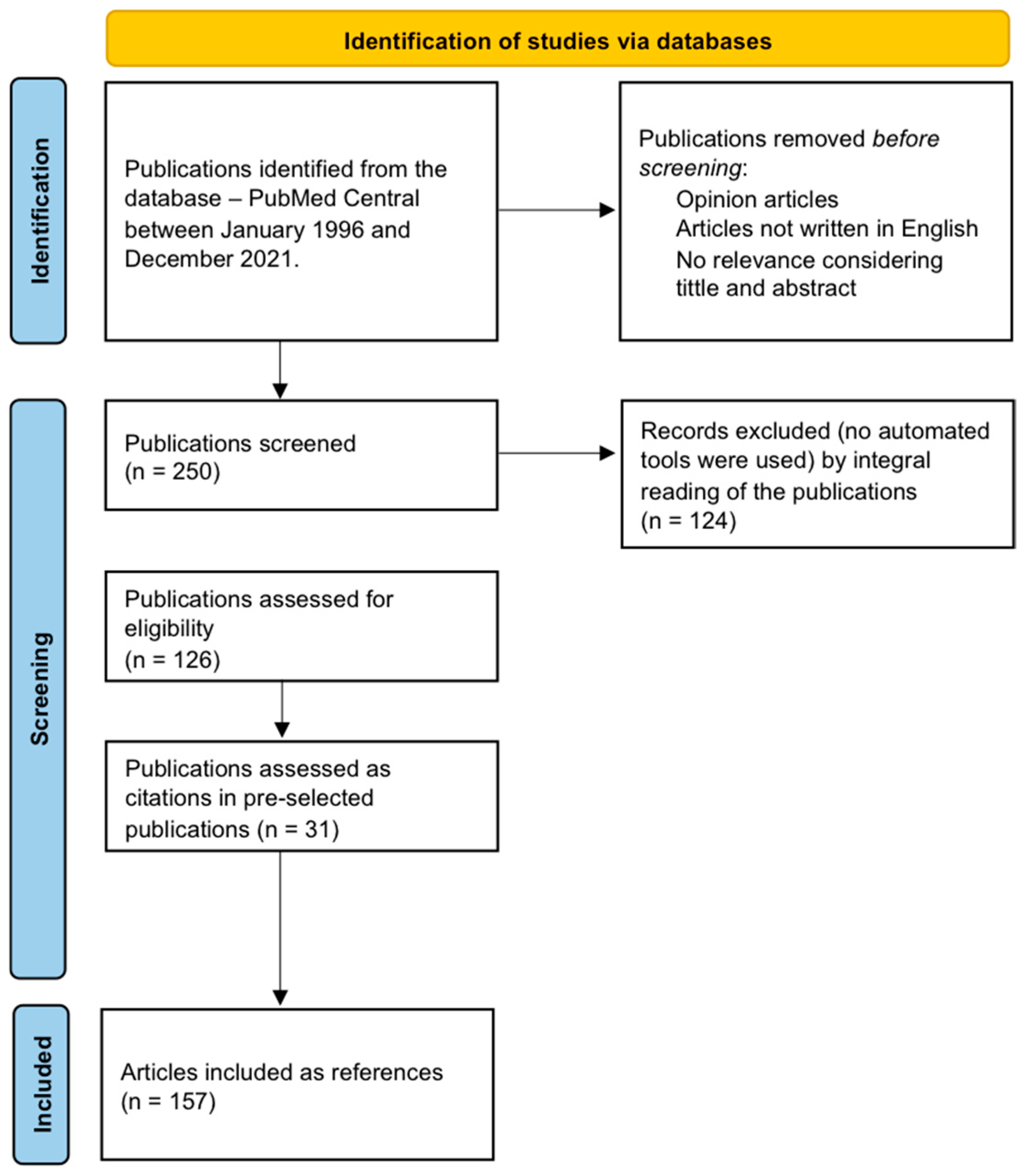
2. Materials and Methods
3. The Fetal Cerebral Circulation
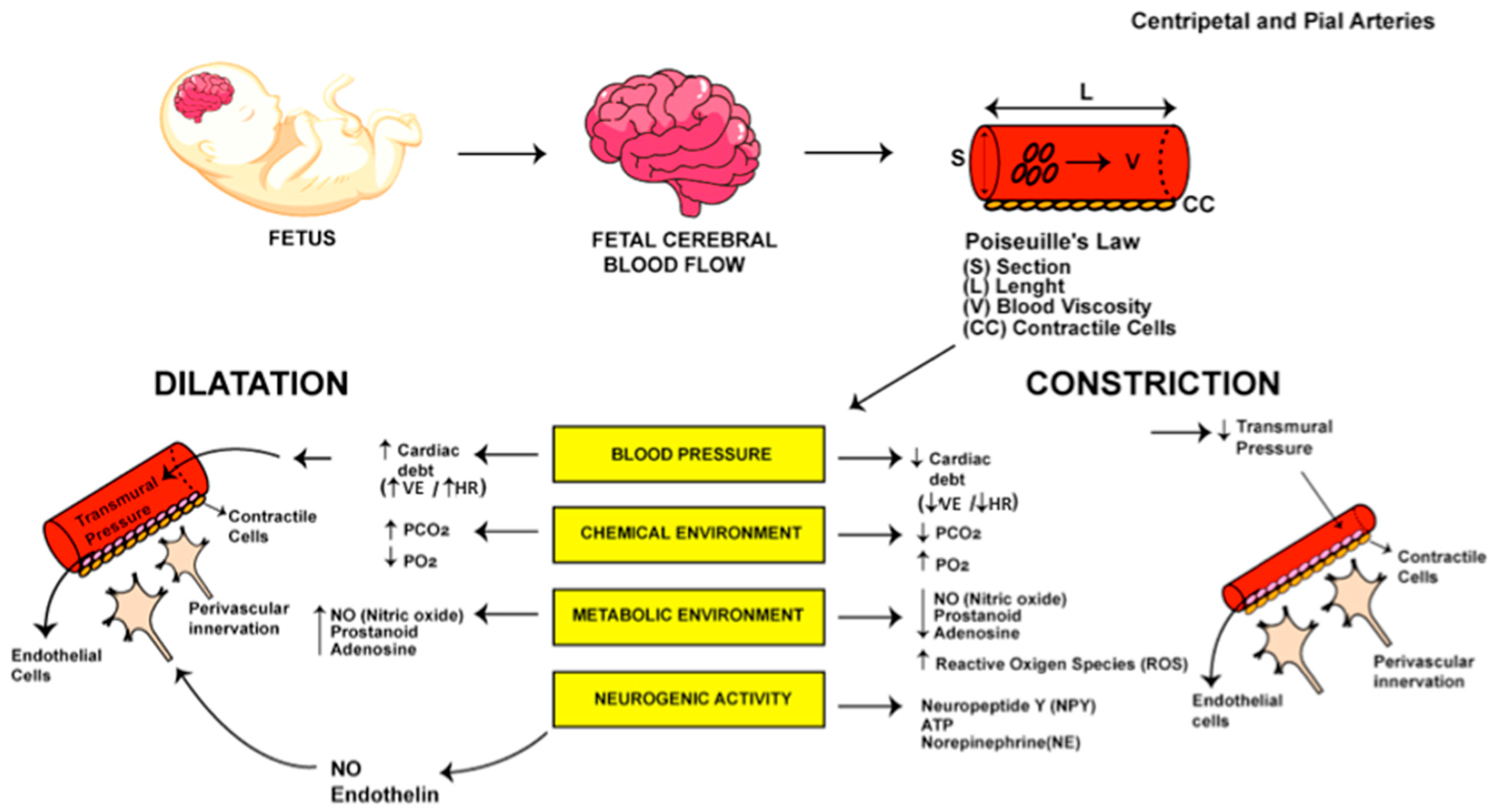
3.1. Fetal Cerebral Autoregulation
3.2. The Effects of Hypoxia on Fetal Cerebral Structure and Function
3.3. Contractile Characteristics of Fetal Cerebrovasculature
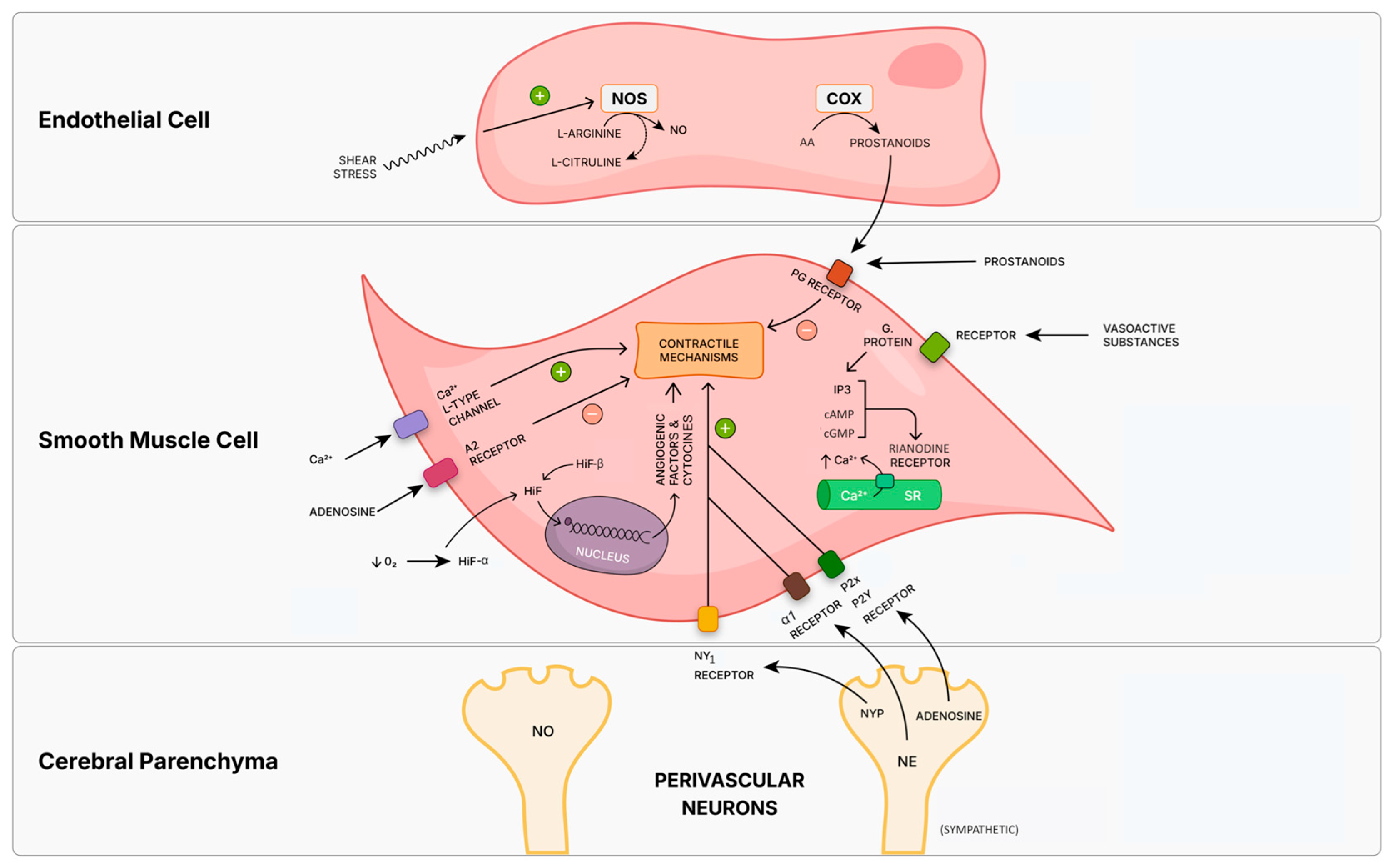
3.4. Fetal Cerebrovascular Signal Transduction
3.4.1. Calcium
3.4.2. Nitric Oxide and Prostaglandins
3.4.3. Adenosine
3.4.4. Reactive Oxygen Species
3.4.5. Hypoxic Inducible Factor-1
3.4.6. Neurovascular Unit and Its Effectors
3.5. Fetal Carotid Arteries Maturation
3.6. The Concept of Fetal Cerebral Blood Flow Redistribution
3.7. Doppler Studies of Foetal Cerebral Blood Flow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| AA | Arachinoid acid |
| ANGII | Angiotensin II |
| ATP | Adenosine triphosphate |
| Ca2+ | Calcium |
| cAMP | Cyclic adenosine monophosphate |
| CBF | Cerebral blood flow |
| CCs | Contractile cells |
| cGMP | Cyclic guanosine monophosphate |
| CO2 | Carbon dioxide |
| COX | Cyclo-oxygenase |
| EEG | Electroencephalogram |
| EPO | Erythropoietin |
| EV | Ejection volume |
| FGF | Fibroblast growth factor |
| FLNA | Filamin isoform A |
| FLNB | Filamin isoform B |
| FBP1 | Formin binding protein 1 |
| HIF | Hypoxia-inducible factor |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HIF-1β | Hypoxia-inducible factor 1-beta |
| HR | Heart rate |
| ICA | Internal carotid artery |
| ICAs | Internal carotid arteries |
| ISUOG | International Society of Ultrasound in Obstetric and Gynecology Guidelines |
| IP3 | Inositol-1,4,5-trisphosphate |
| K+ | Potassium |
| L | Length |
| MCA | Middle cerebral artery |
| MLC | Myosin light chain |
| MLCK | Myosin light chain kinase |
| mRNA | Messenger riboucleic acid |
| Na/K-ATPase | Sodium–potassium adenosine triphosphate desidrogenase |
| NE | Norepinephrine |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NPY | Neuropeptide |
| pCO2 | Carbone dioxide arterial partial pressure |
| PDGF | Platelet-derived growth factor |
| PI | Pulsatility index |
| pO2 | Oxygen arterial partial pressure |
| PG | Prostaglandin |
| ROS | Reactive oxygen species |
| S | Section |
| SR | Sarcoplasmic reticulum |
| US | Ultrasound |
| V | Blood viscosity |
| VEGF | Vascular endothelial growth factor |
References
- Bueno, D.; Parvas, M.; Garcia-Fernàndez, J. The embryonic bloodcerebrospinal fluid barrier function before the formation of the fetal choroid plexus: Role in cerebrospinal fluid formation and homeostasis. Croat. Med. J. 2014, 55, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.; Parvas, M.; Nabiuni, M.; Miyan, J. Embryonic cerebrospinal fluid formation and regulation. Semin. Cell Dev. Biol. 2020, 102, 3–12. [Google Scholar] [CrossRef]
- Vasung, L.; Abaci Turk, E.; Ferradal, S.L.; Sutin, J.; Stout, J.N.; Ahtam, B.; Lin, P.Y.; Grant, P.E. Exploring early human brain development with structural and physiological neuroimaging. Neuroimage 2019, 187, 226–254. [Google Scholar] [CrossRef]
- du Plessis, A.J. Cerebral Blood Flow and Metabolism in the Developing Fetus. Clin. Perinatol. 2009, 36, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Lemire, R.J. Cerebral vasculature. In Normal and Abnormal Development of the Human Nervous System; Medical Department, Harper & Row: New York, NY, USA, 1975; pp. 1–42. [Google Scholar]
- Degani, S. Fetal cerebrovascular circulation: A review of prenatal ultrasound assessment. Gynecol. Obstet. Investig. 2008, 66, 184–196. [Google Scholar] [CrossRef]
- Rudolph, A.M. Impaired cerebral development in fetuses with congenital cardiovascular malformations: Is it the result of inadequate glucose supply? Pediatr. Res. 2016, 80, 172–177. [Google Scholar] [CrossRef]
- Nagaraj, U.D.; Evangelou, I.E.; Donofrio, M.T.; Vezina, L.G.; McCarter, R.; Du Plessis, A.J.; Limperopoulos, C. Impaired global and regional cerebral perfusion in newborns with complex congenital heart disease. J. Pediatr. 2015, 167, 1018–1024. [Google Scholar] [CrossRef]
- Sam, C.; Li, F.F.; Liu, S.L. Inherited neurovascular diseases affecting cerebral blood vessels and smooth muscle. Metab. Brain Dis. 2015, 30, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- McQuillen, P.S.; Miller, S.P. Congenital heart disease and brain development. Ann. N. Y. Acad. Sci. 2010, 1184, 68–86. [Google Scholar] [CrossRef]
- Dupin, E.; Le Douarin, N.M. The neural crest, A multifaceted structure of the vertebrates. Birth Defects Res. Part C-Embryo Today Rev. 2014, 102, 187–209. [Google Scholar] [CrossRef]
- Wu, H.M.; Chuang, Y.M. The clinical relevance of fetal variant of the circle of willis and its influence on the cerebral collateral circulation. Acta Neurol. Taiwan. 2011, 20, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.; Harding, R.; Inder, T. The developmental environment and the origins of neurological disorders. Dev. Orig. Health Dis. 2006, 379–391. [Google Scholar] [CrossRef]
- Rasmussen, J.M.; Thompson, P.M.; Entringer, S.; Buss, C.; Wadhwa, P.D. Fetal programming of human energy homeostasis brain networks: Issues and considerations. Obes. Rev. 2022, 23, 1–27. [Google Scholar] [CrossRef]
- Limperopoulos, C. Disorders of the Fetal Circulation and the Fetal Brain. Clin. Perinatol. 2009, 36, 561–577. [Google Scholar] [CrossRef]
- Tweed, W.A.; Cote, J.; Wade, J.G.; Gregory, G.; Mills, A. Preservation of fetal brain blood flow relative to other organs during hypovolemic hypotension. Pediatr. Res. 1982, 16, 137–140. [Google Scholar] [CrossRef]
- Löhle, M.; Müller, T.; Wicher, C.; Roedel, M.; Schubert, H.; Witte, O.W.; Nathanielsz, P.W.; Schwab, M. Betamethasone effects on fetal sheep cerebral blood flow are not dependent on maturation of cerebrovascular system and pituitary-adrenal axis. J. Physiol. 2005, 564, 575–588. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Benavides Serralde, J.A.; Cruz-Martinez, R. Can anomalies of fetal brain circulation be useful in the management of growth restricted fetuses? Prenat. Diagn. 2012, 32, 103–112. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Stampalija, T.; Figueras, F. Cerebral blood flow studies in the diagnosis and management of intrauterine growth restriction. Curr. Opin. Obstet. Gynecol. 2013, 25, 138–144. [Google Scholar] [CrossRef]
- Bukiya, A.N.; Dopico, A.M. Fetal cerebral circulation as target of maternal alcohol consumption. Alcohol. Clin. Exp. Res. 2019, 42, 1006–1018. [Google Scholar] [CrossRef]
- Bar, J.; Weiner, E.; Levy, M.; Gilboa, Y. The thrifty phenotype hypothesis: The association between ultrasound and Doppler studies in fetal growth restriction and the development of adult disease. Am. J. Obstet. Gynecol. MFM 2021, 3, 100473. [Google Scholar] [CrossRef] [PubMed]
- Mihu, D.; Diculescu, D.; Costin, N.; Mihu, C.M.; Blaga, L.; Ciortea, R.; Mǎluţan, A. Applications of Doppler ultrasound during labor. Med. Ultrason. 2011, 13, 141–149. [Google Scholar]
- Kawakita, T.; Sasaki, H.; Hirabuki, S.; Asamoto, A. Fetal growth restriction and reversed middle cerebral artery end-diastolic flow with subchorionic placental lake. J. Obstet. Gynaecol. Res. 2013, 39, 578–582. [Google Scholar] [CrossRef]
- Nassr, A.A.; Abdelmagied, A.M.; Shazly, S.A.M. Fetal cerebro-placental ratio and adverse perinatal outcome: Systematic review and meta-analysis of the association and diagnostic performance. J. Perinat. Med. 2016, 44, 249–256. [Google Scholar] [CrossRef]
- Hershkovitz, R.; Kingdom, J.C.P.; Geary, M.; Rodeck, C.H. Fetal cerebral blood flow redistribution in late gestation: Identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet. Gynecol. 2000, 15, 209–212. [Google Scholar] [CrossRef]
- Wolf, H.; Stampalija, T.; Lees, C.C.; Arabin, B.; Berger, A.; Bergman, E.; Bhide, A.; Bilardo, C.M.; Breeze, A.C.; Brodszki, J.; et al. Fetal cerebral blood-flow redistribution: Analysis of Doppler reference charts and association of different thresholds with adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2021, 58, 705–715. [Google Scholar] [CrossRef]
- Back, S.A.; Riddle, A.; Dean, J.; Hohimer, A.R. The Instrumented Fetal Sheep as a Model of Cerebral White Matter Injury in the Premature Infant. Neurotherapeutics 2012, 9, 359–370. [Google Scholar] [CrossRef]
- Sinha, A.K.; Cane, C.; Kempley, S.T. Blood flow in the common carotid artery in term and preterm infants: Reproducibility and relation to cardiac output. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, 31–35. [Google Scholar] [CrossRef] [PubMed]
- van den Wijngaard, J.A.G.W.; Reuss, A.; Wladimiroff, J.W. The blood flow velocity waveform in the fetal internal carotid artery in the presence of hydrocephaly. Early Hum. Dev. 1988, 18, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Baerts, W.; Van Bel, F. Brain-Sparing in Intrauterine Growth Restriction: Considerations for the Neonatologist. Neonatology 2015, 108, 269–276. [Google Scholar] [CrossRef]
- Robinson, R.; Iida, H.; O’Brien, T.P.; Pane, M.A.; Traystman, R.J.; Gleason, C.A. Comparison of cerebrovascular effects of intravenous cocaine injection in fetal, newborn, and adult sheep. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tarzamni, M.K.; Nezami, N.; Gatreh-Samani, F.; Vahedinia, S.; Tarzamni, M. Doppler waveform indices of fetal middle cerebral artery in normal 20 to 40 weeks pregnancies. Arch. Iran. Med. 2009, 12, 29–34. [Google Scholar] [PubMed]
- Silverman, A.; Petersen, N.H. Physiology, Cerebral Autoregulation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020; pp. 1–8. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31985976 (accessed on 20 November 2020).
- Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 2005, 81, 423–428. [Google Scholar] [CrossRef]
- Wu, T.W.; Azhibekov, T.; Seri, I. Transitional hemodynamics in preterm neonates: Clinical relevance. Pediatr. Neonatol. 2016, 57, 7–18. [Google Scholar] [CrossRef] [PubMed]
- De Carli, A.; Andresen, B.; Giovannella, M.; Durduran, T.; Contini, D.; Spinelli, L.; Weigel, U.M.; Passera, S.; Pesenti, N.; Mosca, F.; et al. Cerebral oxygenation and blood flow in term infants during postnatal transition: BabyLux project. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F648–F653. [Google Scholar] [CrossRef]
- Altman, D.I.; Perlman, J.M.; Volpe, J.J.; Powers, W.J. Cerebral oxygen metabolism in newborns. Pediatrics 1993, 92, 99–104. [Google Scholar] [CrossRef]
- Altman, D.I.; Powers, W.J.; Perlman, J.M.; Herscovitch, P.; Volpe, S.L.; Volpe, J.J. Cerebral blood flow requirement for brain viability in newborn infants is lower than in adults. Ann. Neurol. 1988, 24, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Niu, Y.; Herrera, E.A.; Richter, H.G.; Camm, E.J.; Thakor, A.S.; Kane, A.D.; Hansell, J.A.; Brain, K.L.; Skeffi, K.L.; et al. The Fetal Cerebral Circulation: Three Decades of Exploration by the LLU Center for Perinatal Biology. Adv. Exp. Med. Biol. 2014, 814, 77–87. [Google Scholar] [CrossRef]
- Bishai, J.M.; Blood, A.B.; Hunter, C.J.; Longo, L.D.; Power, G.G. Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: A comparison of laser Doppler and microsphere measurements of CBF. J. Physiol. 2003, 546, 869–878. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Hernandez, M.J. Perinatal cerebral blood flow. Mead Johns. Symp. Perinat. Dev. Med. 1980, 17–29. Available online: https://pubmed.ncbi.nlm.nih.gov/6808252/#article-details (accessed on 9 October 2025).
- Tweed, W.A.; Pash, M.; Doig, G. Cerebrovascular mechanisms in perinatal asphyxia: The role of vasogenic brain edema. Pediatr. Res. 1981, 15, 44–46. [Google Scholar] [CrossRef]
- Jones, M.D.; Travstman, R.J. Cerebral oxygenation of the fetus, newborn, and adult. Semin. Perinatol. 1984, 8, 205–216. [Google Scholar]
- Purves, M.J.; James, I.M. Observations on the control of cerebral blood flow in the sheep fetus and newborn lamb. Circ. Res. 1969, 25, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Upadhyay, A.; Gothwal, S.; Chaudhary, H.; Tandon, A. Comparison of Umbilical Cord Milking and Delayed Cord Clamping on Cerebral Blood Flow in Term Neonates. Indian J. Pediatr. 2015, 82, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Katheria, A.C.; Szychowski, J.M.; Essers, J.; Mendler, M.R.; Dempsey, E.M.; Schmölzer, G.M.; Arnell, K.; Rich, W.D.; Hassen, K.; Allman, P.; et al. Early Cardiac and Cerebral Hemodynamics with Umbilical Cord Milking Compared with Delayed Cord Clamping in Infants Born Preterm. J. Pediatr. 2020, 223, 51–56.e1. [Google Scholar] [CrossRef]
- Kooi, E.M.W.; Richter, A.E. Cerebral Autoregulation in Sick Infants: Current Insights. Clin. Perinatol. 2020, 47, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, S.; Donofrio, M.T. Circulatory Changes and Cerebral Blood Flow and Oxygenation During Transition in Newborns With Congenital Heart Disease. Semin. Pediatr. Neurol. 2018, 28, 38–47. [Google Scholar] [CrossRef]
- Hahn, G.H.; Maroun, L.L.; Larsen, N.; Hougaard, D.M.; Sorensen, L.C.; Lou, H.C.; Greisen, G. Cerebral autoregulation in the first day after preterm birth: No evidence of association with systemic inflammation. Pediatr. Res. 2012, 71, 253–260. [Google Scholar] [CrossRef]
- Noori, S.; Seri, I. Hemodynamic antecedents of peri/intraventricular hemorrhage in very preterm neonates. Semin. Fetal Neonatal Med. 2015, 20, 232–237. [Google Scholar] [CrossRef]
- Wu, T.W.; Tamrazi, B.; Soleymani, S.; Seri, I.; Noori, S. Hemodynamic Changes During Rewarming Phase of Whole-Body Hypothermia Therapy in Neonates with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2018, 197, 68–74.e2. [Google Scholar] [CrossRef]
- Binder-Heschl, C.; Urlesberger, B.; Schwaberger, B.; Koestenberger, M.; Pichler, G. Borderline hypotension: How does it influence cerebral regional tissue oxygenation in preterm infants? J. Matern. Neonatal Med. 2016, 29, 2341–2346. [Google Scholar] [CrossRef]
- Noori, S.; Anderson, M.; Soleymani, S.; Seri, I. Effect of carbon dioxide on cerebral blood flow velocity in preterm infants during postnatal transition. Acta Paediatr. Int. J. Paediatr. 2014, 103, 334–339. [Google Scholar] [CrossRef]
- Wyatt, J. Cerebral oxygenation and haemodynamics in the foetus and newborn infant. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 697–700. [Google Scholar] [CrossRef]
- Tortora, D.; Severino, M.; Rossi, A. Arterial spin labeling perfusion in neonates. Semin. Fetal Neonatal Med. 2020, 25, 101130. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.S.; Carmichael, L.; Homan, J.; Patrick, J.E. Electrocortical activity, electroocular activity, and breathing movements in fetal sheep with prolonged and graded hypoxemia. Am. J. Obstet. Gynecol. 1992, 167, 553–558. [Google Scholar] [CrossRef]
- Ashwal, S.; Majcher, J.S.; Longo, L.D. Patterns of fetal lamb regional cerebral blood flow during and after prolonged hypoxia: Studies during the posthypoxic recovery period. Am. J. Obstet. Gynecol. 1981, 139, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Park, M.K.; Kuehl, T.J. Relaxant and contractile responses to prostaglandins in premature, newborn and adult baboon cerebral arteries. J. Pharmacol. Exp. Ther. 1985, 233, 628–635. [Google Scholar] [CrossRef]
- Pearce, W.J.; Ashwal, S. Developmental changes in thickness, contractility, and hypoxic sensitivity of newborn lamb cerebral arteries. Pediatr. Res. 1987, 22, 192–196. [Google Scholar] [CrossRef]
- Scher, M.S. Normal and abnormal cerebrovascular development: Gene-environment interactions during early life with later life consequences. In Handbook of Clinical Neurology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 112, pp. 1021–1042. [Google Scholar] [CrossRef]
- Xing, C.Y.; Tarumi, T.; Liu, J.; Zhang, Y.; Turner, M.; Riley, J.; Tinajero, C.D.; Yuan, L.J.; Zhang, R. Distribution of cardiac output to the brain across the adult lifespan. J. Cereb. Blood Flow Metab. 2017, 37, 2848–2856. [Google Scholar] [CrossRef]
- Williams, L.R.; Leggett, R.W. Reference values for resting blood flow to organs of man. Clin. Phys. Physiol. Meas. 1989, 10, 187–217. [Google Scholar] [CrossRef]
- Northington, F.J.; Tobin, J.R.; Harris, A.P.; Traystman, R.J.; Koehler, R.C. Developmental and regional differences in nitric oxide synthase activity and blood flow in the sheep brain. J. Cereb. Blood Flow Metab. 1997, 17, 109–115. [Google Scholar] [CrossRef] [PubMed]
- El-Dib, M.; Soul, J.S. Monitoring and management of brain hemodynamics and oxygenation. In Handbook of Clinical Neurology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 162, pp. 295–314. [Google Scholar] [CrossRef]
- Akundi, R.S.; Rivkees, S.A. Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells. PLoS ONE 2009, 4, e4739. [Google Scholar] [CrossRef]
- Low, J.A. Intrapartum fetal asphyxia: Definition, diagnosis, and classification. Am. J. Obstet. Gynecol. 1997, 176, 957–959. [Google Scholar] [CrossRef]
- Pearce, W. Hypoxic regulation of the fetal cerebral circulation. J. Appl. Physiol. 2006, 100, 731–738. [Google Scholar] [CrossRef]
- Fahey, J.; King, T.L. Intrauterine asphyxia: Clinical implications for providers of intrapartum care. J. Midwifery Women’s Health 2005, 50, 498–506. [Google Scholar] [CrossRef]
- Rudolph, A.M. Circulatory changes during gestational development of the sheep and human fetus. Pediatr. Res. 2018, 84, 348–351. [Google Scholar] [CrossRef]
- Rudolph, A.M. Cerebral glucose deficiency versus oxygen deficiency in neonatal encephalopathy. J. Neonatal. Perinatal. Med. 2018, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hüppi, P.S.; Warfield, S.; Kikinis, R.; Barnes, P.D.; Zientara, G.P.; Jolesz, F.A.; Tsuji, M.K.; Volpe, J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998, 43, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.K.; Hernandez-Andrade, E.; Krishnamurthy, U.; Buch, S.; Jella, P.; Trifan, A.; Yeo, L.; Hassan, S.S.; Haacke, E.M.; Romero, R.; et al. Dual-Imaging Modality Approach to Evaluate Cerebral Hemodynamics in Growth-Restricted Fetuses: Oxygenation and Perfusion. Fetal Diagn. Ther. 2020, 47, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wolfberg, A.J.; du Plessis, A.J. Near-Infrared Spectroscopy in the Fetus and Neonate. Clin. Perinatol. 2006, 33, 707–728. [Google Scholar] [CrossRef]
- Segovia, K.N.; Mcclure, M.; Moravec, M.; Luo, N.L.; Wan, Y.; Gong, X.; Riddle, A.; Craig, A.; Struve, J.; Sherman, L.S.; et al. Arrested Oligodendrocyte Lineage Maturation in chronic perinatal white matter injury. Ann. Neurol. 2008, 63, 520–530. [Google Scholar] [CrossRef]
- Fan, X.; Heijnen, C.J.; van der Kooij, M.A.; Groenendaal, F.; van Bel, F. The role and regulation of hypoxia-inducible factor-1α expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res. Rev. 2009, 62, 99–108. [Google Scholar] [CrossRef]
- Alonso-Spilsbury, M.; Mota-Rojas, D.; Villanueva-García, D.; Martínez-Burnes, J.; Orozco, H.; Ramírez-Necoechea, R.; Mayagoitia, A.L.; Trujillo, M.E. Perinatal asphyxia pathophysiology in pig and human: A review. Anim. Reprod. Sci. 2005, 90, 1–30. [Google Scholar] [CrossRef]
- Tomimatsu, T.; Kakigano, A.; Mimura, K.; Kanayama, T.; Koyama, S.; Fujita, S.; Taniguchi, Y.; Kanagawa, T.; Kimura, T. Maternal carbon dioxide level during labor and its possible effect on fetal cerebral oxygenation: Mini review. J. Obstet. Gynaecol. Res. 2013, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.M. Maternal hyperoxygenation for the human fetus: Should studies be curtailed? Pediatr. Res. 2020, 87, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, T.; Pereyra Peňa, J.; Hatran, D.P.; Longo, L.D. Maternal oxygen administration and fetal cerebral oxygenation: Studies on near-term fetal lambs at both low and high altitude. Am. J. Obstet. Gynecol. 2006, 195, 535–541. [Google Scholar] [CrossRef]
- Müller, J.J.; Antonow-Schlorke, I.; Kroegel, N.; Rupprecht, S.; Rakers, F.; Witte, O.W.; Schwab, M. Cardiovascular effects of prenatal stress—Are there implications for cerebrovascular, cognitive and mental health outcome? Neurosci. Biobehav. Rev. 2020, 117, 78–97. [Google Scholar] [CrossRef]
- Nathaniel, T.I. Brain-regulated metabolic suppression during hibernation: A neuroprotective mechanism for perinatal hypoxia-ischemia. Int. J. Stroke 2008, 3, 98–104. [Google Scholar] [CrossRef]
- Pourcyrous, M. Cerebral hemodynamic measurements in acute versus chronic asphyxia. Clin. Perinatol. 1999, 26, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.D.; Pearce, W.J. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 1998, 119, 683–694. [Google Scholar] [CrossRef]
- Rakers, F.; Rupprecht, S.; Dreiling, M.; Bergmeier, C.; Witte, O.W.; Schwab, M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2020, 117, 185–197. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Weinstock, M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem. Res. 2007, 32, 1730–1740. [Google Scholar] [CrossRef]
- Anwar, M.A.; Schwab, M.; Poston, L.; Nathanielsz, P.W. Betamethasone-mediated vascular dysfunction and changes in hematological profile in the ovine fetus. Am. J. Physiol.-Heart Circ. Physiol. 1999, 276, 1137–1143. [Google Scholar] [CrossRef]
- Malhotra, A.; Ditchfield, M.; Fahey, M.C.; Castillo-Melendez, M.; Allison, B.J.; Polglase, G.R.; Wallace, E.M.; Hodges, R.; Jenkin, G.; Miller, S.L. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr. Res. 2017, 82, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Dabertrand, F.; Nelson, M.T.; Brayden, J.E. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ. Res. 2012, 110, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Niño Cruz, G.I.; Ramirez Varela, A.; da Silva, I.C.M.; Hallal, P.C.; Santos, I.S. Physical activity during pregnancy and offspring neurodevelopment: A systematic review. Paediatr. Perinat. Epidemiol. 2018, 32, 369–379. [Google Scholar] [CrossRef]
- Antonow-Schlorke, I.; Schwab, M.; Cox, L.A.; Li, C.; Stuchlik, K.; Witte, O.W.; Nathanielsz, P.W.; McDonald, T.J. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc. Natl. Acad. Sci. USA 2011, 108, 3011–3016. [Google Scholar] [CrossRef]
- Colberg, C.; Antonow-Schlorke, I.; Müller, T.; Schubert, H.; Witte, O.W.; Schwab, M. Recovery of glucocorticoid-related loss of synaptic density in the fetal sheep brain at 0.75 of gestation. Neurosci. Lett. 2004, 364, 130–134. [Google Scholar] [CrossRef]
- Schwab, M.; Schmidt, K.; Roedel, M.; Mueller, T.; Schubert, H.; Anwar, M.A.; Nathanielsz, P.W. Non-linear changes of electrocortical activity after antenatal betamethasone treatment in fetal sheep. J. Physiol. 2001, 531, 535–543. [Google Scholar] [PubMed]
- Salihagić-Kadić, A.; Medić, M.; Jugović, D.; Kos, M.; Latin, V.; Jukić, M.K.; Arbeille, P. Fetal cerebrovascular response to chronic hypoxia—Implications for the prevention of brain damage. J. Matern. Neonatal Med. 2006, 19, 387–396. [Google Scholar] [CrossRef]
- Korček, P.; Širc, J.; Straňák, Z. Cerebral oxygenation reflects fetal development in preterm monochorionic and dichorionic twins. Early Hum. Dev. 2020, 144, 105025. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Huppi, P.S.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.; Liu, J. Neurodevelopment in children with intrauterine growth restriction: Adverse effects and interventions. J. Matern. Neonatal Med. 2016, 29, 660–668. [Google Scholar] [CrossRef]
- Longo, L.; Goyal, R. Cerebral Artery Signal Transduction Mechanisms: Developmental Changes in Dynamics and Ca2+ Sensitivity. Curr. Vasc. Pharmacol. 2013, 11, 655–711. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.; Gilles, F.H. Human telencephalic angiogenis. Ann. Neurol. 1985, 17, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Leviton, A. How Much of Neonatal Encephalopathy Is due to Birth Asphyxia? Am. J. Dis. Child. 1991, 145, 1325–1331. [Google Scholar] [CrossRef]
- Boegehold, M.A. Endothelium-dependent control of vascular tone during early postnatal and juvenile growth. Microcirculation 2010, 17, 394–406. [Google Scholar] [CrossRef]
- Goyal, R.; Mittal, A.; Chu, N.; Arthur, R.A.; Zhang, L.; Longo, L.D. Maturation and long-term hypoxia-induced acclimatization responses in PKC-mediated signaling pathways in ovine cerebral arterial contractility. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R1377–R1386. [Google Scholar] [CrossRef]
- Longo, L.D.; Pearce, W.J. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: A model for prenatal programming of adult disease? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, 16–25. [Google Scholar] [CrossRef]
- Nauli, S.M.; Williams, J.M.; Akopov, S.E.; Zhang, L.; Pearce, W.J. Developmental changes in ryanodine- and IP3-sensitive Ca2+ pools in ovine basilar artery. Am. J. Physiol.-Cell Physiol. 2001, 281, C1785–C1796. [Google Scholar] [CrossRef]
- Long, W.; Zhang, L.; Longo, L.D. Cerebral artery sarcoplasmic reticulum Ca2+ stores and contractility: Changes with development. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, 860–873. [Google Scholar] [CrossRef]
- Adeoye, O.O.; Silpanisong, J.; Williams, J.M.; Pearce, W.J. Role of the sympathetic autonomic nervous system in hypoxic remodeling of the fetal cerebral vasculature. J. Cardiovasc. Pharmacol. 2015, 65, 308–316. [Google Scholar] [CrossRef]
- Tao, X.; Lin, M.T.; Thorington, G.U.; Wilson, S.M.; Longo, L.D.; Hessinger, D.A. Long-term hypoxia increases calcium affinity of bk channels in ovine fetal and adult cerebral artery smooth muscle. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, 707–722. [Google Scholar] [CrossRef]
- Behringer, E.J.; Leite, L.D.; Buchholz, N.E.; Keeney, M.G.; Pearce, W.J.; Vanterpool, C.K.; Wilson, S.M.; Buchholz, J.N. Maturation and long-term hypoxia alters Ca2+-induced Ca2+ release in sheep cerebrovascular sympathetic neurons. J. Appl. Physiol. 2009, 107, 1223–1234. [Google Scholar] [CrossRef]
- Geary, G.G.; Osol, G.J.; Longo, L.D. Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries. J. Physiol. 2004, 558, 883–896. [Google Scholar] [CrossRef]
- Jenkins, D.D.; Wiest, D.B.; Mulvihill, D.M.; Hlavacek, A.M.; Majstoravich, S.J.; Brown, T.R.; Taylor, J.J.; Buckley, J.R.; Turner, R.P.; Rollins, L.G.; et al. Fetal and Neonatal Effects of N-Acetylcysteine When Used for Neuroprotection in Maternal Chorioamnionitis. J. Pediatr. 2016, 168, 67–76.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-T.; Chang, Y.C.; Wang, L.Y.; Wang, S.T.; Huang, C.C.; Ho, C.J. cAMP response element-binding protein activation in ligation preconditioning in neonatal brain. Ann. Neurol. 2004, 56, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Blood, A.B.; Hunter, C.J.; Power, G.G. Adenosine mediates decreased cerebral metabolic rate and increased cerebral flow during acute moderate hypoxia in the near-term fetal sheep. J. Physiol. 2003, 553, 935–945. [Google Scholar] [CrossRef]
- Koos, B.J. Adenosine A2a receptors and O2 sensing in development. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R601–R622. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Uliana, M.; Virdis, A. Angiotensin II and vascular damage in hypertension: Role of oxidative stress and sympathetic activation. Vascul. Pharmacol. 2019, 115, 13–17. [Google Scholar] [CrossRef]
- Wood, C.E.; Walker, C.D. Fetal and neonatal HPA axis. Compr. Physiol. 2016, 6, 33–62. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Functions of corticotropin-releasing hormone in anthropoid primates: From brain to placenta. Am. J. Hum. Biol. 2006, 18, 431–447. [Google Scholar] [CrossRef]
- Reynolds, R.M. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis-2012 Curt Richter Award Winner. Psychoneuroendocrinology 2013, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salceda, S.; Caro, J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997, 272, 22642–22647. [Google Scholar] [CrossRef]
- Ke, Q.; Costa, M. Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 2007, 9–12. [Google Scholar] [CrossRef]
- Buchholz, J.; Edwards-Teunissen, K.; Duckles, S.P. Impact of development and chronic hypoxia on NE release from adrenergic nerves in sheep arteries. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 276, R799–R808. [Google Scholar] [CrossRef]
- Teng, G.Q.; Williams, J.; Zhang, L.; Purdy, R.; Pearce, W.J. Effects of maturation, artery size, and chronic hypoxia on 5-HT receptor type in ovine cranial arteries. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 275, 742–753. [Google Scholar] [CrossRef]
- Carstens, M.H. Development of the facial midline. J. Craniofac. Surg. 2002, 13, 129–187. [Google Scholar] [CrossRef] [PubMed]
- Bertulli, L.; Robert, T. Embryological development of the human cranio-facial arterial system: A pictorial review. Surg. Radiol. Anat. 2021, 43, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.W.; Deshpande, S. Metabolic adaptation at birth. Semin. Fetal Neonatal Med. 2005, 10, 341–350. [Google Scholar] [CrossRef]
- Booth, L.C.; Tummers, L.; Jensen, E.C.; Barrett, C.J.; Malpas, S.C.; Gunn, A.J.; Bennet, L. Differential effects of the adenosine A1 receptor agonist adenosine amine congener on renal, femoral and carotid vascular conductance in preterm fetal sheep. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1316–1320. [Google Scholar] [CrossRef]
- Sorensen, D.W.; Injeti, E.R.; Mejia-Aguilar, L.; Williams, J.M.; Pearce, W.J. Postnatal development alters functional compartmentalization of myosin light chain kinase in ovine carotid arteries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, 441–453. [Google Scholar] [CrossRef]
- Injeti, E.R.; Sandoval, R.J.; Williams, J.M.; Smolensky, A.V.; Ford, L.E.; Pearce, W.J. Maximal stimulation-induced in situ myosin light chain kinase activity is upregulated in fetal compared with adult ovine carotid arteries. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, 2289–2299. [Google Scholar] [CrossRef]
- Goyal, R.; Longo, L.D. Gene expression in sheep carotid arteries: Major changes with maturational development. Pediatr. Res. 2012, 72, 137–146. [Google Scholar] [CrossRef]
- Kilavuz, Ö.; Vetter, K. Is the liver of the fetus the 4th preferential organ for arterial blood supply besides brain, heart, and adrenal glands? J. Perinat. Med. 1999, 27, 103–106. [Google Scholar] [CrossRef]
- Giussani, D.A. The fetal brain sparing response to hypoxia: Physiological mechanisms. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Marín, M.J.; Marín-Clavijo, J.; Blanco-Elena, J.A.; Jiménez-López, J.; González-Mesa, E. Brain sparing effect on neurodevelopment in children with intrauterine growth restriction: A systematic review. Children 2021, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.; Hernandez-Andrade, E.; Basheer, S.N.; Lees, C. Impact of cerebral redistribution on neurodevelopmental outcome in small-for-gestational-age or growth-restricted babies: A systematic review. Ultrasound Obstet. Gynecol. 2015, 46, 398–404. [Google Scholar] [CrossRef]
- Roza, S.J.; Steegers, E.A.P.; Verburg, B.O.; Jaddoe, V.W.V.; Moll, H.A.; Hofman, A.; Verhulst, F.C.; Tiemeier, H. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am. J. Epidemiol. 2008, 168, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Y.; Milligan, N.; Keating, S.; Windrim, R.; Keunen, J.; Thakur, V.; Ohman, A.; Portnoy, S.; Sled, J.G.; Kelly, E.; et al. The hemodynamics of late-onset intrauterine growth restriction by MRI. Am. J. Obstet. Gynecol. 2016, 214, 367.e1–367.e17. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse-Delmas, H.; Grimaud, O.; Moscoso, G.; Ville, Y. Color Doppler study of the venous circulation in the fetal brain and hemodynamic study of the cerebral transverse sinus. Ultrasound Obstet. Gynecol. 1999, 13, 34–42. [Google Scholar] [CrossRef]
- Morales-Roselló, J.; Khalil, A.; Morlando, M.; Hervás-Marín, D.; Perales-Marín, A. Doppler reference values of the fetal vertebral and middle cerebral arteries, at 19-41 weeks gestation. J. Matern. Neonatal Med. 2015, 28, 338–343. [Google Scholar] [CrossRef]
- Brȩborowicz, A.; Dubiel, M.; Pietryga, M.; Brȩborowicz, G.H.; Gudmundsson, S. Fetal pulmonary and cerebral artery Doppler velocimetry in normal and high risk pregnancy. Ginekol. Pol. 2014, 85, 26–30. [Google Scholar] [CrossRef]
- Harman, C.R.; Baschat, A.A. Comprehensive assessment of fetal wellbeing: Which Doppler tests should be performed? Curr. Opin. Obstet. Gynecol. 2003, 15, 147–157. [Google Scholar] [CrossRef]
- Kiserud, T.; Acharya, G. The fetal circulation. Prenat. Diagn. 2004, 24, 1049–1059. [Google Scholar] [CrossRef]
- Bada, H.S.; Hajjar, W.; Chua, C.; Sumner, D.S. Noninvasive diagnosis of neonatal asphyxia and intraventricular hemorrhage by Doppler ultrasound. J. Pediatr. 1979, 95, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Dubiel, M. Doppler velocimetry in the evaluation of fetal hypoxia. J. Perinat. Med. 2001, 29, 399–407. [Google Scholar] [CrossRef]
- Wladimiroff, J.W.; Tonge, H.M.; Stewart, P.A. Doppler ultrasound assessment of cerebral blood flow in the human fetus. BJOG Int. J. Obstet. Gynaecol. 1986, 93, 471–475. [Google Scholar] [CrossRef]
- Gailloud, P.; Albayram, S.; Fasel, J.H.D.; Beauchamp, N.J.; Murphy, K.J. Angiographic and embryologic considerations in five cases of middle cerebral artery fenestration. Am. J. Neuroradiol. 2002, 23, 585–587. [Google Scholar] [PubMed]
- Bhide, A.; Acharya, G.; Baschat, A.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Ebbing, C.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, J.; et al. ISUOG Practice Guidelines (updated): Use of Doppler velocimetry in obstetrics. Ultrasound Obstet. Gynecol. 2021, 58, 331–339. [Google Scholar] [CrossRef]
- Polavarapu, S.R.; Fitzgerald, G.D.; Contag, S.; Hoffman, S.B. Utility of prenatal Doppler ultrasound to predict neonatal impaired cerebral autoregulation. J. Perinatol. 2018, 38, 474–481. [Google Scholar] [CrossRef]
- Morales-ROSELLOó, J.; Khalil, A.; Morlando, M.; Bhide, A.; Papageorghiou, A.; Thilaganathan, B. Poor neonatal acid-base status in term fetuses with low cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2015, 45, 156–161. [Google Scholar] [CrossRef]
- Monteith, C.; Flood, K.; Mullers, S.; Unterscheider, J.; Breathnach, F.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; et al. Evaluation of normalization of cerebro-placental ratio as a potential predictor for adverse outcome in SGA fetuses. Am. J. Obstet. Gynecol. 2017, 216, 285.e1–285.e6. [Google Scholar] [CrossRef] [PubMed]
- Twickler, D.M.; McIntire, D.D.; Alexander, J.M.; Leveno, K.J. Effects of magnesium sulfate on preterm fetal cerebral blood flow using Doppler analysis: A randomized controlled trial. Obstet. Gynecol. 2010, 115, 21–25. [Google Scholar] [CrossRef]
- Manaa, E.M.; Romeih, M.S. Fetal responses to epidural analgesia as evidenced by Doppler indices. Middle East J. Anesthesiol. 2008, 19, 1321–1336. [Google Scholar]
- Aghajanian, P.; Assaf, S.A.; Korst, L.M.; Miller, D.A.; Chmait, R.H. Fetal middle cerebral artery Doppler fluctuations after laser surgery for twin-twin transfusion syndrome. J. Perinatol. 2011, 31, 368–372. [Google Scholar] [CrossRef]
- Oros, D.; Figueras, F.; Cruz-Martinez, R.; Padilla, N.; Meler, E.; Hernandez-Andrade, E.; Gratacos, E. Middle versus anterior cerebral artery Doppler for the prediction of perinatal outcome and neonatal neurobehavior in term small-for-gestational-age fetuses with normal umbilical artery Doppler. Ultrasound Obstet. Gynecol. 2010, 35, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Serralde, J.A.; Hernández-Andrade, E.; Figueroa-Diesel, H.; Oros, D.; Feria, L.A.; Scheier, M.; Figueras, F.; Gratacós, E. Reference values for Doppler parameters of the fetal anterior cerebral artery throughout gestation. Gynecol. Obstet. Investig. 2010, 69, 33–39. [Google Scholar] [CrossRef]
- Barbosa, M.M.; Carvalho, F.H.C.; Júnior, E.A.; Nardozza, L.M.M.; Santana, R.M.; Torloni, M.R.; Moron, A.F. Prediction of acidemia at birth by Doppler assessment of fetal cerebral transverse sinus in pregnancies with placental insufficiency. Ultrasound Obstet. Gynecol. 2009, 33, 188–192. [Google Scholar] [CrossRef]
- Cruz-Martinez, R.; Figueras, F.; Hernandez-Andrade, E.; Benavides-Serralde, A.; Gratacos, E. Normal reference ranges of fetal regional cerebral blood perfusion as measured by fractional moving blood volume. Ultrasound Obstet. Gynecol. 2011, 37, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Figueroa-Diesel, H.; Jansson, T.; Rangel-Nava, H.; Gratacos, E. Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2008, 32, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.K.; Liang, S.T.; Lo, R.L.S.; Chan, F.Y. Middle cerebral arterv Doppler flow velocity waveforms. Obstet. Gynecol. 1987, 70, 613–616. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, C.; Guedes-Martins, L. Fetal Cerebral Blood Flow (Dys)autoregulation. Diagnostics 2025, 15, 2592. https://doi.org/10.3390/diagnostics15202592
Moreira C, Guedes-Martins L. Fetal Cerebral Blood Flow (Dys)autoregulation. Diagnostics. 2025; 15(20):2592. https://doi.org/10.3390/diagnostics15202592
Chicago/Turabian StyleMoreira, Cristiana, and Luís Guedes-Martins. 2025. "Fetal Cerebral Blood Flow (Dys)autoregulation" Diagnostics 15, no. 20: 2592. https://doi.org/10.3390/diagnostics15202592
APA StyleMoreira, C., & Guedes-Martins, L. (2025). Fetal Cerebral Blood Flow (Dys)autoregulation. Diagnostics, 15(20), 2592. https://doi.org/10.3390/diagnostics15202592






