Analysing the Renal Vasculature Using Super-Resolution Ultrasound Imaging: Considerations for Clinical and Research Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Animal Population
2.3. SRUS Data Acquisition and Experimental Variables
2.3.1. MB Detections and MB Tracks
2.3.2. Physiological Parameters
2.3.3. MB Flow Velocities
2.4. Ex Vivo µCT
2.5. Image Segmentation and Co-Registration
2.5.1. Identification and Isolation of Co-Registered μCT Image Planes
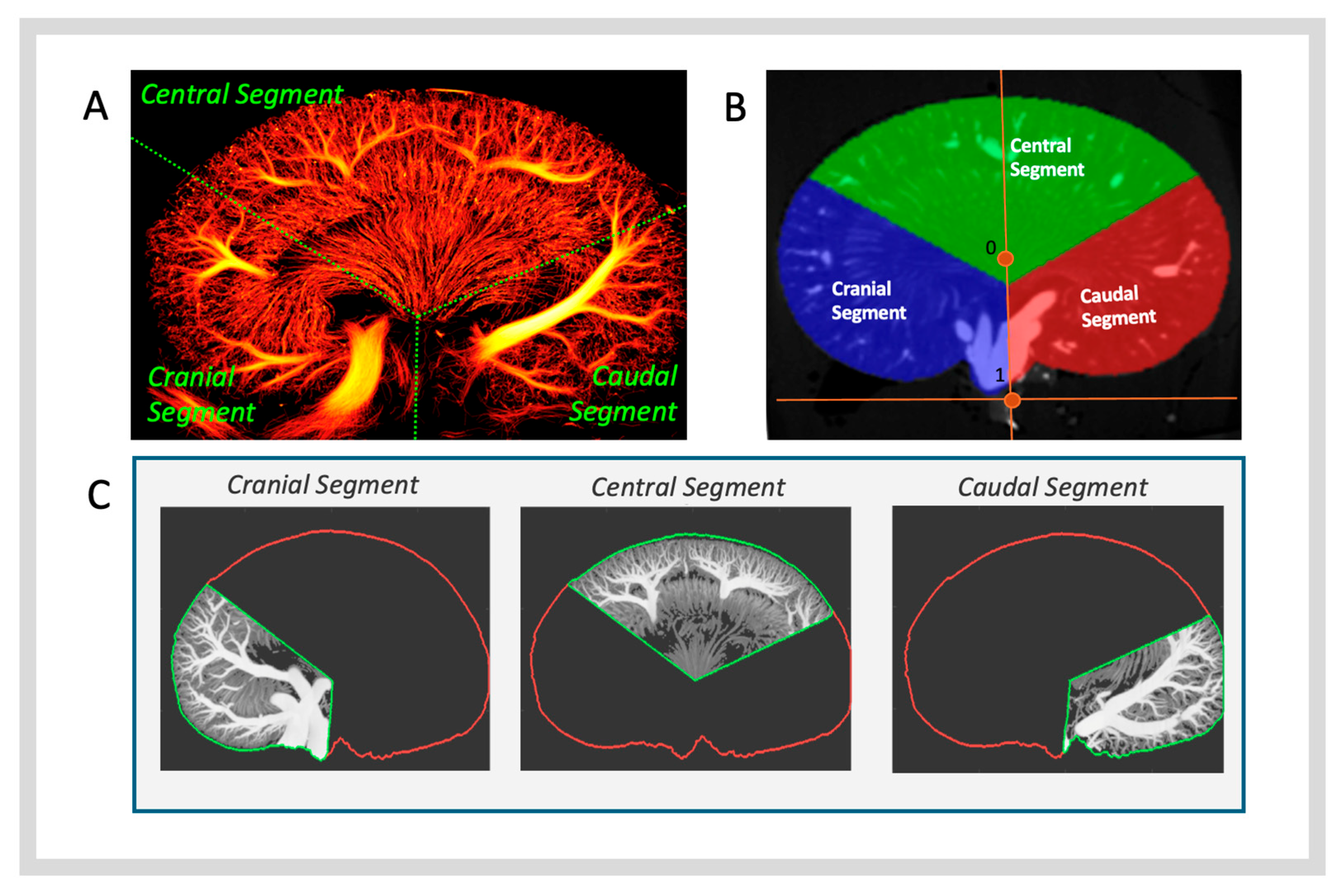
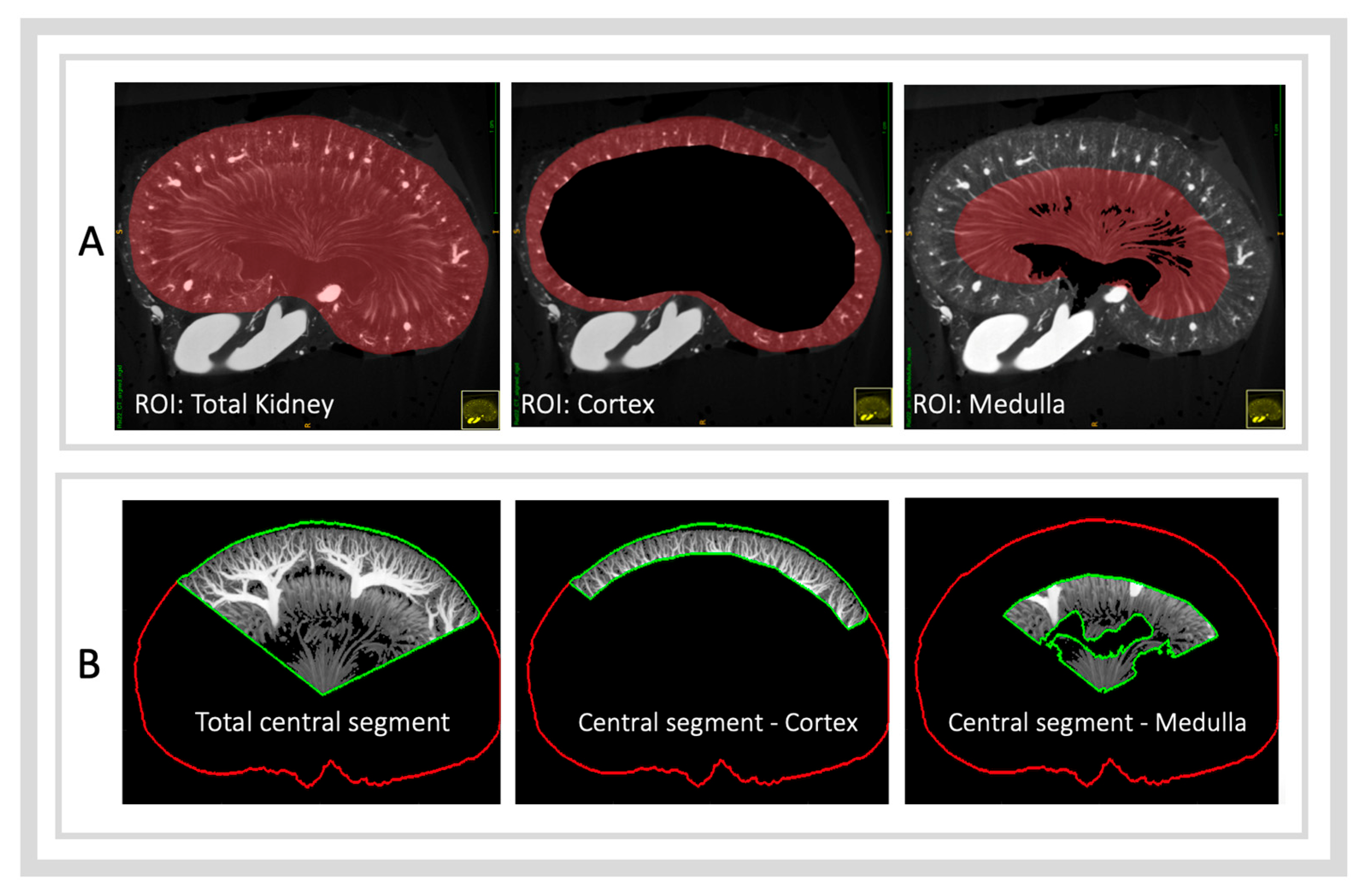
2.5.2. Anatomical Regions of Interest
- ROI—Total Kidney: Derived directly from the kidney segmentation mask.
- ROI—Cortex: Created by subtracting the arcuate artery boundary mask from the kidney mask.
- ROI—Medulla: Created by subtracting the pelvic mask from the vasa recta boundary mask.
2.6. Quantifying Vascular Density
2.6.1. µCT Post-Processing and Vascular Density Assessment
2.6.2. SRUS Post-Processing and Vascular Density Assessment
2.7. Statistical Analysis
3. Results
3.1. The Impact of Experimental Variables on SRUS Imaging
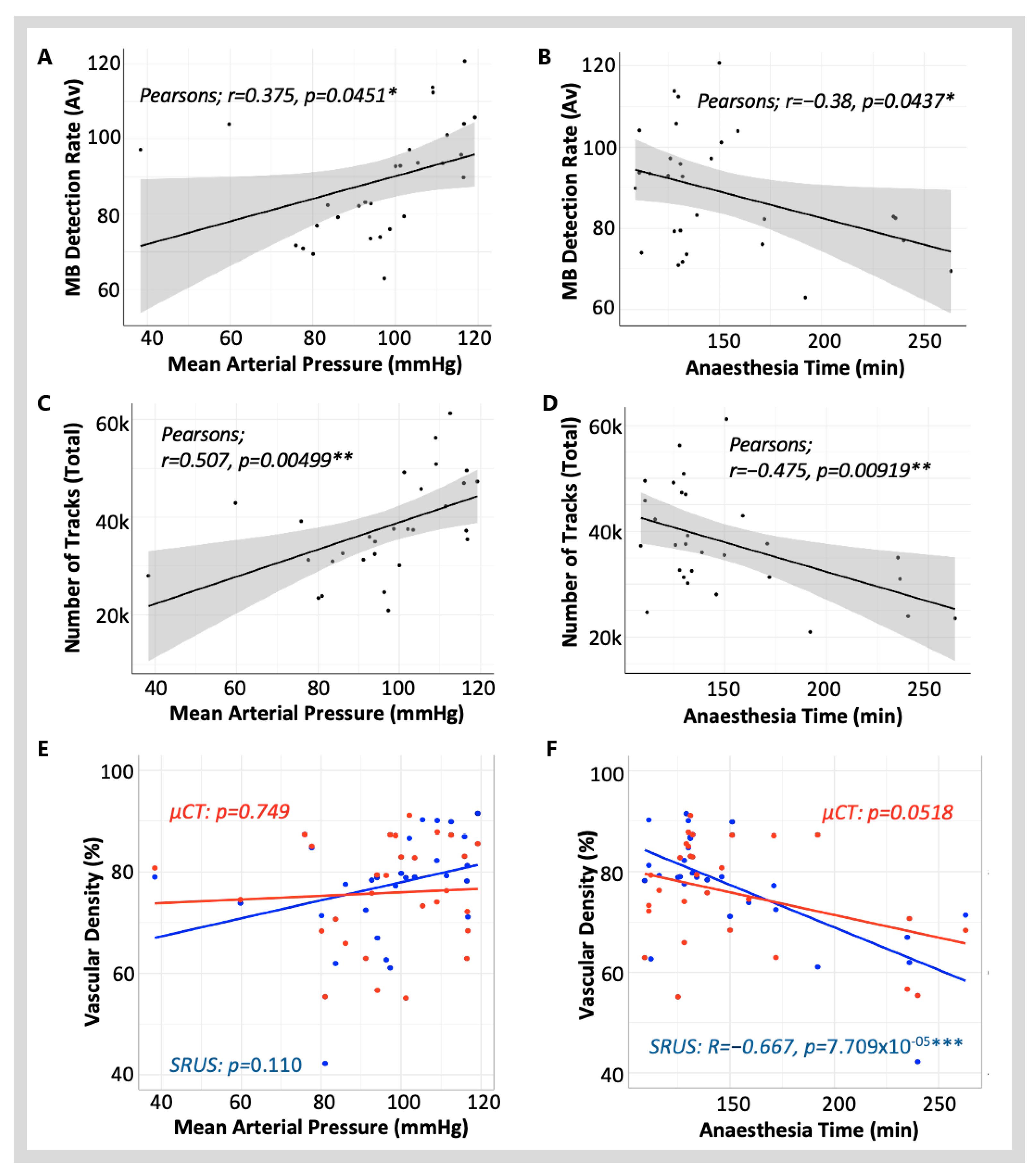
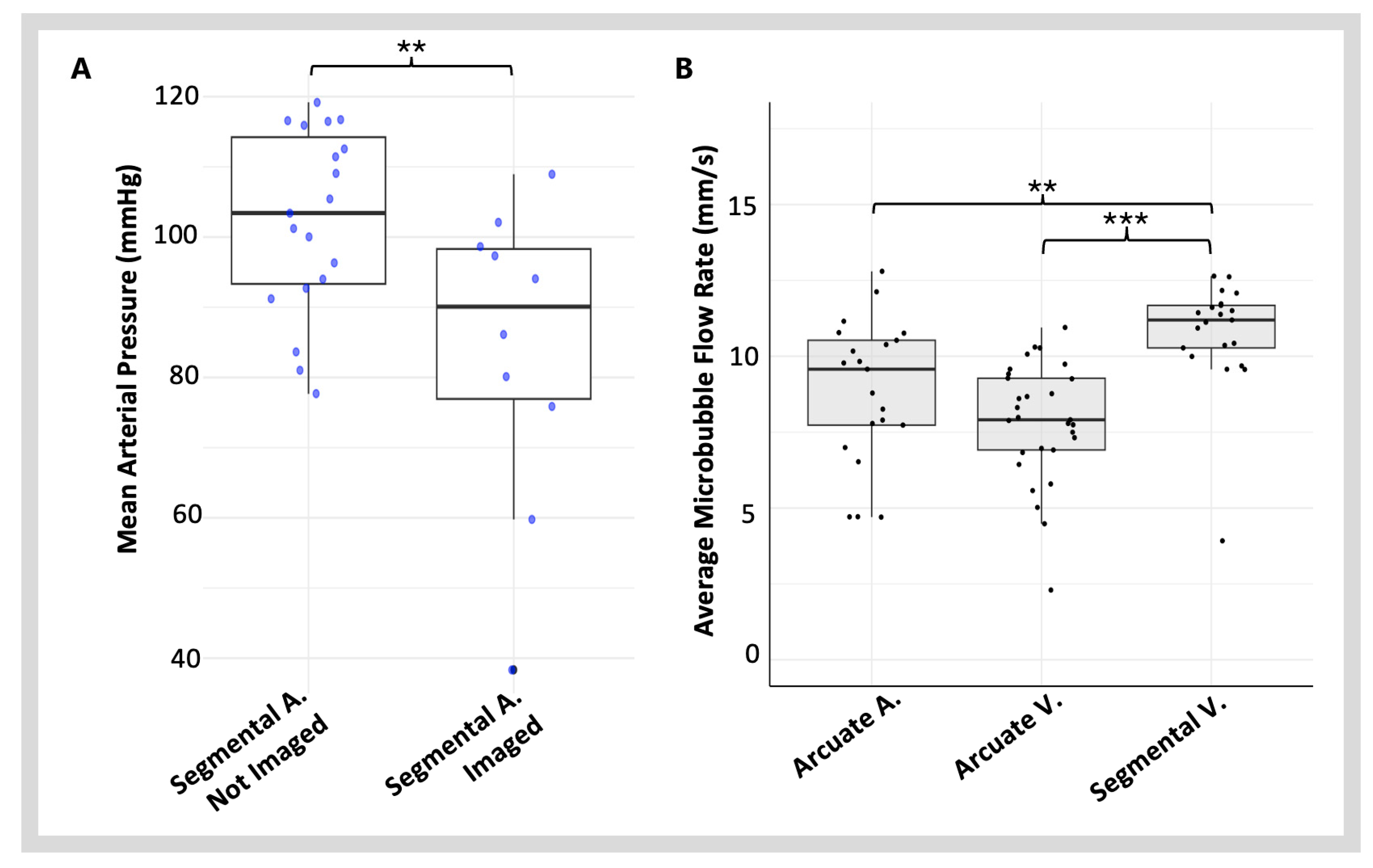
3.1.1. Blood Pressure and Heart Rate
3.1.2. Anaesthesia Time
3.1.3. Other Clinical Variables
3.2. Imaging Blood Vessels with Varying Blood Flow Velocities
3.3. Vascular Density
3.3.1. Regional Variations in Vascular Density Measured with SRUS
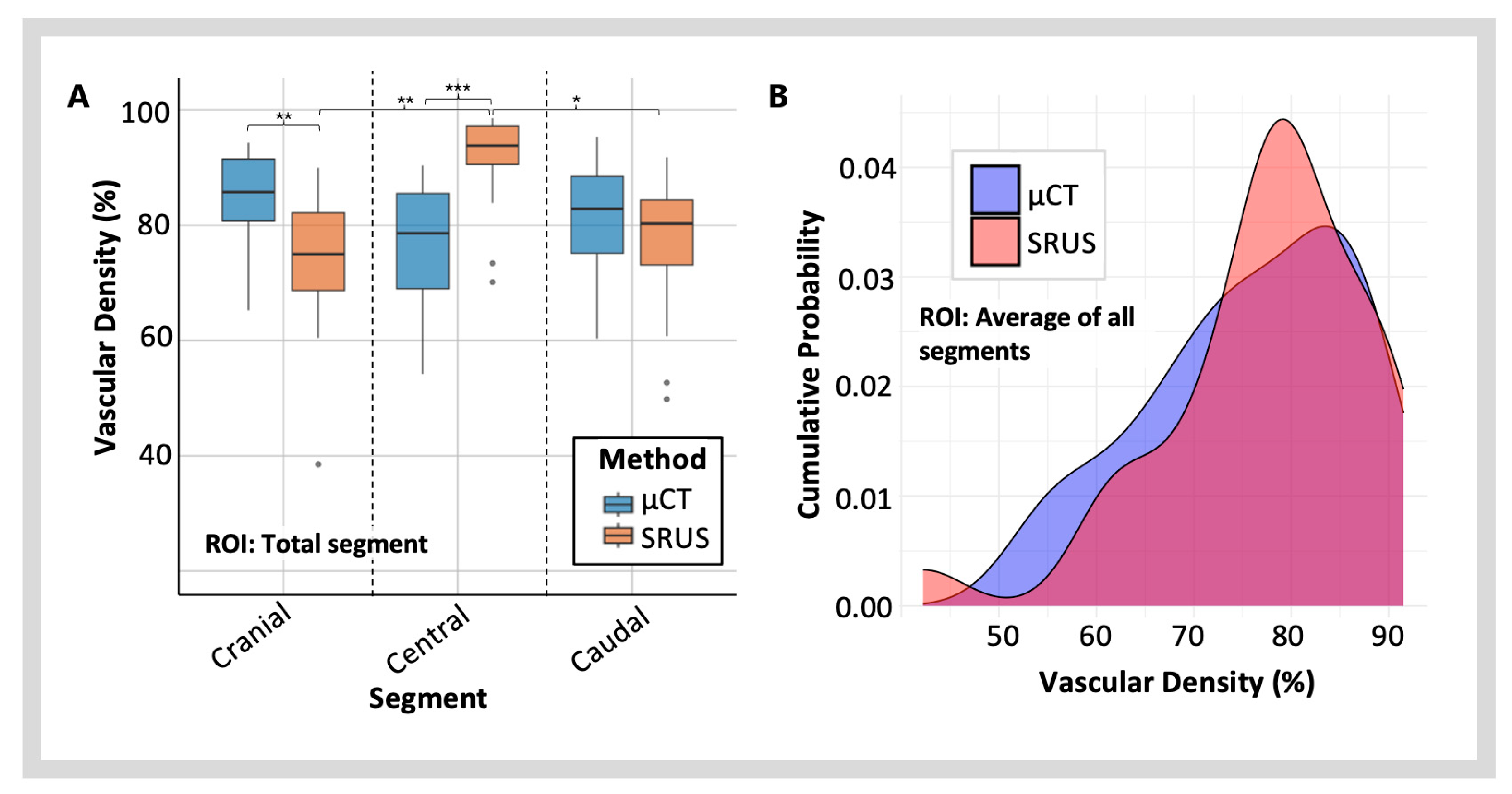
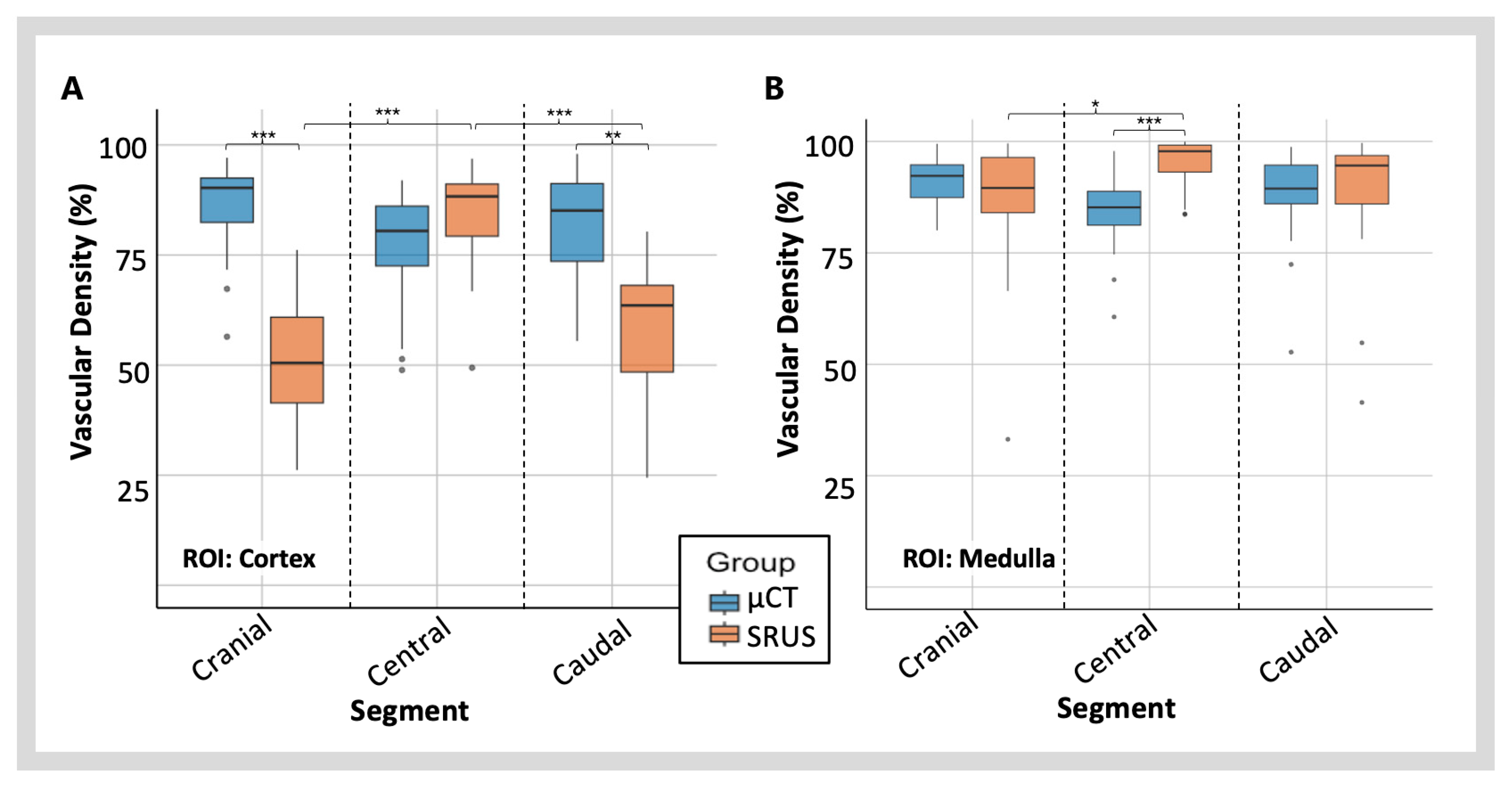
3.3.2. Vascular Density Measured with SRUS vs. µCT
4. Discussion
4.1. The Impact of Experimental Variables on SRUS Imaging
4.2. Limitations of SRUS Renal Imaging
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SRUS | Super-resolution ultrasound |
| MB | Microbubble |
| µCT | Micro-computed tomography |
| MAP | Mean arterial blood pressure |
| ROI | Region of interest |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ANOVA | Analysis of variance |
| ANCOVA | Analysis of covariance |
References
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.; Girirajan, T.; Mason, M. Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J. 2007, 91, 4258–4272. [Google Scholar] [CrossRef]
- Couture, O.; Bannouf, S.; Montaldo, G.; Aubry, J.F.; Fink, M.; Tanter, M. Ultrafast imaging of ultrasound contrast agents. Ultrasound Med. Biol. 2009, 35, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.X.; et al. Super-resolution Ultrasound Imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef]
- Hingot, V.; Errico, C.; Heiles, B.; Rahal, L.; Tanter, M.; Couture, O. Microvascular flow dictates the compromise between spatial resolution and acquisition time in Ultrasound Localization Microscopy. Sci. Rep. 2019, 9, 2456. [Google Scholar] [CrossRef]
- Errico, C.; Pierre, J.; Pezet, S.; Desailly, Y.; Lenkei, Z.; Couture, O.; Tanter, M. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 2015, 527, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Browning, R.J.; Tang, M.X.; Dunsby, C.; Eckersley, R.J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 2015, 34, 433–440. [Google Scholar] [CrossRef]
- Harput, S.; Christensen-Jeffries, K.; Brown, J.; Li, Y.; Williams, K.J.; Davies, A.H.; Eckersley, R.J.; Dunsby, C.; Tang, M.X.; Christensen-Jeffries, K.; et al. Two-Stage Motion Correction for Super-Resolution Ultrasound Imaging in Human Lower Limb. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 803–814. [Google Scholar] [CrossRef]
- Opacic, T.; Dencks, S.; Theek, B.; Piepenbrock, M.; Ackermann, D.; Rix, A.; Lammers, T.; Stickeler, E.; Delorme, S.; Schmitz, G.; et al. Motion model ultrasound localization microscopy for preclinical and clinical multiparametric tumor characterization. Nat. Commun. 2018, 9, 1527. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Gong, P.; Lok, U.W.; Tang, S.; Yin, T.; Zhang, X.; Zhu, L.; Sang, M.; Song, P.; et al. Super-resolution ultrasound localization microscopy based on a high frame-rate clinical ultrasound scanner: An in-human feasibility study. Phys. Med. Biol. 2021, 66, 08NT01. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Dencks, S.; Lowerison, M.; Hansen-Shearer, J.; Shin, Y.; Schmitz, G.; Song, P.; Tang, M.X. Super-Resolution Ultrasound: From Data Acquisition and Motion Correction to Localization, Tracking, and Evaluation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2025, 72, 408–426. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Kirchin, M.A.; Monteith, J.; Storto, M.L.; Spinazzi, A. Safety of Lumason(R) (SonoVue(R)) in special populations and critically ill patients. Front. Cardiovasc. Med. 2023, 10, 1225654. [Google Scholar] [CrossRef]
- Dayton, P.A.; Morgan, K.E.; Klibanov, A.L.; Brandenburger, G.H.; Ferrara, K.W. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999, 46, 220–232. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Brown, J.; Christensen-Jeffries, K.; Harput, S.; Zhang, G.; Zhu, J.; Dunsby, C.; Tang, M.X.; Eckersley, R.J. Investigation of Microbubble Detection Methods for Super-Resolution Imaging of Microvasculature. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 676–691. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.Y.; Yu, X.Y.; Lei, S.; Ma, C.; Wang, X.; Xue, X.D.; Ding, Y.; Ma, T.; Zhu, B.P. Deep learning for fast super-resolution ultrasound microvessel imaging. Phys. Med. Biol. 2023, 68, 245023. [Google Scholar] [CrossRef]
- Tang, S.; Song, P.; Trzasko, J.D.; Lowerison, M.; Huang, C.; Gong, P.; Lok, U.W.; Manduca, A.; Chen, S. Kalman Filter-Based Microbubble Tracking for Robust Super-Resolution Ultrasound Microvessel Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1738–1751. [Google Scholar] [CrossRef]
- Guo, X.; Ta, D.; Xu, K. Frame rate effects and their compensation on super-resolution microvessel imaging using ultrasound localization microscopy. Ultrasonics 2023, 132, 107009. [Google Scholar] [CrossRef]
- Holliger, C.; Lemley, K.V.; Schmitt, S.L.; Thomas, F.C.; Robertson, C.R.; Jamison, R.L. Direct determination of vasa recta blood flow in the rat renal papilla. Circ. Res. 1983, 53, 401–413. [Google Scholar] [CrossRef]
- Crawford, C.; Kennedy-Lydon, T.; Sprott, C.; Desai, T.; Sawbridge, L.; Munday, J.; Unwin, R.J.; Wildman, S.S.; Peppiatt-Wildman, C.M. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron Physiol. 2012, 120, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.B.; Taghavi, I.; Kjer, H.M.; Sogaard, S.B.; Gundlach, C.; Dahl, V.A.; Nielsen, M.B.; Dahl, A.B.; Jensen, J.A.; Sorensen, C.M. Evaluation of 2D super-resolution ultrasound imaging of the rat renal vasculature using ex vivo micro-computed tomography. Sci. Rep. 2021, 11, 24335. [Google Scholar] [CrossRef]
- Sogaard, S.B.; Andersen, S.B.; Taghavi, I.; Schou, M.; Christoffersen, C.; Jacobsen, J.C.B.; Kjer, H.M.; Gundlach, C.; McDermott, A.; Jensen, J.A.; et al. Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease. Diagnostics 2023, 13, 3197. [Google Scholar] [CrossRef]
- Taghavi, I.; Andersen, S.B.; Sogaard, S.B.; Nielsen, M.B.; Sorensen, C.M.; Stuart, M.B.; Jensen, J.A. Automatic Classification of Arterial and Venous Flow in Super-resolution Ultrasound Images of Rat Kidneys. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021. [Google Scholar]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Taghavi, I.; Andersen, S.B.; Hoyos, C.A.V.; Nielsen, M.B.; Sorensen, C.M.; Jensen, J.A. In Vivo Motion Correction in Super-Resolution Imaging of Rat Kidneys. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.M.; Connolly, L.A.; Klauck, J.A. Inhalation anesthesiology and volatile liquid anesthetics: Focus on isoflurane, desflurane, and sevoflurane. Pharmacotherapy 2005, 25, 1773–1788. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Mari, J.M.; Wells, P.N.; Eckersley, R.J. Attenuation correction in ultrasound contrast agent imaging: Elementary theory and preliminary experimental evaluation. Ultrasound Med. Biol. 2008, 34, 1998–2008. [Google Scholar] [CrossRef][Green Version]
- Tang, M.X.; Mulvana, H.; Gauthier, T.; Lim, A.K.; Cosgrove, D.O.; Eckersley, R.J.; Stride, E. Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus. 2011, 1, 520–539. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Conzen, P.; Rehm, M. A rational approach to perioperative fluid management. Anesthesiology 2008, 109, 723–740. [Google Scholar] [CrossRef]
- Johnson, P.R.; Stern, J.S.; Horwitz, B.A.; Harris, R.E., Jr.; Greene, S.F. Longevity in obese and lean male and female rats of the Zucker strain: Prevention of hyperphagia. Am. J. Clin. Nutr. 1997, 66, 890–903. [Google Scholar] [CrossRef]
- Qian, X.; Huang, C.; Li, R.; Song, B.J.; Tchelepi, H.; Shung, K.K.; Chen, S.; Humayun, M.S.; Zhou, Q. Super-Resolution Ultrasound Localization Microscopy for Visualization of the Ocular Blood Flow. IEEE Trans. Biomed. Eng. 2022, 69, 1585–1594. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Yan, J.; Shu, H.; Li, Z.; Ye, C.; Chen, L.; Feng, C.; Zheng, Y. Non-invasive ultrasound localization microscopy (ULM) in azoospermia: Connecting testicular microcirculation to spermatogenic functions. Theranostics 2024, 14, 4967–4982. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, I.; Amin-Naji, M.; Schou, M.; Ommen, M.L.; Steenberg, K.; Larsen, N.B.; Thomsen, E.V.; Jensen, J.A. Compensation for Velocity Underestimation in 2D Super-Resolution Ultrasound. In Proceedings of the 2022 IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022. [Google Scholar]
- Bezer, J.H.; Prentice, P.; Lim Kee Chang, W.; Morse, S.V.; Christensen-Jeffries, K.; Rowlands, C.J.; Kozlov, A.S.; Choi, J.J. Microbubble dynamics in brain microvessels. PLoS ONE 2025, 20, e0310425. [Google Scholar] [CrossRef]
- Lindner, J.R.; Song, J.; Jayaweera, A.R.; Sklenar, J.; Kaul, S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J. Am. Soc. Echocardiogr. 2002, 15, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ringgaard, S.; Christiansen, T.; Bak, M.; Pedersen, E.M.; Stodkilde-Jorgensen, H.; Flyvbjerg, A. Measurement of renal vein blood flow in rats by high-field magnetic resonance. Kidney Int. 1997, 52, 1359–1363. [Google Scholar] [CrossRef]
- Bellini, M.H.; Silva, R.M.D.; Ko, G.M.; Santos, J.E.M.; Boim, M.A.; Ikegami, A. Evaluation of the applicability of doppler velocimetry for monitoring acute kidney injury in rats. Sociedade Brasileira de Ciência em Animais de Laboratório 2016, 4, 9–14. [Google Scholar]
- Seely, J.C.; Hard, G.C.; Blankenship, B. Chapter 11—Kidney. In Boorman’s Pathology of the Rat, 2nd ed.; Suttie, A.W., Ed.; Academic Press: Boston, MA, USA, 2018; pp. 125–166. [Google Scholar] [CrossRef]
- Cupples, W.A.; Braam, B. Assessment of renal autoregulation. Am. J. Physiol. Renal Physiol. 2007, 292, F1105–F1123. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Swanevelder, J. Resolution in ultrasound imaging. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 186–192. [Google Scholar] [CrossRef]
- Hong, S.H.; Herman, A.M.; Stephenson, J.M.; Wu, T.; Bahadur, A.N.; Burns, A.R.; Marrelli, S.P.; Wythe, J.D. Development of barium-based low viscosity contrast agents for micro CT vascular casting: Application to 3D visualization of the adult mouse cerebrovasculature. J. Neurosci. Res. 2020, 98, 312–324. [Google Scholar] [CrossRef]
- Fan, L.; Wang, S.; He, X.; Gonzalez-Fernandez, E.; Lechene, C.; Fan, F.; Roman, R.J. Visualization of the intrarenal distribution of capillary blood flow. Physiol. Rep. 2019, 7, e14065. [Google Scholar] [CrossRef]
- Jensen, J.A.; Tomov, B.G.; Haslund, L.E.; Panduro, N.S.; Sorensen, C.M. Universal Synthetic Aperture Sequence for Anatomic, Functional, and Super Resolution Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Tomov, B.G.D.; Daigle, R.; Jensen, J.A. Ten-minute Continuous Acquisitions with the Verasonics Vantage Research Scanner. In Proceedings of the 2023 IEEE International Ultrasonics Symposium, Montreal, QC, Canada, 3–8 September 2023. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDermott, A.; Panduro, N.S.; Taghavi, I.; Kjer, H.M.; Søgaard, S.B.; Nielsen, M.B.; Jensen, J.A.; Sørensen, C.M. Analysing the Renal Vasculature Using Super-Resolution Ultrasound Imaging: Considerations for Clinical and Research Applications. Diagnostics 2025, 15, 1515. https://doi.org/10.3390/diagnostics15121515
McDermott A, Panduro NS, Taghavi I, Kjer HM, Søgaard SB, Nielsen MB, Jensen JA, Sørensen CM. Analysing the Renal Vasculature Using Super-Resolution Ultrasound Imaging: Considerations for Clinical and Research Applications. Diagnostics. 2025; 15(12):1515. https://doi.org/10.3390/diagnostics15121515
Chicago/Turabian StyleMcDermott, Amy, Nathalie Sarup Panduro, Iman Taghavi, Hans Martin Kjer, Stinne Byrholdt Søgaard, Michael Bachmann Nielsen, Jørgen Arendt Jensen, and Charlotte Mehlin Sørensen. 2025. "Analysing the Renal Vasculature Using Super-Resolution Ultrasound Imaging: Considerations for Clinical and Research Applications" Diagnostics 15, no. 12: 1515. https://doi.org/10.3390/diagnostics15121515
APA StyleMcDermott, A., Panduro, N. S., Taghavi, I., Kjer, H. M., Søgaard, S. B., Nielsen, M. B., Jensen, J. A., & Sørensen, C. M. (2025). Analysing the Renal Vasculature Using Super-Resolution Ultrasound Imaging: Considerations for Clinical and Research Applications. Diagnostics, 15(12), 1515. https://doi.org/10.3390/diagnostics15121515






