Median Nerve Diameter Ratio on Ultrasound as a Complementary Tool to Electrodiagnostic Testing in Carpal Tunnel Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
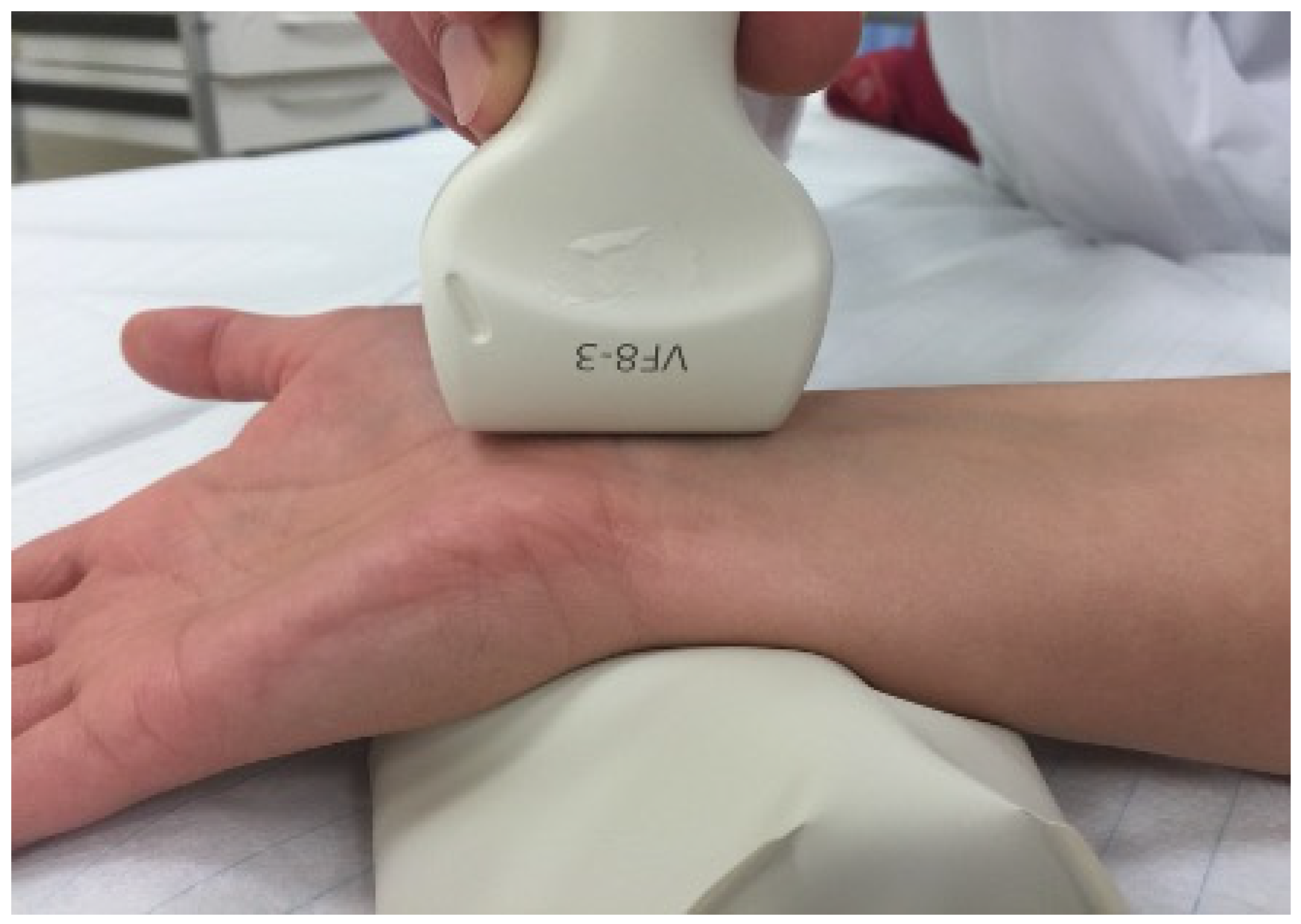
2.2. Ultrasound Technique
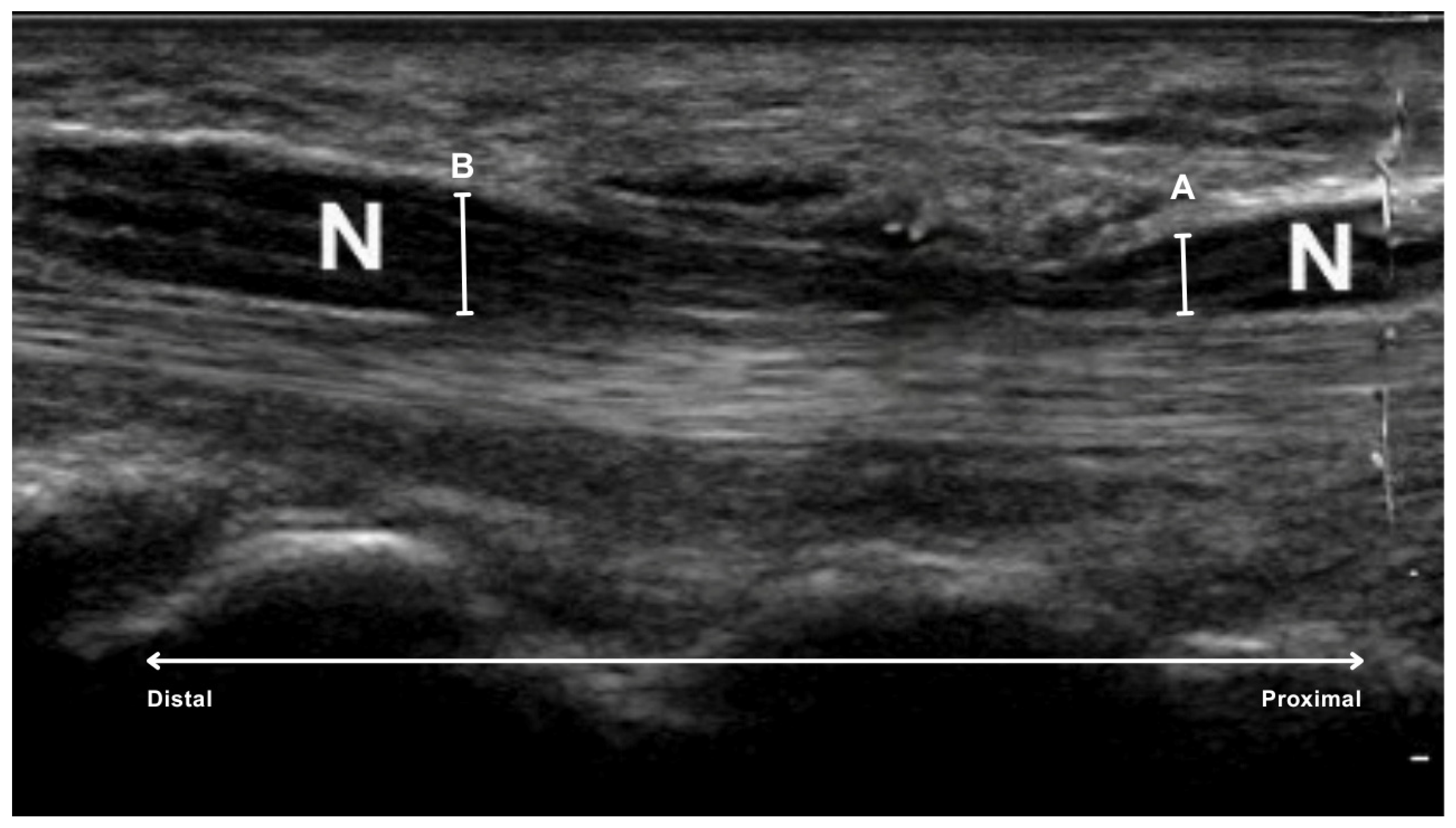
2.3. Measurement of Median Nerve Diameters
- proximal to the carpal tunnel, at the level of the first carpal row, specifically over the os lunatum, the os triquetrum and the base of the os scaphoideum (A in Figure 2).
- inside the carpal tunnel, centrally under the flexor retinaculum, at the level of the tuberosity of the scaphoid and trapezium bones on the radial side and the hamulus of the hamate and pisiform bones on the ulnar side (B in Figure 2).
2.4. Statistical Analysis
3. Results
3.1. Correlation Between Carpal Tunnel Ratio and Prolonged Distal Motor Latency
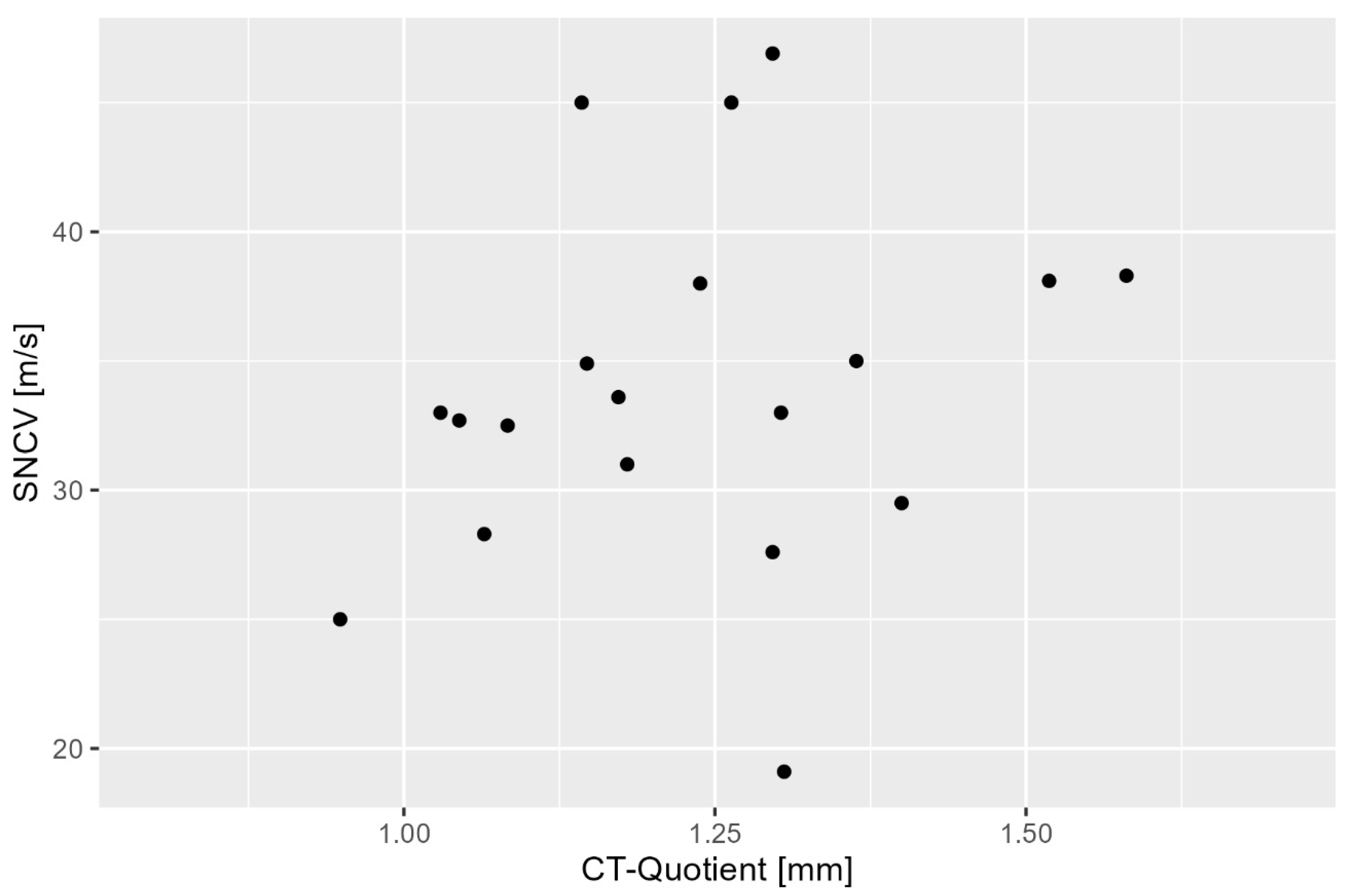
3.2. Correlation Between CT Ratio and Sensory Nerve Conduction Velocity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTS | Carpal tunnel syndrome |
| CSA | Cross-sectional area |
| DML | Distal motor latency |
| SNCV | Sensory nerve conduction velocity |
References
- Antoniadis, G.; Bischoff, C.; Dumont, C.; Dillmann, U.; Frick, A.; Kastrup, O.; Langer, M.; Mailander, P.; Schadel-Hopfner, M.; Schwerdtfeger, K.; et al. Diagnostik und Therapie des Karpaltunnelsyndroms—S3-Leitlinie. AWMF-Register-Nr. 005/003. Deutsche Gesellschaft für Handchirurgie (DGH), Deutsche Gesellschaft für Neurochirurgie (DGNC), Deutsche Gesellschaft für Neurologie (DGN), Deutsche Gesellschaft für Orthopädie und Unfallchirurgie (DGOU); Version 2022, gültig bis 01.01.2027. Available online: https://www.awmf.org/leitlinien/detail/ll/005-003.html (accessed on 14 May 2025).
- Mondelli, M.; Aprile, I.; Ballerini, M.; Ginanneschi, F.; Reale, F.; Romano, C.; Rossi, S.; Padua, L. Sex differences in carpal tunnel syndrome: Comparison of surgical and non-surgical populations. Eur. J. Neurol. 2005, 12, 976–983. [Google Scholar] [CrossRef]
- Donati, D.; Boccolari, P.; Tedeschi, R. Manual Therapy vs. Surgery: Which Is Best for Carpal Tunnel Syndrome Relief? Life 2024, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Atcheson, S.G.; Ward, J.R.; Lowe, W. Concurrent medical disease in work-related carpal tunnel syndrome. Arch. Intern. Med. 1998, 158, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Sánchez, F.; Gómez-Menéndez, A.I.; López-Veloso, M.; Calvo-Simal, S.; Lloria-Gil, M.C.; González-Santos, J.; Muñoz-Alcaraz, M.N.; Jiménez-Vilchez, A.J.; González-Bernal, J.J.; García-López, B. A Proposal for Neurography Referral in Patients with Carpal Tunnel Syndrome Based on Clinical Symptoms and Demographic Variables of 797 Patients. Diagnostics 2024, 14, 297. [Google Scholar] [CrossRef]
- Beekman, R.; Visser, L.H. Sonography in the diagnosis of carpal tunnel syndrome: A critical review of the literature. Muscle Nerve 2003, 27, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mhoon, J.T.; Juel, V.C.; Hobson-Webb, L.D. Median nerve ultrasound as a screening tool in carpal tunnel syndrome: Correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle Nerve 2012, 46, 871–878. [Google Scholar] [CrossRef]
- Donati, D.; Goretti, C.; Tedeschi, R.; Boccolari, P.; Ricci, V.; Farì, G.; Vita, F.; Tarallo, L. Comparing endoscopic and conventional surgery techniques for carpal tunnel syndrome: A retrospective study. JPRAS Open 2024, 41, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lew, H.L.; Date, E.S.; Pan, S.S.; Wu, P.; Ware, P.F.; Kingery, W.S. Sensitivity, specificity, and variability of nerve conduction velocity measurements in carpal tunnel syndrome. Arch. Phys. Med. Rehabil. 2005, 86, 12–16. [Google Scholar] [CrossRef]
- Graham, B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J. Bone Jt. Surg. 2008, 90, 2587–2593. [Google Scholar] [CrossRef]
- Olchowy, C.; Solinski, D.; Lasecki, M.; Dabrowski, P.; Urban, S.; Zaleska-Dorobisz, U. Wrist ultrasound examination—Scanning technique and ultrasound anatomy. Part 2: Ventral wrist. J. Ultrason. 2017, 17, 123–128. [Google Scholar] [CrossRef]
- Kopf, H.; Loizides, A.; Mostbeck, G.H.; Gruber, H. Diagnostic sonography of peripheral nerves: Indications, examination techniques and pathological findings. Ultraschall. Med. 2011, 32, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Lo Monaco, M.; Gregori, B.; Valente, E.M.; Padua, R.; Tonali, P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol. Scand. 1997, 96, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Lo Monaco, M.; Valente, E.M.; Tonali, P.A. A useful electrophysiologic parameter for diagnosis of carpal tunnel syndrome. Muscle Nerve 1996, 19, 48–53. [Google Scholar] [CrossRef]
- Cartwright, M.S.; Hobson-Webb, L.D.; Boon, A.J.; Alter, K.E.; Hunt, C.H.; Flores, V.H.; Werner, R.A.; Shook, S.J.; Thomas, T.D.; Primack, S.J.; et al. Evidence-based guideline: Neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve 2012, 46, 287–293. [Google Scholar] [CrossRef]
- Zyluk, A.; Szlosser, Z. Are conduction studies in the median nerve obligatory for the diagnosis of the carpal tunnel syndrome: A review. Chir. Narzadow. Ruchu Ortop. Pol. 2009, 74, 174–179. [Google Scholar]
- Kohara, N. Clinical and electrophysiological findings in carpal tunnel syndrome. Brain Nerve 2007, 59, 1229–1238. [Google Scholar]
- Seror, P. Sonography and electrodiagnosis in carpal tunnel syndrome diagnosis: An analysis of the literature. Eur. J. Radiol. 2008, 67, 146–152. [Google Scholar] [CrossRef]
- Baiee, R.H.; Al-Mukhtar, N.J.; Al-Rubiae, S.J.; Hammoodi, Z.H.; Abass, F.N. Neurophysiological findings in patients with carpal tunnel syndrome by nerve conduction study in comparing with ultrasound study. J. Nat. Sci. Res. 2015, 5, 9–13. [Google Scholar]
- Lin, T.Y.; Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasonography for the diagnosis of carpal tunnel syndrome: An umbrella review. J. Neurol. 2022, 269, 4663–4675. [Google Scholar] [CrossRef]
- Linehan, C.; Childs, J.; Quinton, A.E.; Aziz, A. Ultrasound parameters to identify and diagnose carpal tunnel syndrome: A review of the literature. Australas J. Ultrasound Med. 2020, 23, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.J.; McKell, J.S. Sonographic measurement of median nerve cross-sectional area to determine severity of carpal tunnel syndrome: A cautionary tale. J. Ultrasound Med. 2024, 43, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, A.; Stelitano, C.; Bollani, P.; Giardini, A.; Vanzetti, E.; Ferrari, M. Carpal tunnel sonography. J. Ultrasound 2020, 23, 337–347. [Google Scholar] [CrossRef]
- Crasto, J.A.; Scott, M.E.; Fowler, J.R. Ultrasound measurement of the cross-sectional area of the median nerve: The effect of teaching on measurement accuracy. Hand 2019, 14, 155–162. [Google Scholar] [CrossRef]
- O’Riordan, C.; Van De Ven, P.; Nelson, J.; McCreesh, K.; Clifford, A. Reliability of a measurement method for the cross-sectional area of the longus colli using real-time ultrasound imaging. Ultrasound 2016, 24, 154–162. [Google Scholar] [CrossRef]
- Kele, H.; Verheggen, R.; Bittermann, H.J.; Reimers, C.D. The potential value of ultrasonography in the evaluation of carpal tunnel syndrome. Neurology 2003, 61, 389–391. [Google Scholar] [CrossRef]
- Hobson-Webb, L.D.; Padua, L. Median nerve ultrasonography in carpal tunnel syndrome: Findings from two laboratories. Muscle Nerve 2009, 40, 94–97. [Google Scholar] [CrossRef]
- Wong, S.; Griffith, J.; Hui, A.; Tang, A.; Wong, K. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002, 46, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Ziswiler, H.R.; Reichenbach, S.; Vogelin, E.; Bachmann, L.M.; Villiger, P.M.; Juni, P. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: A prospective study. Arthritis Rheum. 2005, 52, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Arsova, M.; Matthes, K.; Husman, J.; Toppe, D.; Kober, J.; Trittler, T.; Swist, D.; Dorausch, E.M.G.; Urbig, A.; et al. Technical assessment of resolution of handheld ultrasound devices and clinical implications. Ultraschall Med. 2024, 45, 405–411. [Google Scholar] [CrossRef]
- Ozsoy-Unubol, T.; Bahar-Ozdemir, Y.; Yagci, I. Diagnosis and grading of carpal tunnel syndrome with quantitative ultrasound: Is it possible? J. Clin. Neurosci. 2020, 75, 25–29. [Google Scholar] [CrossRef]
- Paliwal, P.R.; Therimadasamy, A.K.; Chan, Y.C.; Wilder-Smith, E.P. Does measuring the median nerve at the carpal tunnel outlet improve ultrasound CTS diagnosis? J. Neurol. Sci. 2014, 339, 47–51. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, C.; Alexander, M.; Kane, D. The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: A new paradigm. Rheumatology 2015, 54, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.; Filippou, G.; Gallom, A.; Frediani, B. Diagnostic utility of ultrasonography versus nerve conduction studies in mild carpal tunnel syndrome. Arthritis Rheum. 2008, 59, 357–366. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnhardt, T.; Soost, C.; Graw, J.A.; Burchard, R.; Bliemel, C.; Barsumyan, A. Median Nerve Diameter Ratio on Ultrasound as a Complementary Tool to Electrodiagnostic Testing in Carpal Tunnel Syndrome. Diagnostics 2025, 15, 2464. https://doi.org/10.3390/diagnostics15192464
Lehnhardt T, Soost C, Graw JA, Burchard R, Bliemel C, Barsumyan A. Median Nerve Diameter Ratio on Ultrasound as a Complementary Tool to Electrodiagnostic Testing in Carpal Tunnel Syndrome. Diagnostics. 2025; 15(19):2464. https://doi.org/10.3390/diagnostics15192464
Chicago/Turabian StyleLehnhardt, Thorsten, Christian Soost, Jan Adriaan Graw, Rene Burchard, Christopher Bliemel, and Artur Barsumyan. 2025. "Median Nerve Diameter Ratio on Ultrasound as a Complementary Tool to Electrodiagnostic Testing in Carpal Tunnel Syndrome" Diagnostics 15, no. 19: 2464. https://doi.org/10.3390/diagnostics15192464
APA StyleLehnhardt, T., Soost, C., Graw, J. A., Burchard, R., Bliemel, C., & Barsumyan, A. (2025). Median Nerve Diameter Ratio on Ultrasound as a Complementary Tool to Electrodiagnostic Testing in Carpal Tunnel Syndrome. Diagnostics, 15(19), 2464. https://doi.org/10.3390/diagnostics15192464




