Novel Handheld Device for Remote Monitoring of Dry Macular Degeneration and Patient Usability Assessment
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
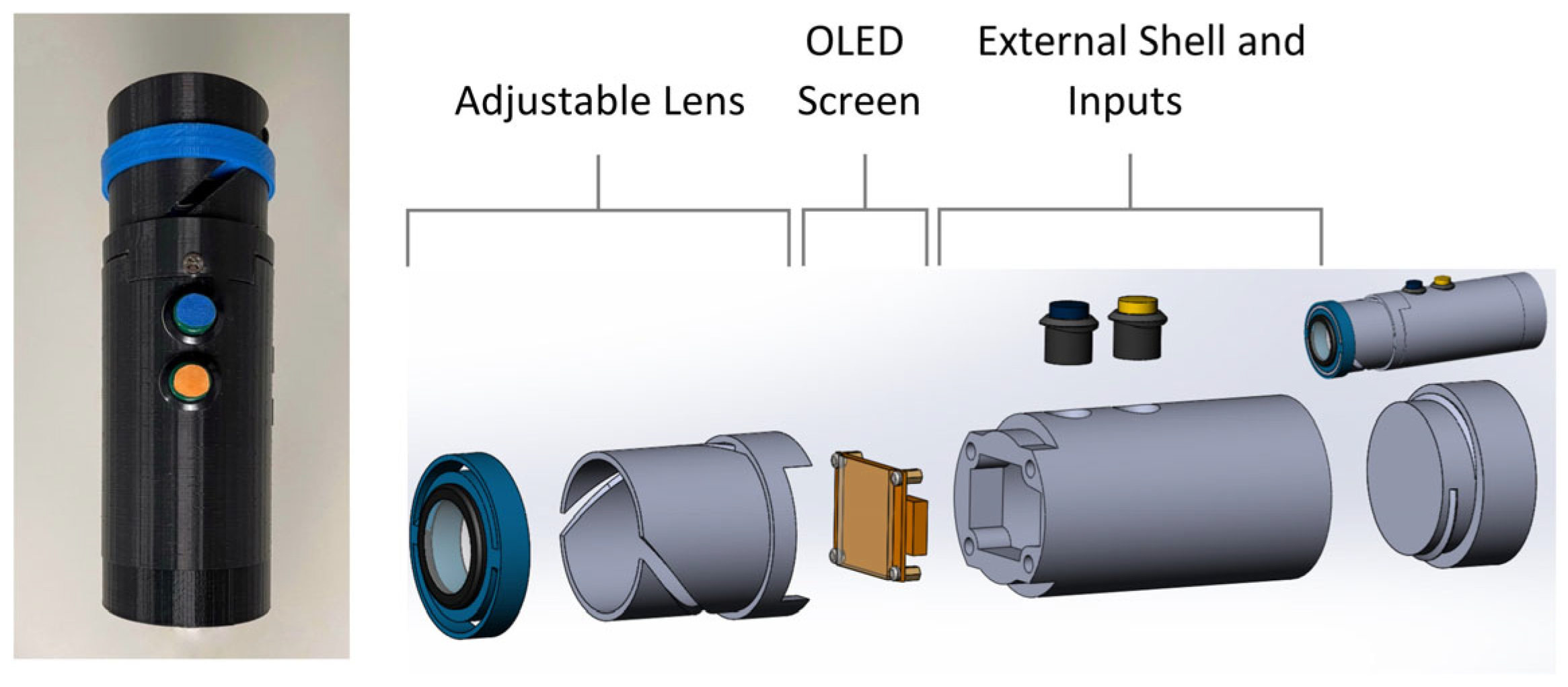
3.1. Device
3.2. Patient Characteristics
3.3. Testing Duration
3.4. Device and SUS Surveys Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| UM | University of Michigan |
| IRB | Institutional Review Board |
| SUS | System Usability Scale |
| STD | Standard deviation |
| OCT | optical coherence tomography |
| FDA | United States Food and Drug Administration |
| MDDT | minimum distortion-detection threshold |
| RGB | Red green blue |
| OLED | Organic light-emitting diode |
| SD | Standard deviation |
References
- Bressler, N.M. Age-Related Macular Degeneration Is the Leading Cause of Blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N. Causes and Prevalence of Visual Impairment among Adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef]
- Ambati, J.; Ambati, B.K.; Yoo, S.H.; Ianchulev, S.; Adamis, A.P. Age-Related Macular Degeneration: Etiology, Pathogenesis, and Therapeutic Strategies. Surv. Ophthalmol. 2003, 48, 257–293. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Jensen, S.C.; Meuer, S.M. The Five-year Incidence and Progression of Age-related Maculopathy: The Beaver Dam Eye Study. Ophthalmology 1997, 104, 7–21. [Google Scholar] [CrossRef]
- Ferris, F.L., III; Fine, S.L.; Hyman, L. Age-Related Macular Degeneration and Blindness due to Neovascular Maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P1–P65. [Google Scholar] [CrossRef]
- Loewenstein, A.; Malach, R.; Goldstein, M.; Leibovitch, I.; Barak, A.; Baruch, E.; Alster, Y.; Rafaeli, O.; Avni, I.; Yassur, Y. Replacing the Amsler grid: A new method for monitoring patients with age-related macular degeneration. Ophthalmology 2003, 110, 966–970. [Google Scholar] [CrossRef]
- Schuchard, R.A. Validity and Interpretation of Amsler Grid Reports. Arch. Ophthalmol. 1993, 111, 776–780. [Google Scholar] [CrossRef]
- Kreatsoulas, J. Monitoring AMD With the ForeseeHome. Retina Today 2011, 17–19. [Google Scholar]
- Chew, E.Y.; Clemons, T.E.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Heier, J.S.; Kim, J.E.; Garfinke, R. Randomized Trial of a Home Monitoring System for Early Detection of Choroidal Neovascularization Home Monitoring of the Eye (HOME) Study. Ophthalmology 2014, 121, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Mathai, M.; Reddy, S.; Elman, M.J.; Elman, M.J.; Garfinkel, R.A.; Ladd, B.; Wagner, A.L.; Sanborn, G.E.; Jacobs, J.H.; Busquets, M.A.; et al. Analysis of the Long-term Visual Outcomes of ForeseeHome Remote Telemonitoring: The ALOFT Study. Ophthalmol. Retina 2022, 6, 922–929. [Google Scholar] [CrossRef]
- Blinder, K.J.; Calhoun, C.; Maguire, M.G.; Glassman, A.R.; Mein, C.E.; Baskin, D.E.; Vieyra, G.; Jampol, L.M.; Chica, M.A.; Sun, J.K.; et al. Home OCT Imaging for Newly Diagnosed Neovascular Age-Related Macular Degeneration: A Feasibility Study. Ophthalmol. Retina 2024, 8, 376–387. [Google Scholar] [CrossRef]
- von der Burchard, C.; Moltmann, M.; Tode, J.; Ehlken, C.; Sudkamp, H.; Theisen-Kunde, D.; König, I.; Hüttmann, G.; Roider, J. Self-examination low-cost full-field OCT (SELFF-OCT) for patients with various macular diseases. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1503–1511. [Google Scholar] [CrossRef]
- Balaskas, K.; Drawnel, F.; Khanani, A.; Knox, P.; Mavromaras, G.; Wang, Y.Z. Home vision monitoring in patients with maculopathy: Current and future options for digital technologies. Eye 2023, 37, 3108–3120. [Google Scholar] [CrossRef]
- Labowsky, M.; Stinnett, S.; Luhmann, U.F.O.; Vajzovic, L.; Horne, A.; Toth, C.A.; Cousins, S.W.; Lad, E.M. Use of mobile MyVisionTrack (mVT) technology as a remote visual function metric in Early and Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2430. [Google Scholar]
- Korot, E.; Pontikos, N.; Drawnel, F.M.; Jaber, A.; Fu, D.J.; Zhang, G.; Miranda, M.A.; Liefers, B.; Glinton, S.; Wagner, S.K.; et al. Enablers and Barriers to Deployment of Smartphone-Based Home Vision Monitoring in Clinical Practice Settings. JAMA Ophthalmol. 2022, 140, 153–160. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An Empirical Evaluation of the System Usability Scale. Int. J. Hum. Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Sauro, J. A Practical Guide to Measuring Usability: 72 Answers to the Most Common Questions About Quantifying the Usability of Websites and Software; Measuring Usability LLC: Denver, CO, USA, 2010. [Google Scholar]
- Brooke, J. SUS: A Retrospective. J. Usability Stud. 2013, 8, 29–40. [Google Scholar]
- Denno, S.; Isle, B.A.; Ju, G.; Koch, C.G.; Metz, S.V.; Penner, R.; Wang, L.; Ward, J. Human Factors Design Guidelines for the Elderly and People with Disabilities Revision 3 Draft; Honeywell Inc.: Minneapolis, MN, USA, 1992. [Google Scholar]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar] [PubMed]
- Cohen, S.Y.; Lamarque, F.; Saucet, J.C.; Provent, P.; Langram, C.; LeGargasson, J.F. Filling-in phenomenon in patients with age-related macular degeneration: Differences regarding uni- or bilaterality of central scotoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Zur, D.; Ullman, S. Filling-in of retinal scotomas. Vision. Res. 2003, 43, 971–982. [Google Scholar] [CrossRef]
- Crossland, M.D.; Silva, R.S.; Macedo, A.F. Smartphone, tablet computer and e-reader use by people with vision impairment. Ophthalmic Physiol. Opt. 2014, 34, 552–557. [Google Scholar] [CrossRef]
- Faes, L.; Golla, K.; Islam, M.; Lienhard, K.R.; Schmid, M.K.; Sim, D.A.; Bachmann, L.M. System usability, user satisfaction and long-term adherence to mobile hyperacuity home monitoring—Prospective follow-up study. Eye 2023, 37, 650–654. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wilson, E.; Locke, K.G.; Edwards, A.O. Shape discrimination in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2055–2062. [Google Scholar] [PubMed]
- Pitrelli Vazquez, N.; Harding, S.P.; Heimann, H.; Czanner, G.; Knox, P.C. Radial shape discrimination testing for new-onset neovascular age-related macular degeneration in at-risk eyes. PLoS ONE 2018, 13, e0207342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lott, L.A.; Schneck, M.E.; Haegerstrom-Portnoy, G.; Hewlett, S.; Stepien-Bernabe, N.; Gauer, B.M.; Zaidi, A.; Fu, A.D.; Brabyn, J.A. Simple Vision Function Tests that Distinguish Eyes with Early to Intermediate Age-related Macular Degeneration. Ophthalmic Epidemiol. 2021, 28, 93–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joseph, A.; Bullimore, M.; Drawnel, F.; Miranda, M.; Morgan, Z.; Wang, Y.Z. Remote Monitoring of Visual Function in Patients with Maculopathy: The Aphelion Study. Ophthalmol. Ther. 2024, 13, 409–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, H.J.; Kiernan, D.F.; Eichenbaum, D.; Sheth, V.S.; Wykoff, C.C. Home Monitoring of Age-Related Macular Degeneration: Utility of the ForeseeHome Device for Detection of Neovascularization. Ophthalmol. Retina 2021, 5, 348–356. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Age (years), mean (SD) | 77.4 (7.9) |
| Age Group | |
| 50–64 years of age (%) | 3 (10.0) |
| 65–74 years of age (%) | 8 (26.7) |
| 75–84 years of age (%) | 13 (43.3) |
| 85+ years of age (%) | 6 (20.0) |
| Female gender (%) | 15 (50) |
| Race * | |
| White | 30 (100%) |
| Ethnicity | |
| Non-Hispanic | 27 (90%) |
| Unknown | 3 (10%) |
| Eyes | 35 |
| Right eye (%) | 20 (57.1) |
| Left eye (%) | 15 (42.9) |
| Both eyes (%) | 5 (14.3) |
| Statement Number | Statement | Mean Likert Score *, (SD) |
|---|---|---|
| DS 1 | The device was comfortable to hold for the duration of the test. | 4.4 (0.9) |
| DS 2 | The device weight was acceptable. | 4.8 (0.4) |
| DS 3 | The buttons were easy to push. | 4.9 (0.3) |
| DS 4 | I found it easy to learn how to use the device. | 4.7 (0.7) |
| DS 5 | The time it took to complete the test was acceptable. | 4.8 (0.8) |
| DS 6 | The time it took for the screen to transition was acceptable. | 4.2 (1.3) |
| DS 7 | The time it took for the circle to appear on the screen was acceptable. | 4.4 (0.9) |
| DS 8 | The time it took for the device to turn on and be ready for use was acceptable. | 4.9 (0.3) |
| SUS 1 | I think that I would like to use the device frequently. | 3.6 (1.2) |
| SUS 2 | I found the device unnecessarily complex. | 1.3 (0.6) |
| SUS 3 | I thought the device was easy to use. | 4.8 (0.6) |
| SUS 4 | I think that I would need the support of a technical person to be able to use the device. | 1.2 (0.4) |
| SUS 5 | I found the various functions in the device were well integrated. | 4.5 (0.8) |
| SUS 6 | I thought there was too much inconsistency in the device. | 1.5 (0.9) |
| SUS 7 | I would imagine that most people would learn to use the device very quickly | 4.9 (0.3) |
| SUS 8 | I found the device very cumbersome (awkward) to use. | 1.5 (1.1) |
| SUS 9 | I felt very confident using the device. | 4.0 (1.2) |
| SUS 10 | I needed to learn a lot of things before I could get going with the device. | 1.4 (0.8) |
| Statement | Participant | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 |
| DS 1 | 1 | 5 | 4 | 3 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 3 | 5 | 5 | 4 | 4 | 4 | 5 | 5 | 5 |
| DS 2 | 4 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| DS 3 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| DS 4 | 2 | 5 | 3 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 5 | 5 |
| DS 5 | 5 | 5 | 4 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 5 | 5 |
| DS 6 | 5 | 2 | 2 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 2 | 5 | 4 | 5 | 3 | 5 | 5 | 5 | 2 | 4 | 1 | 5 | 5 | 4 | 5 | 5 | 5 |
| DS 7 | 4 | 4 | 4 | 5 | 4 | 5 | 5 | 3 | 5 | 5 | 3 | 5 | 2 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 2 | 4 | 5 | 5 | 4 | 4 | 5 | 5 | 5 |
| DS 8 | 4 | 5 | 4 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| SUS 1 | 2 | 2 | 4 | 5 | 2 | 2 | 2 | 4 | 5 | 4 | 4 | 4 | 4 | 2 | 5 | 5 | 5 | 4 | 3 | 2 | 5 | 5 | 3 | 5 | 2 | 4 | 3 | 5 | 5 | 2 |
| SUS 2 | 1 | 3 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| SUS 3 | 4 | 2 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| SUS 4 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| SUS 5 | 4 | 4 | 5 | 5 | 4 | 2 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 3 | 5 | 5 | 5 | 5 | 5 | 3 | 5 | 4 | 5 | 5 | 5 | 3 | 4 | 5 | 5 | 5 |
| SUS 6 | 2 | 4 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| SUS 7 | 4 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 |
| SUS 8 | 5 | 1 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 4 | 1 | 1 | 1 |
| SUS 9 | 1 | 2 | 5 | 5 | 2 | 5 | 5 | 5 | 4 | 3 | 4 | 5 | 3 | 2 | 5 | 5 | 5 | 4 | 5 | 3 | 5 | 3 | 5 | 2 | 4 | 4 | 4 | 5 | 5 | 5 |
| SUS 10 | 4 | 1 | 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, A.C.; Azzouz, L.; Paulus, Y.M. Novel Handheld Device for Remote Monitoring of Dry Macular Degeneration and Patient Usability Assessment. Diagnostics 2025, 15, 1353. https://doi.org/10.3390/diagnostics15111353
Yim AC, Azzouz L, Paulus YM. Novel Handheld Device for Remote Monitoring of Dry Macular Degeneration and Patient Usability Assessment. Diagnostics. 2025; 15(11):1353. https://doi.org/10.3390/diagnostics15111353
Chicago/Turabian StyleYim, Angela C., Lyna Azzouz, and Yannis M. Paulus. 2025. "Novel Handheld Device for Remote Monitoring of Dry Macular Degeneration and Patient Usability Assessment" Diagnostics 15, no. 11: 1353. https://doi.org/10.3390/diagnostics15111353
APA StyleYim, A. C., Azzouz, L., & Paulus, Y. M. (2025). Novel Handheld Device for Remote Monitoring of Dry Macular Degeneration and Patient Usability Assessment. Diagnostics, 15(11), 1353. https://doi.org/10.3390/diagnostics15111353






