Serum Galectin-3 and Presepsin Levels in Pediatric Familial Mediterranean Fever Patients During Remission: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Sample
2.3. Data Collection
2.4. Study’s Outcomes
2.5. Sample Size Calculations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CBC | Complete blood count |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| ESR | Erythrocyte sedimentation rate |
| FMF | Familial Mediterranean fever |
| LDH | Lactate dehydrogenase |
| MEFV | Mediterranean fever gene |
| PFAPA | Periodic fever, aphthous stomatitis, pharyngitis, and adenitis |
| PRINTO | Pediatric Rheumatology International Trials Organization |
| SAA | Serum amyloid A |
References
- Batu, E.D.; Vezir, E.; Öğüş, E.; Özbaş Demirel, Ö.; Akpınar, G.; Demir, S.; Özen, S. Galectin-3: A New Biomarker for Differentiating Periodic Fever, Adenitis, Pharyngitis, Aphthous Stomatitis (PFAPA) Syndrome from Familial Mediterranean Fever? Rheumatol. Int. 2022, 42, 71–80. [Google Scholar] [CrossRef]
- Yilmaz, H.; Inan, O.; Darcin, T.; Bilgic, M.A.; Akcay, A. Serum Galectin-3 Levels Were Associated with Proteinuria in Patients with Familial Mediterranean Fever. Clin. Exp. Nephrol. 2015, 19, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Yel, S.; Dursun, İ.; Çetin, F.; Baştuğ, F.; Tülpar, S.; Düşünsel, R.; Gündüz, Z.; Poyrazoğlu, H.; Yılmaz, K. Increased Circulating Endothelial Microparticles in Children with FMF. Biomarkers 2018, 23, 558–562. [Google Scholar] [CrossRef]
- Saccon, F.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Role of Galectin-3 in Autoimmune and Non-Autoimmune Nephropathies. Autoimmun. Rev. 2017, 16, 34–47. [Google Scholar] [CrossRef]
- Chen, P.K.; Lan, J.L.; Li, J.P.; Chang, C.K.; Chang, S.H.; Huang, P.H.; Yeo, K.J.; Chen, D.Y. Elevated Plasma Galectin-3 Levels and Their Correlation with Disease Activity in Adult-Onset Still’s Disease. Clin. Rheumatol. 2020, 39, 1945–1952. [Google Scholar] [CrossRef]
- Mahalleh, M.; Behnoush, A.H.; Khalaji, A.; Gouravani, M.; Assempoor, R.; Jamshidi, A.; Mahmoudi, M.; Farhadi, E. Serum Galectin-3 Level in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Iran. J. Allergy Asthma Immunol. 2024, 23, 476–488. [Google Scholar] [CrossRef]
- Tural, D.A.; Emiralioglu, N.; Akin, S.; Alboga, D.; Ozsezen, B.; Buyuksahin, H.N.; Guzelkas, I.; Kasikci, M.; Sunman, B.; Gungor, I.; et al. Galectin-3 levels in children with cystic fibrosis. Eur. J. Pediatr. 2024, 183, 2333–2342, Correction in Eur. J. Pediatr. 2024, 183, 3633–3634. [Google Scholar] [CrossRef]
- Hashimoto, K.; Morishima, T.; Watanabe, K.; Ikemoto, T.; Nakamura, Y.; Takahashi, N. Serum Presepsin Might Not Detect Periprosthetic Joint Infection After Hip Arthroplasty. J. Clin. Med. 2025, 14, 4246. [Google Scholar] [CrossRef] [PubMed]
- Mišura Jakobac, K.; Milunović, V.; Kušec, V.; HraAbač, P.; Martinović, M.; Radić-Krišto, D.; Ostojić Kolonić, S.; Pavliša, G. Biomarkers Affecting Treatment Outcomes of Febrile Neutropenia in Hematological Patients with Lymphomas: Is Presepsin the New Promising Diagnostic and Prognostic Biomarker? J. Clin. Med. 2025, 14, 2238. [Google Scholar] [CrossRef] [PubMed]
- Juneja, D.; Jain, N.; Singh, O.; Goel, A.; Arora, S. Comparison between Presepsin, Procalcitonin, and CRP as Biomarkers to Diagnose Sepsis in Critically Ill Patients. J. Anaesthesiol. Clin. Pharmacol. 2023, 39, 458–462. [Google Scholar] [CrossRef]
- Chenevier-Gobeaux, C.; Borderie, D.; Weiss, N.; Mallet-Coste, T.; Claessens, Y.E. Presepsin (SCD14-ST), an Innate Immune Response Marker in Sepsis. Clin. Chim. Acta 2015, 450, 97–103. [Google Scholar] [CrossRef]
- Vodnik, T.; Kaljevic, G.; Tadic, T.; Majkic-Singh, N. Presepsin (SCD14-ST) in Preoperative Diagnosis of Abdominal Sepsis. Clin. Chem. Lab. Med. 2013, 51, 2053–2062. [Google Scholar] [CrossRef]
- Nakamura, M.; Takeuchi, T.; Naito, K.; Shirakawa, K.; Hosaka, Y.; Yamasaki, F.; Furusako, S. Early Elevation of Plasma Soluble CD14 Subtype, a Novel Biomarker for Sepsis, in a Rabbit Cecal Ligation and Puncture Model. Crit. Care 2008, 12, P194. [Google Scholar] [CrossRef]
- Gedik, M.S.; Kilci, A.I.; Hakkoymaz, H.; Seyithanoğlu, M.; Orakçı, M.A.; Basan, N.M.; Aksu, A.; Küçük, Ö.F. The Role of Ischemia-Modified Albumin, Presepsin, Delta Neutrophil Index, and Inflammatory Markers in Diagnosing Acute Cholecystitis. Ulus. Travma Acil Cerrahi Derg. 2024, 30, 242–247. [Google Scholar] [CrossRef]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E.; et al. Classification Criteria for Autoinflammatory Recurrent Fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef]
- Elhani, I.; Backes, S.; Kallinich, T.; Amaryan, G.; Belot, A.; Berendes, R.; Berger, T.; Dressler, F.; Foell, D.; Fühner, S.; et al. Inflammatory Biomarker Analysis Confirms Reduced Disease Severity in Heterozygous Patients with Familial Mediterranean Fever. RMD Open 2024, 10, e004677. [Google Scholar] [CrossRef]
- Livneh, A.; Langevitz, P.; Zemer, D.; Zaks, N.; Kees, S.; Lidar, T.; Migdal, A.; Padeh, S.; Pras, M. Criteria for the Diagnosis of Familial Mediterranean Fever. Arthritis Rheum. 1997, 40, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Job, K.M.; Gamalo, M.; Ward, R.M. Pediatric Age Groups and Approach to Studies. Ther. Innov. Regul. Sci. 2019, 53, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Accetturo, M.; D’Uggento, A.M.; Portincasa, P.; Stella, A. Improvement of MEFV Gene Variants Classification to Aid Treatment Decision Making in Familial Mediterranean Fever. Rheumatology 2020, 59, 754–761. [Google Scholar] [CrossRef]
- Ellergezen, P.; Coşkun, B.N.; Bozkurt, Z.Y.; Çeçen, G.S.; Ağca, H.; Pehlivan, Y.; Dalkılıç, H.E.; Çavun, S.; Yanar, Y.B. A9 B1 Integrin & Its Ligands as New Potential Biomarkers in FMF. Indian J. Med. Res. 2024, 160, 102–108. [Google Scholar] [CrossRef]
- Karadag, S.I.K.; Eren, F.; Gündüz, Z.G.; Yıldıran, A. Adaptive Immune System Evaluation in Familial Mediterranean Fever: Clinical and Immunological Analysis. Allergol. Immunopathol. 2025, 53, 26–31. [Google Scholar] [CrossRef]
- Biyik, Z.; Yavuz, Y.C.; Altintepe, L.; Cizmecioglu, A.; Yakşı, E.; Korez, M.K.; Yılmaz, S. Immature Granulocyte: A Novel Inflammatory Biomarker in Familial Mediterranean Fever. Int. J. Rheum. Dis. 2025, 28, e70149. [Google Scholar] [CrossRef]
- Sipahioglu, H.; Sen, O.; Koyuncu, S.; Kuzugüden, S. Serum Asprosin Level as a New Biomarker in Differentiating Familial Mediterranean Fever Attacks. Cureus 2023, 15, e35342. [Google Scholar] [CrossRef] [PubMed]
- Yildirim Borazan, F.; Ergun, I.; Candar, T.; Oguz, A.K. Association between Serum Uric Acid Levels and Galectin-3 in Patients with Uncomplicated Type 2 Diabetes. Intern. Med. J. 2025, 55, e70070. [Google Scholar] [CrossRef] [PubMed]
- Ten Oever, J.; Giamarellos-Bourboulis, E.J.; Van De Veerdonk, F.L.; Stelma, F.F.; Simon, A.; Janssen, M.; Johnson, M.; Pachot, A.; Kullberg, B.J.; Joosten, L.A.B.; et al. Circulating Galectin-3 in Infections and Non-Infectious Inflammatory Diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1605–1610. [Google Scholar] [CrossRef]
- Bajraktari, G.; Elger, T.; Huss, M.; Loibl, J.; Albert, A.; Kandulski, A.; Müller, M.; Tews, H.C.; Buechler, C. Serum Galectin-3 as a Non-Invasive Marker for Primary Sclerosing Cholangitis. Int. J. Mol. Sci. 2024, 25, 4765. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Geng, N.; Liu, Z.; Pan, W.; Zhu, Y.; Shan, J.; Shi, H.; Han, Y.; Ma, Y.; Liu, B. Presepsin as a Prognostic Biomarker in COVID-19 Patients: Combining Clinical Scoring Systems and Laboratory Inflammatory Markers for Outcome Prediction. Virol. J. 2024, 21, 96. [Google Scholar] [CrossRef]
| FMF Group (n = 74) | Control Group (n = 67) | p * | ||
|---|---|---|---|---|
| Age at admission (year) X ± SD | 10.8 ± 4.1 | 10.1 ± 4.1 | 0.344 | |
| Age groups n (%) | Early childhood (2–5 years) | 10 (13.5) | 7 (10.4) | 0.892 |
| Middle childhood (6–12 years) | 37 (50.0) | 37 (55.2) | ||
| Adolescents (13–18 years) | 27 (36.5) | 23 (34.3) | ||
| Sex n (%) | Female | 38 (51.4) | 39 (58.2) | 0.517 |
| Male | 36 (48.6) | 28 (41.8) | ||
| Body mass index (kg/m2) X ± SD | 18.5 ± 3.8 | 17.5 ± 3.3 | 0.084 | |
| Familial Mediterranean Fever Group (n = 74) | |||||
|---|---|---|---|---|---|
| MEFV Gene Mutation Spectrum | |||||
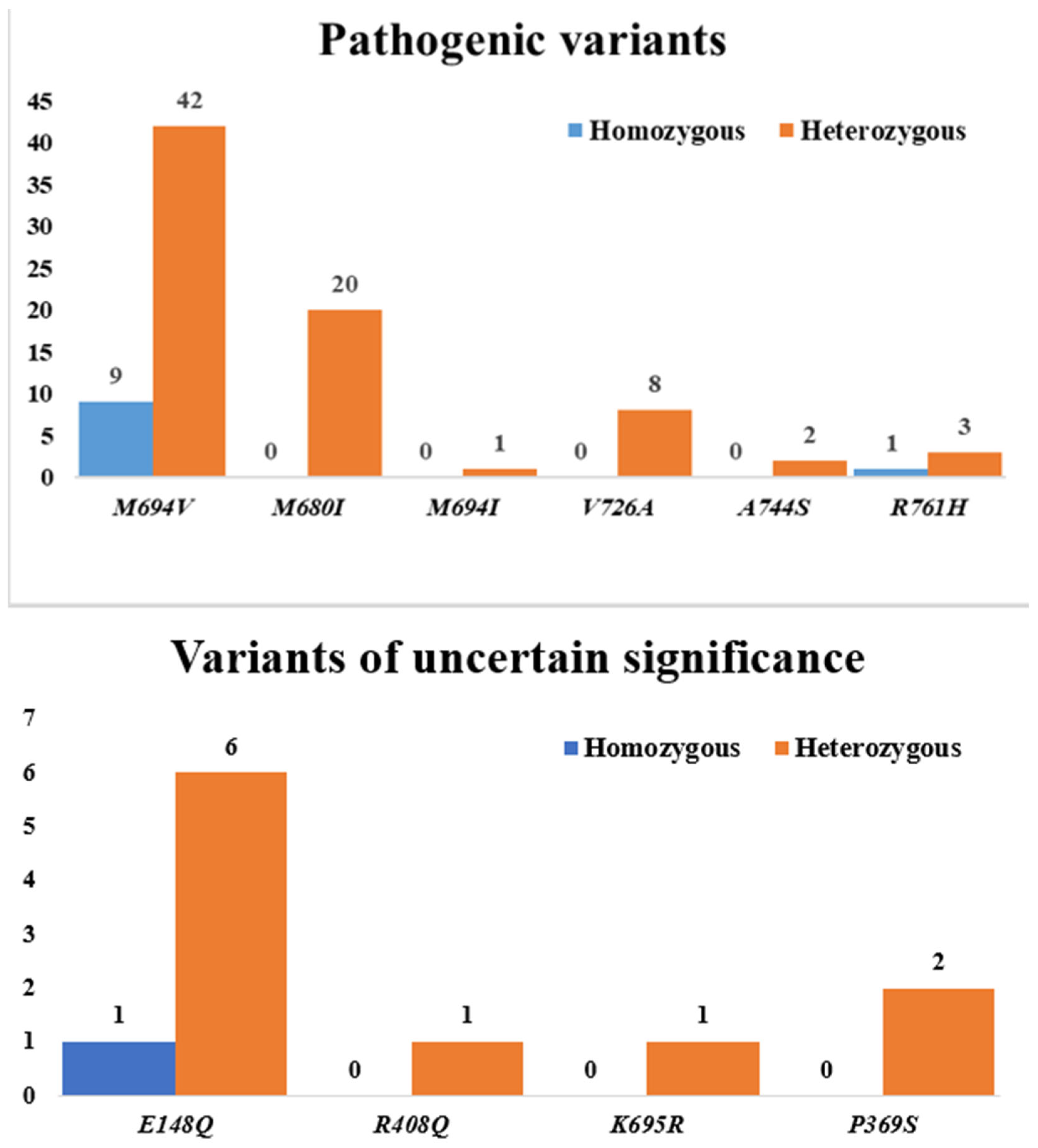
| Variants | MEFV Mutations | n (%) Ω | Mutation Types | ||
| Pathogenic variants | M694V | 51 (68.9) | Homozygous n (%) | 9 (17.6) | |
| Heterozygous n (%) | 42 (82.4) | ||||
| M680I | 20 (27.0) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 20 (100) | ||||
| M694I | 1 (1.4) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 1 (100) | ||||
| V726A | 8 (10.8) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 8 (100) | ||||
| A744S | 2 (2.7) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 2 (100) | ||||
| R761H | 4 (5.4) | Homozygous n (%) | 1 (25) | ||
| Heterozygous n (%) | 3 (75) | ||||
| Variants of uncertain significance | E148Q | 7 (9.5) | Homozygous n (%) | 1 (14.3) | |
| Heterozygous n (%) | 6 (85.7) | ||||
| R408Q | 1 (1.4) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 1 (100) | ||||
| K695R | 1 (1.4) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 1 (100) | ||||
| P369S | 2 (2.7) | Homozygous n (%) | 0 | ||
| Heterozygous n (%) | 2 (100) | ||||
| Clinical manifestations | |||||
| Family history of FMF n (%) | 55 (74.3) | ||||
| Main presenting symptom n (%) Ω | Abdominal pain | 58 (78.4) | |||
| Arthralgia | 42 (56.8) | ||||
| Chest pain | 25 (33.8) | ||||
| Fever | 55 (74.3) | ||||
| Headache | 9 (12.2) | ||||
| Nausea/vomiting | 9 (12.2) | ||||
| Diarrhea | 3 (4.1) | ||||
| FMF Group (n = 74) | Control Group (n = 67) | p * | |
|---|---|---|---|
| Hemoglobin (g/dL) § | 12.4 [5.2–16.0] | 12.6 [5.2–15.2] | 0.843 |
| Leukocyte count (103/μL) § | 7.2 [3.9–12.6] | 7.2 [5.2–11.4] | 0.806 |
| Platelet count (103/μL) § | 325.0 [192.0–627.0] | 330.0 [192.0–627.0] | 0.934 |
| Blood urea nitrogen (mg/dL) § | 22.7 [13.3–39.8] | 23.5 [15.1–39.8] | 0.095 |
| Creatinine (mg/dL) § | 0.5 [0.3–0.9] | 0.3 [0.1–0.7] | <0.001 |
| Alanine aminotransferase (U/L) § | 15.0 [6.0–40.0] | 14.0 [7.0–40.0] | 0.432 |
| Aspartate aminotransferase (U/L) § | 22.0 [12.0–43.0] | 20.0 [16.0–43.0] | 0.285 |
| Lactate dehydrogenase (U/L) § | 210.0 [138.0–347.0] | 203.0 [150.0–324.0] | 0.733 |
| Erythrocyte sedimentation rate (mm/h) § | 8.0 [1.0–37.0] | 3.0 [1.0–9.0] | <0.001 |
| C-reactive protein (mg/L) § | 0.6 [0.1–155.0] | 1.0 [0.2–6.5] | 0.818 |
| Serum amyloid A (mcg/mL) § | 0.5 [0.2–84.3] | 0.4 [0.2–3.5] | 0.350 |
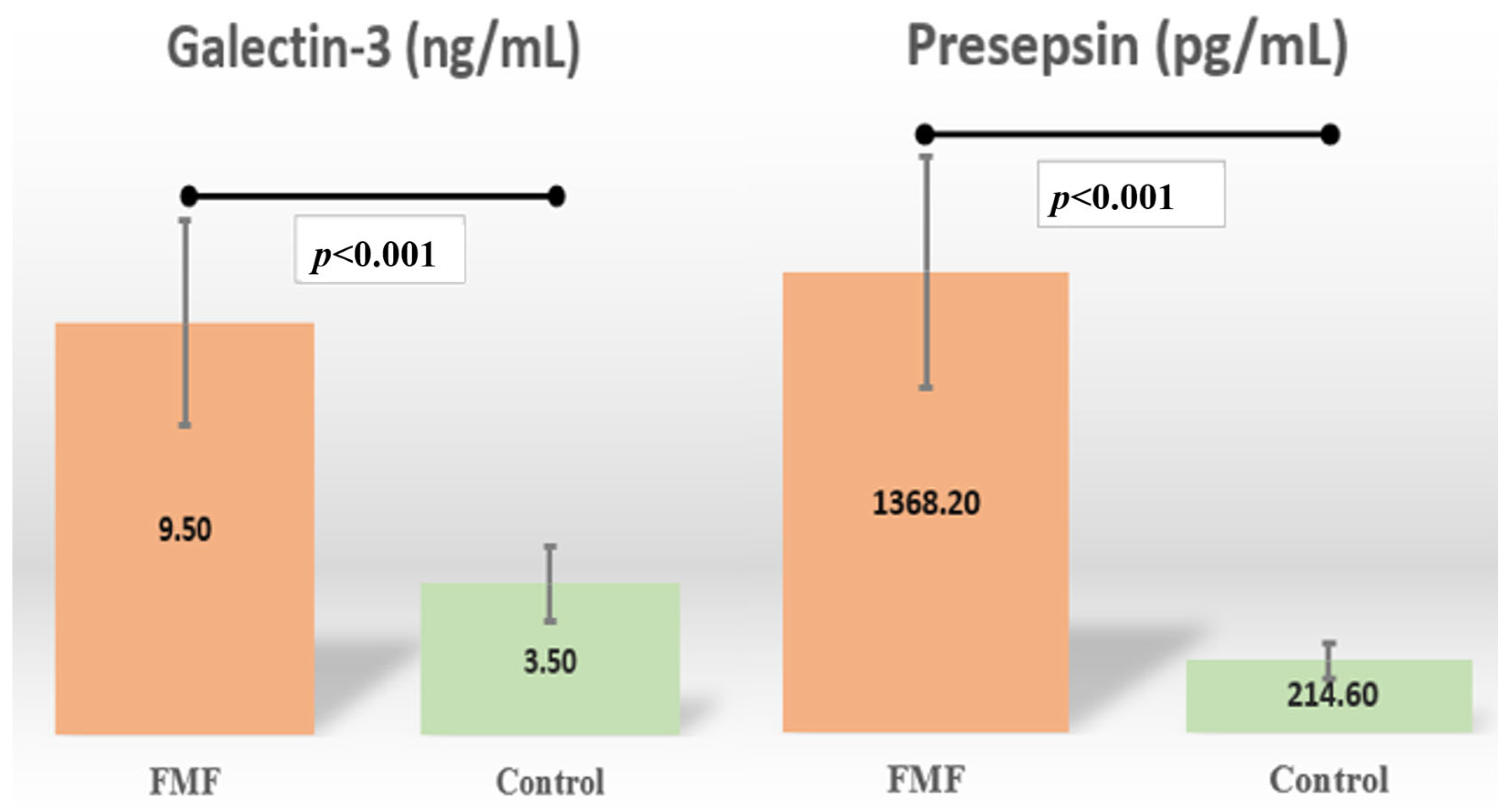
| Galectin-3 (ng/mL) § | 9.3 [0.0–50.2] | 3.5 [1.2–29.7] | <0.001 |
| Presepsin (pg/mL) § | 1368.2 [610.7–13,066.0] | 214.6 [24.2–2022.8] | <0.001 |
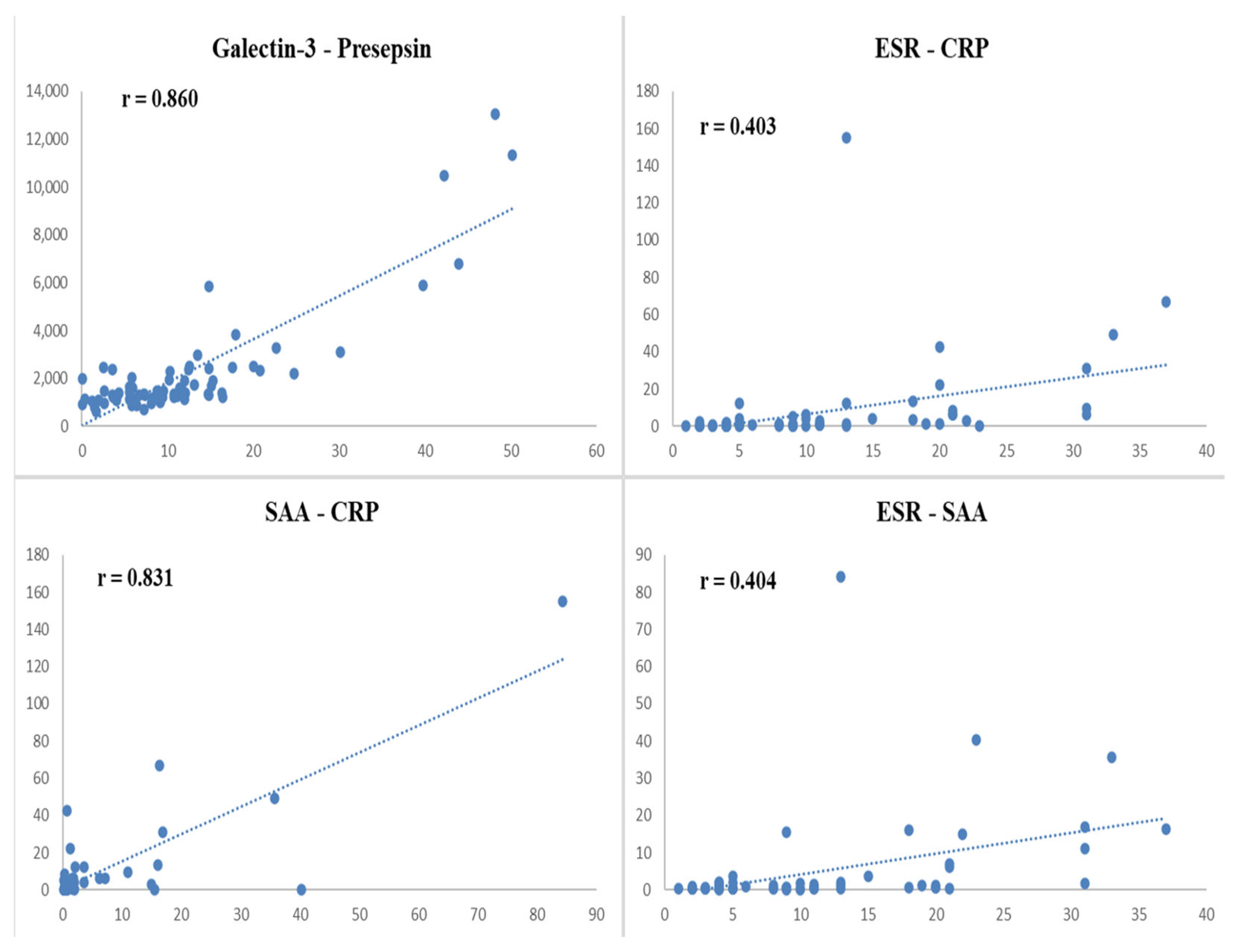
| Presepsin | ESR | SAA | CRP | |
|---|---|---|---|---|
| Galectin-3 | r = 0.860 | r = 0.088 | r = −0.019 | r = −0.030 |
| p < 0.001 | p = 0.457 | p = 0.871 | p = 0.801 | |
| Presepsin | - | r = 0.035 | r = 0.021 | r = −0.001 |
| p = 0.766 | p = 0.862 | p = 0.992 | ||
| ESR | - | - | r = 0.404 | r = 0.403 |
| p < 0.001 | p < 0.001 | |||
| SAA | - | - | - | r = 0.831 |
| p < 0.001 |
| Homozygous Subgroup (n = 11) | Heterozygous Subgroup (n = 42) | Compound Heterozygous Subgroup (n = 21) | p * | |
|---|---|---|---|---|
| Family history of FMF n (%) | 8 (72.7) | 33 (78.6) | 14 (66.7) | 0.589 |
| Creatinine (mg/dL) § | 0.6 [0.3–0.8] | 0.5 [0.3–0.9] | 0.5 [0.3–0.8] | 0.297 |
| Erythrocyte sedimentation rate (mm/h) § | 5.0 [2.0–31.0] | 5.0 [1.0–37.0] | 11.0 [4.0–31.0] | 0.042 |
| Galectin-3 (ng/mL) § | 7.3 [1.2–50.2] | 9.4 [0.3–43.9] | 10.8 [0.04–22.7] | 0.903 |
| Presepsin (pg/mL) § | 1372.3 [718.6–13,066.0] | 1395.0 [767.4–110,497.4] | 1360.0 [610.7–3255.7] | 0.775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogantan, S.; Perk, P.; Sekerci Yuksel, A.; Koc, R.; Keskin, A. Serum Galectin-3 and Presepsin Levels in Pediatric Familial Mediterranean Fever Patients During Remission: A Prospective Study. Diagnostics 2025, 15, 2403. https://doi.org/10.3390/diagnostics15182403
Dogantan S, Perk P, Sekerci Yuksel A, Koc R, Keskin A. Serum Galectin-3 and Presepsin Levels in Pediatric Familial Mediterranean Fever Patients During Remission: A Prospective Study. Diagnostics. 2025; 15(18):2403. https://doi.org/10.3390/diagnostics15182403
Chicago/Turabian StyleDogantan, Seyda, Peren Perk, Arzu Sekerci Yuksel, Rahime Koc, and Adem Keskin. 2025. "Serum Galectin-3 and Presepsin Levels in Pediatric Familial Mediterranean Fever Patients During Remission: A Prospective Study" Diagnostics 15, no. 18: 2403. https://doi.org/10.3390/diagnostics15182403
APA StyleDogantan, S., Perk, P., Sekerci Yuksel, A., Koc, R., & Keskin, A. (2025). Serum Galectin-3 and Presepsin Levels in Pediatric Familial Mediterranean Fever Patients During Remission: A Prospective Study. Diagnostics, 15(18), 2403. https://doi.org/10.3390/diagnostics15182403






