A Novel Virtual Reality-Based System for Measuring Deviation Angle in Strabismus: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Populations
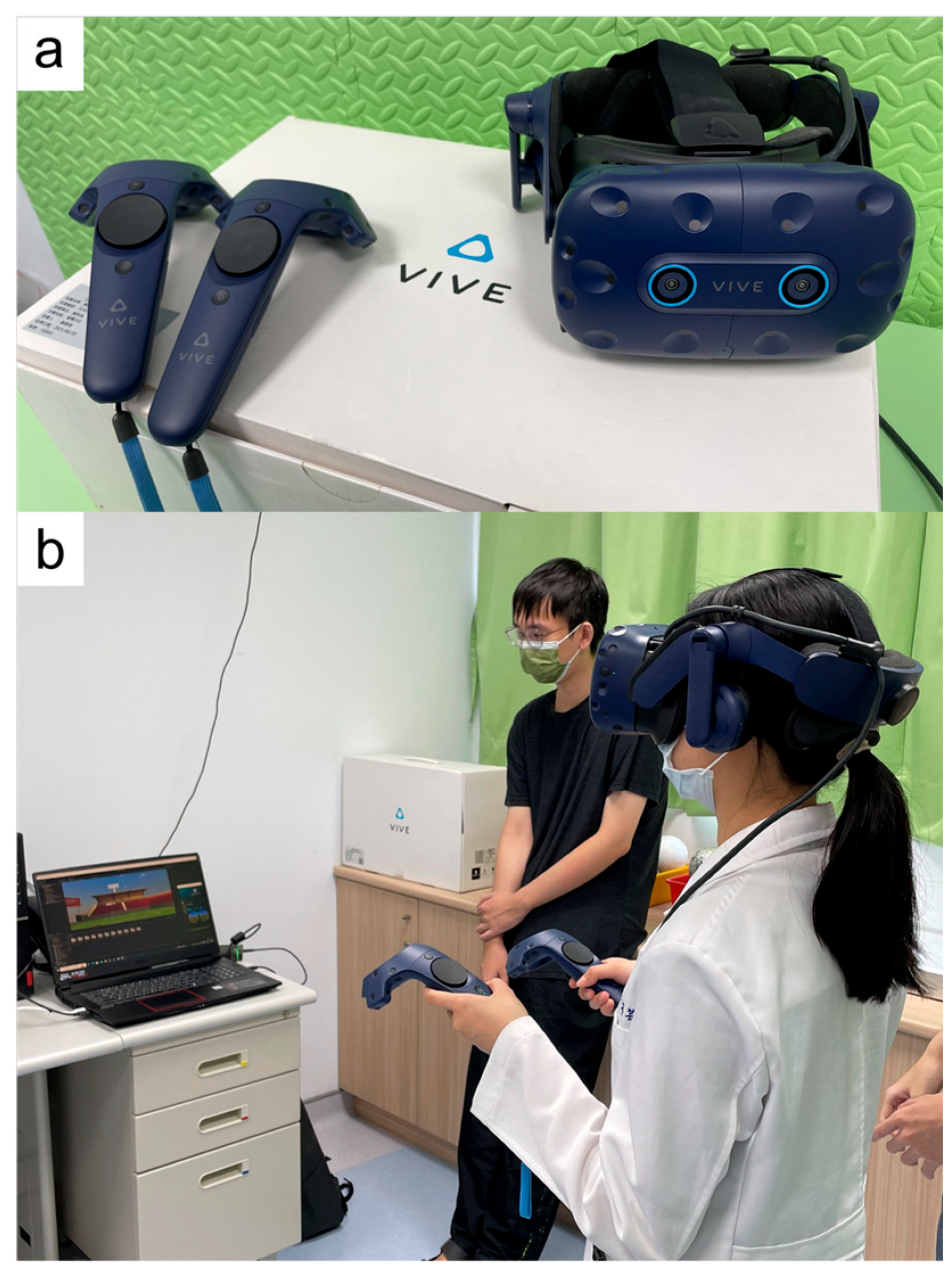
2.3. VR Equipment
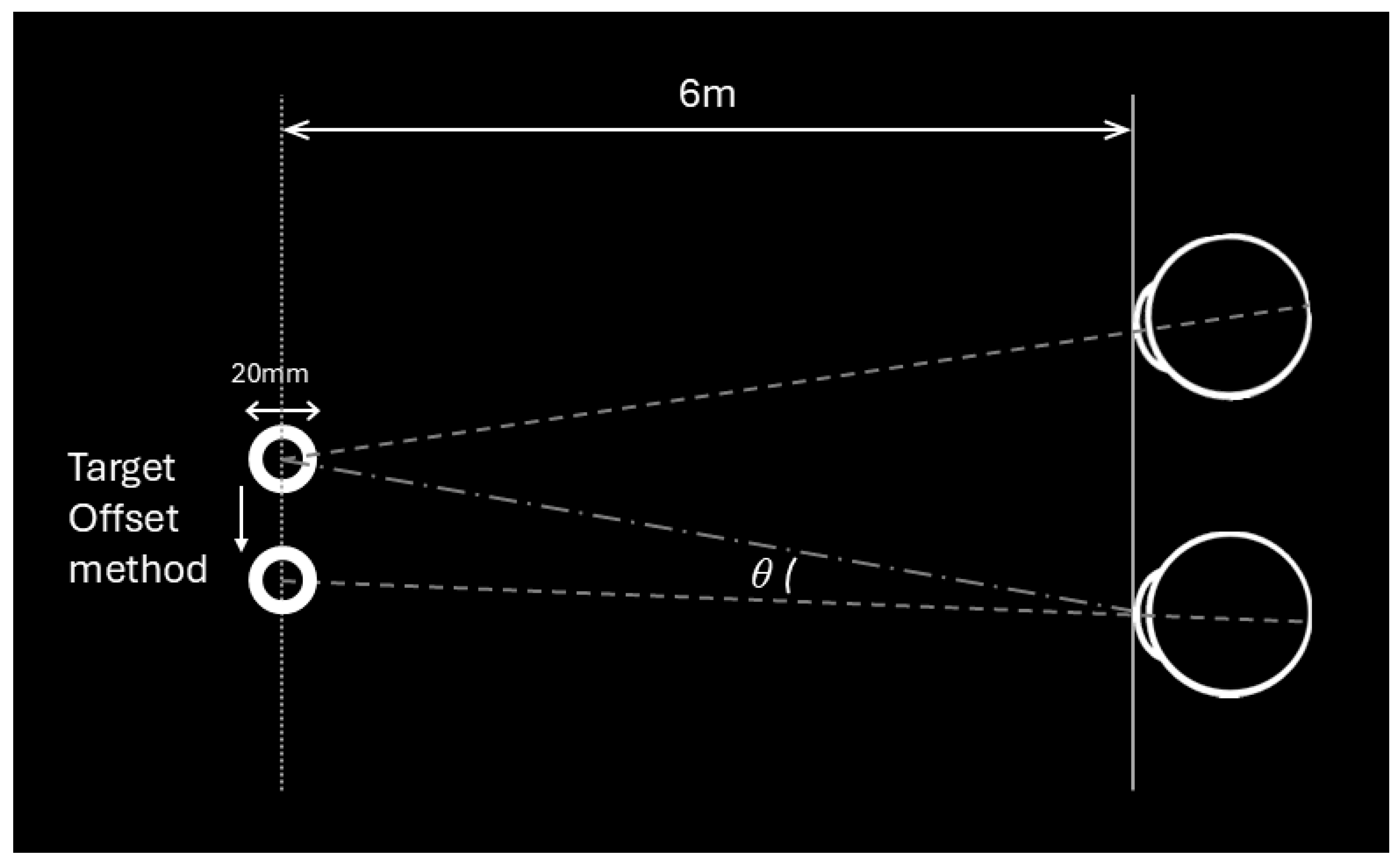
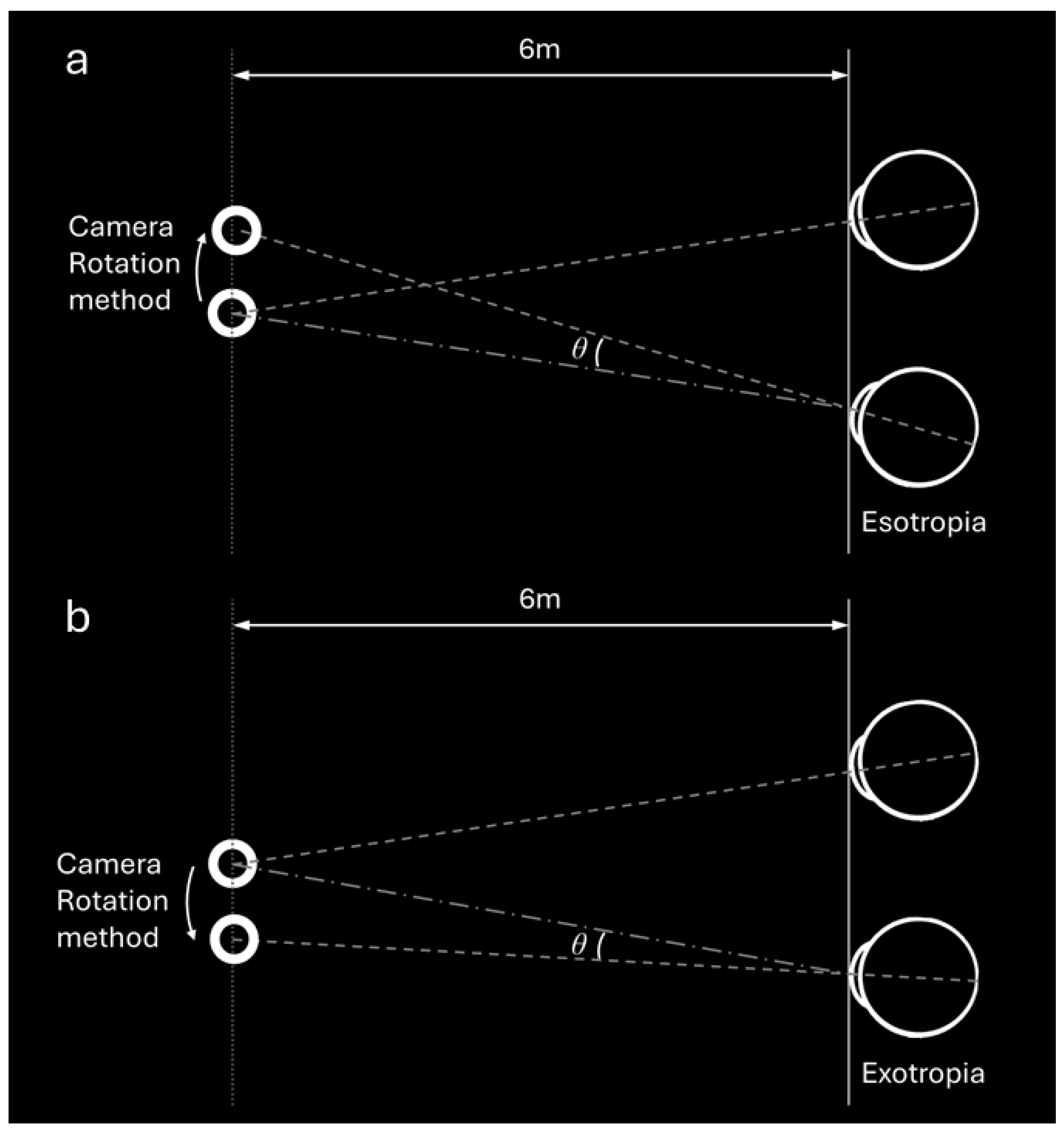
2.4. Deviation Angle Measurements by VR Using Two Methods
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristic of the Test Subjects
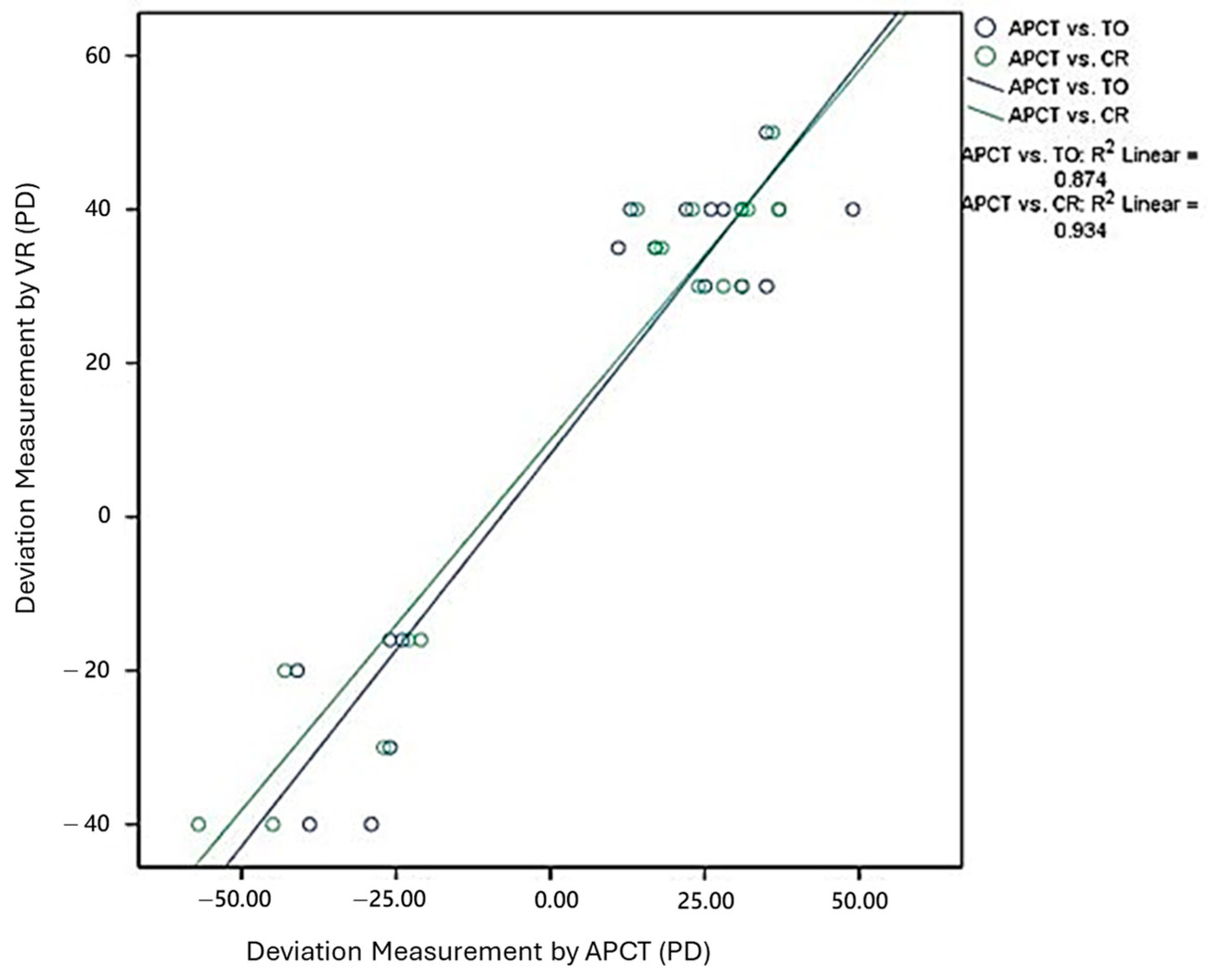
3.2. Deviation Angle Measurements by APCT and VR Using Two Methods
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VR | Virtual Reality |
| XT | exotropia |
| ET | esotropia |
| APCT | alternate prism cover test |
| TO | target offset |
| CR | camera rotation |
| VA | visual acuity |
| PD | prism diopters |
References
- Dagi, L.R.; Velez, F.G.; Holmes, J.M.; Archer, S.M.; Strominger, M.B.; Pineles, S.L.; Paysse, E.A.; Pihlblad, M.S.; Atalay, H.T.; Campolattaro, B.N.; et al. American Academy of Ophthalmology Preferred Practice Pattern Adult Strabismus Panel. Adult Strabismus Preferred Practice Pattern®. Ophthalmology 2024, 131, P306–P403. [Google Scholar] [CrossRef]
- Huang, Y.T.; Lin, S.C.; Huang, L.Y.; Rujikajorn, K.; Chen, P.J.; Chen, J.J.; Wu, M.Y.; Lin, H.J.; Wan, L. Incidence, Risk Factors and Management of Postoperative Complications in Horizontal Strabismus Surgery. Semin. Ophthalmol. 2024, 39, 143–149. [Google Scholar] [CrossRef]
- O’Brien, C.; Marsh, R. Pediatric ophthalmology: Analysis of fellowships and the job market. Invest. Ophthalmol. Vis. Sci. 2015, 56, 145. [Google Scholar]
- Bernstein, B.K.; Nelson, L.B. Workforce Issues in Pediatric Ophthalmology. J. Pediatr. Ophthalmol. Strabismus. 2020, 57, 9–11. [Google Scholar] [CrossRef]
- Walsh, H.L.; Parrish, A.; Hucko, L.; Sridhar, J.; Cavuoto, K.M. Access to Pediatric Ophthalmological Care by Geographic Distribution and US Population Demographic Characteristics in 2022. JAMA Ophthalmol. 2023, 141, 242–249. [Google Scholar] [CrossRef]
- Aydındoğan, G.; Kavaklı, K.; Şahin, A.; Artal, P.; Ürey, H. Applications of augmented reality in ophthalmology [Invited]. Biomed. Opt. Express. 2020, 12, 511–538. [Google Scholar] [CrossRef]
- Zou, L.; Tian, T.; Wygnanski-Jaffe, T.; Yehezkel, O.; Wang, S.; Moshkovitz, A.; Sun, X.; Liu, H.; Liu, R. Effectiveness and repeatability of eye-tracking-based test in strabismus measurement of children. Semin. Ophthalmol. 2022, 37, 502–508. [Google Scholar] [CrossRef]
- Yehezkel, O.; Belkin, M.; Wygnanski-Jaffe, T. Automated Diagnosis and Measurement of Strabismus in Children. Am. J. Ophthalmol. 2020, 213, 226–234. [Google Scholar] [CrossRef]
- Li, J.; Nguyen, A.M.; Kolin, T.; Chang, M.Y.; Reid, M.W.; Lee, T.C.; Nallasamy, S. Evaluation of Streamed Hardware-to-Software Telemedicine Strabismus Consultations Utilizing Video Glasses. Clin. Ophthalmol. 2022, 16, 3927–3933. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Feng, K.M.; Chuang, P.C.; Chen, Y.H.; Chien, K.H. Preliminary data on a novel smart glasses system for measuring the angle of deviation in strabismus. Eye 2023, 37, 2700–2706. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.; Rai, K.; Shetty, S.; Mathangi, V. Comparison of measurements between manual and automated eyetracking systems in patients with strabismus—A preliminary study. Indian J. Ophthalmol. 2022, 70, 3625–3628. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.M.; Kuchhangi, A.; Tame, J.; Cooper, K.; Hajkazemshirazi, L.; Indaram, M.; Keenan, J.D.; Oatts, J.T. Evaluation of a Novel Virtual Reality Simulated Alternate Cover Test to Assess Strabismus: A Prospective, Masked Study. Am. J. Ophthalmol. 2025, 269, 266–272. [Google Scholar] [CrossRef]
- Jonnakuti, V.S.; Frankfort, B.J. Seeing beyond reality: Considering the impact of mainstream virtual reality adoption on ocular health and the evolving role of ophthalmologists. Eye 2024, 38, 1401–1402. [Google Scholar] [CrossRef]
- Miao, Y.; Jeon, J.Y.; Park, G.; Park, S.W.; Heo, H. Virtual reality-based measurement of ocular deviation in strabismus. Comput. Methods Programs Biomed. 2020, 185, 105132. [Google Scholar] [CrossRef]
- Yeh, P.H.; Liu, C.H.; Sun, M.H.; Chi, S.C.; Hwang, Y.S. To measure the amount of ocular deviation in strabismus patients with an eye-tracking virtual reality headset. BMC Ophthalmol. 2021, 21, 246. [Google Scholar] [CrossRef]
- VIVE Pro Eye Specs & User Guide. 2024. Available online: https://developer.vive.com/us/ (accessed on 12 January 2025).
- Nesaratnam, N.; Thomas, P.; Vivian, A. Stepping into the virtual unknown: Feasibility study of a virtual reality-based test of ocular misalignment. Eye 2017, 31, 1503–1506. [Google Scholar] [CrossRef]
- Nixon, N.; Thomas, P.B.M.; Jones, P.R. Feasibility study of an automated Strabismus screening Test using Augmented Reality and Eye-tracking (STARE). Eye 2023, 37, 3609–3614. [Google Scholar] [CrossRef]
- Cantó-Cerdán, M.; Martínez-Abad, A.; Siverio-Colomina, A.; Díez, R.; Amesty, M.A. Comparative Analysis of Strabismus Measurement Using a Video Oculagraphy System and Alternate Prism Cover Test. Asia-Pac. J. Ophthalmol. 2023, 12, 582–590. [Google Scholar] [CrossRef]
- Park, N.; Park, B.; Oh, M.; Moon, S.; Kim, M. A quantitative analysis method for comitant exotropia using video-oculography with alternate cover. BMC Ophthalmol. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Narváez Palazón, C.; Sánchez Ventosa, Á.; Nieves Moreno, M.; Redondo Ibáñez, A.; Gómez de Liaño Sánchez, R. Study of reliability and validity of VOG Perea® and GazeLab® and calculation of the variability of their measurements. Arch. Soc. Esp. Oftalmol. 2021, 96, 127–132. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Neveu, P.; Priot, A.E.; Plantier, J.; Roumes, C. Short exposure to telestereoscope affects the oculomotor system. Ophthalmic Physiol. Opt. 2010, 30, 806–815. [Google Scholar] [CrossRef]
- Xiao, S.; Angjeli, E.; Wu, H.C.; Gaier, E.D.; Gomez, S.; Travers, D.A.; Binenbaum, G.; Langer, R.; Hunter, D.G.; Repka, M.X. Luminopia Pivotal Trial Group. Randomized Controlled Trial of a Dichoptic Digital Therapeutic for Amblyopia. Ophthalmology 2022, 129, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.R.; Lawrence, J.; Williams, D., Jr.; Mainous, A., III. Population-Level Interest and Telehealth Capacity of US Hospitals in Response to COVID-19: Cross-Sectional Analysis of Google Search and National Hospital Survey Data. JMIR Public Health Surveill. 2020, 6, e18961. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Titiyal, J.S. Three-dimensional heads up display in anterior segment surgeries- Expanding frontiers in the COVID-19 era. Indian J. Ophthalmol. 2020, 68, 2338–2340. [Google Scholar] [CrossRef] [PubMed]
- Iskander, M.; Ogunsola, T.; Ramachandran, R.; McGowan, R.; Al-Aswad, L.A. Virtual Reality and Augmented Reality in Ophthalmology: A Contemporary Prospective. Asia-Pac. J. Ophthalmol. 2021, 10, 244–252. [Google Scholar] [CrossRef]
| Age in Years, Mean (Range), Median (IQR) | 22.3 (9–46), 21.5 (17–25) |
|---|---|
| Female (%) | 77.8% |
| Measurements obtained in glasses n (%) | 12, (61.1%) |
| Manifest strabismus, n (%) | 5, (27.8%) |
| Esotropia, n (%) | 6, (33.3%) |
| Exotropia, n (%) | 12, (66.7%) |
| Methods | Number | Deviation Angle (PD) | p Value |
| APCT | 18 | 16.00 ± 32.23 | |
| TO | 18 | 7.67 ± 29.54 | 0.604 |
| CR | 18 | 6.22 ± 32.36 | |
| Subgroup | Methods | Paired Difference in Deviation Angle (PD) | p Value |
| Esotropia n = 6 | TO-CR | 5.12 ± 11.69 | 0.328 |
| APCT-TO | 3.83 ± 11.44 | 0.499 | |
| APCT-CR | 9.00 ± 9.38 | 0.066 | |
| Exotropia n = 12 | TO-CR | −0.43 ± 5.88 | 0.811 |
| APCT-TO | 10.58 ± 11.23 | 0.008 1 | |
| APCT-CR | 10.17 ± 8.19 | 0.001 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.-Y.; Liu, Y.-C.; Lu, J.-B.; Tsai, M.-H.; Liao, W.-L.; Wang, I.-M.; Lin, H.-J.; Huang, Y.-T. A Novel Virtual Reality-Based System for Measuring Deviation Angle in Strabismus: A Prospective Study. Diagnostics 2025, 15, 2402. https://doi.org/10.3390/diagnostics15182402
Lu J-Y, Liu Y-C, Lu J-B, Tsai M-H, Liao W-L, Wang I-M, Lin H-J, Huang Y-T. A Novel Virtual Reality-Based System for Measuring Deviation Angle in Strabismus: A Prospective Study. Diagnostics. 2025; 15(18):2402. https://doi.org/10.3390/diagnostics15182402
Chicago/Turabian StyleLu, Jhih-Yi, Yin-Cheng Liu, Jui-Bang Lu, Ming-Han Tsai, Wen-Ling Liao, I-Ming Wang, Hui-Ju Lin, and Yu-Te Huang. 2025. "A Novel Virtual Reality-Based System for Measuring Deviation Angle in Strabismus: A Prospective Study" Diagnostics 15, no. 18: 2402. https://doi.org/10.3390/diagnostics15182402
APA StyleLu, J.-Y., Liu, Y.-C., Lu, J.-B., Tsai, M.-H., Liao, W.-L., Wang, I.-M., Lin, H.-J., & Huang, Y.-T. (2025). A Novel Virtual Reality-Based System for Measuring Deviation Angle in Strabismus: A Prospective Study. Diagnostics, 15(18), 2402. https://doi.org/10.3390/diagnostics15182402







