An Unveiling of the Misdiagnosis of Granulomatosis with Polyangiitis as Acute Sinusitis: A Case Report
Abstract
1. Introduction
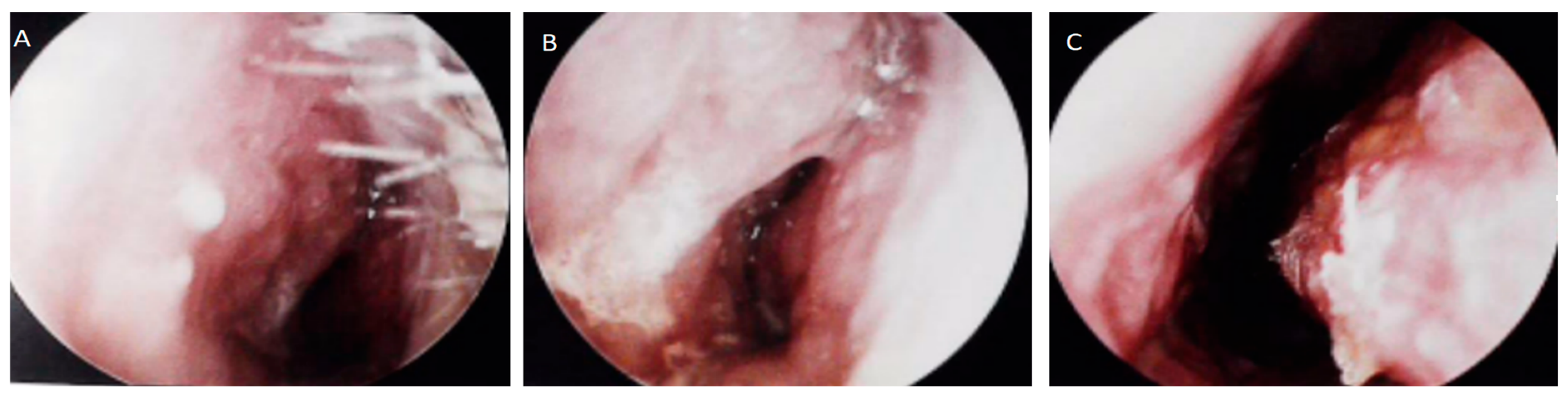
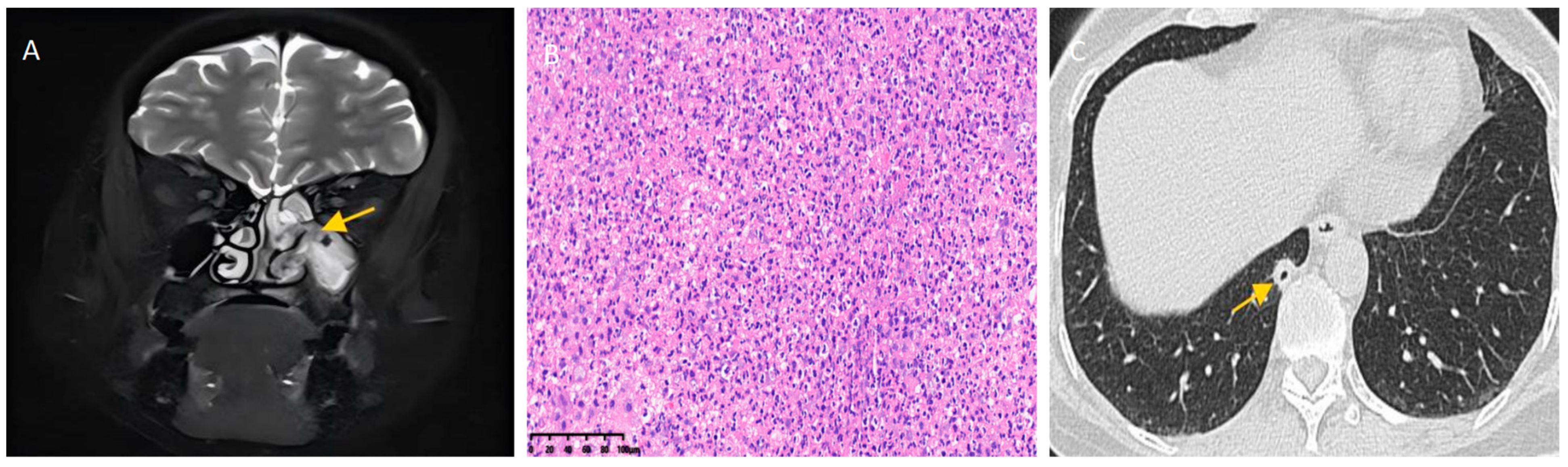
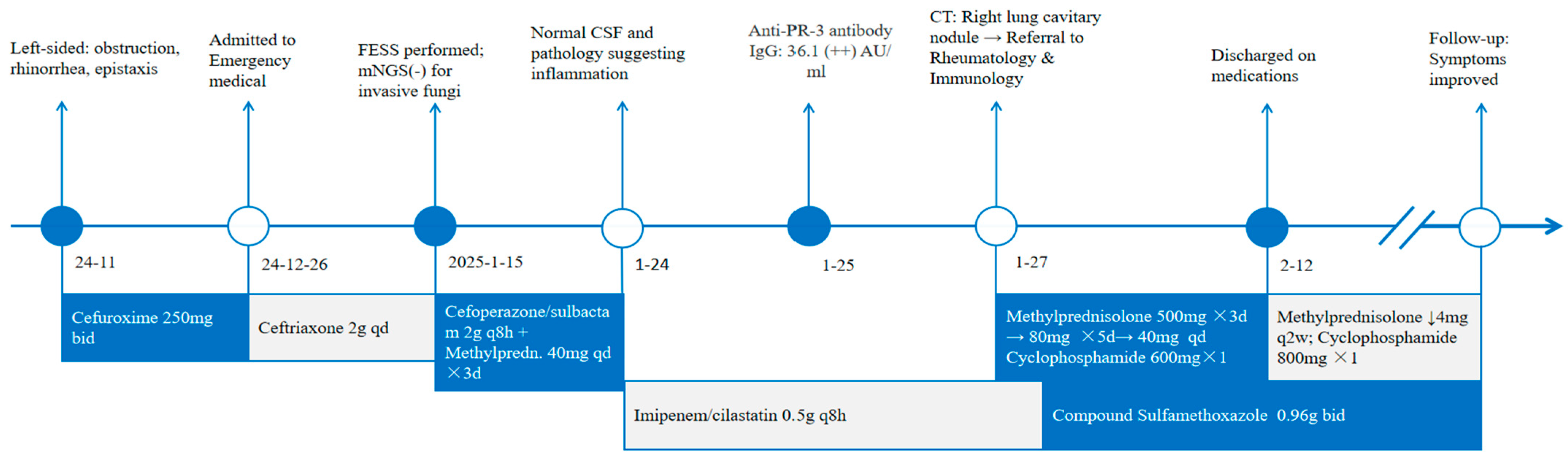
2. Report of a Case
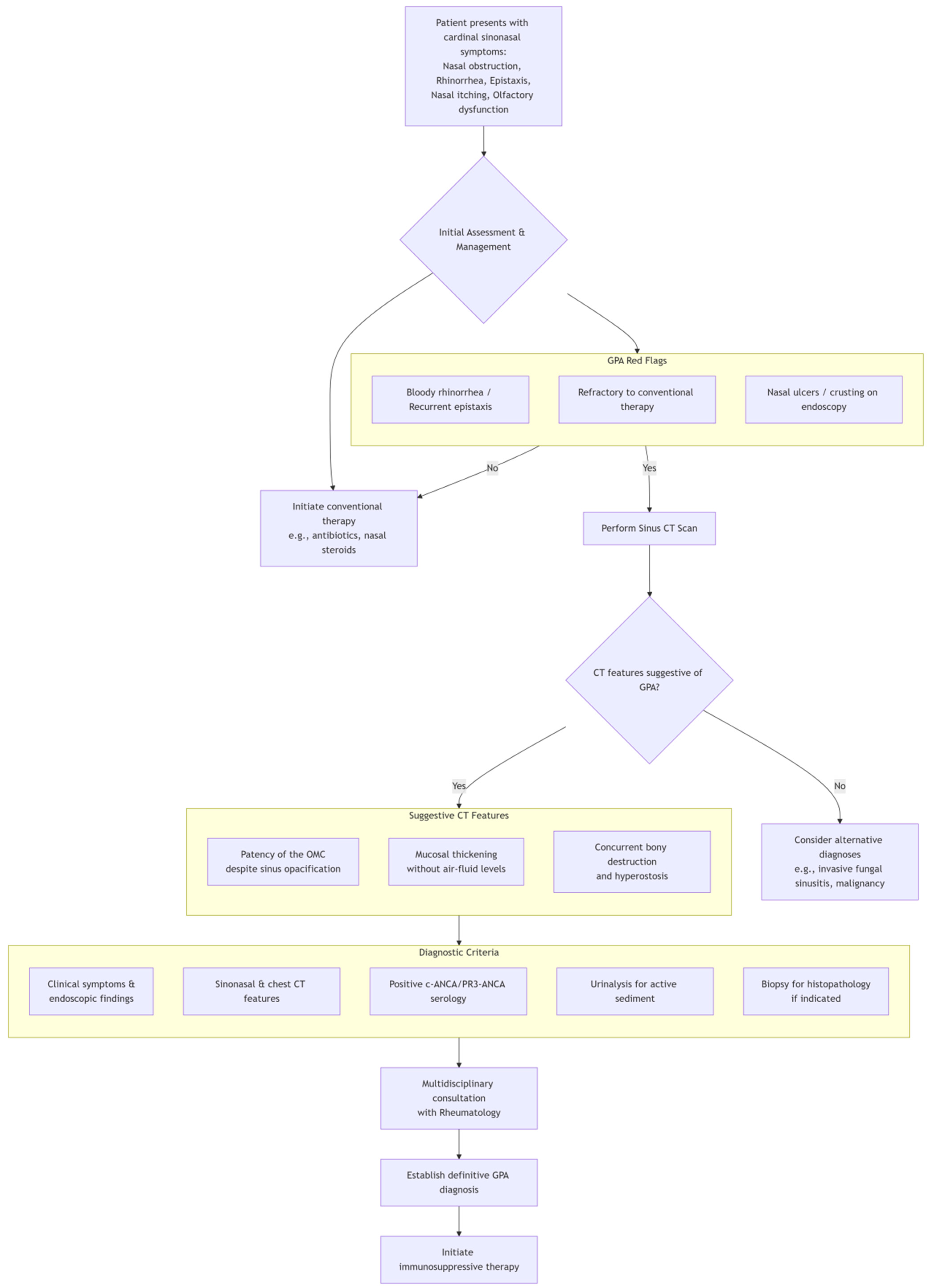
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Greco, A.; Marinelli, C.; Fusconi, M.; Macri, G.F.; Gallo, A.; De Virgilio, A.; Zambetti, G.; de Vincentiis, M. Clinic manifestations in granulomatosis with polyangiitis. Int. J. Immunopathol. Pharmacol. 2016, 29, 151–159. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Takagi, D.; Nakamaru, Y.; Maguchi, S.; Furuta, Y.; Fukuda, S. Otologic manifestations of Wegener’s granulomatosis. Laryngoscope 2002, 112, 1684–1690. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fujimoto, S.; Takahashi, K.; Suzuki, K. Anti-neutrophil cytoplasmic antibody-associated vasculitis, large vessel vasculitis and Kawasaki disease in Japan. Kidney Blood Press Res. 2010, 33, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Sreih, A.G.; Cronin, K.; Shaw, D.G.; Young, K.; Burroughs, C.; Kullman, J.; Machireddy, K.; McAlear, C.A.; Merkel, P.A. Diagnostic delays in vasculitis and factors associated with time to diagnosis. Orphanet J. Rare Dis. 2021, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Bajema, I.M.; Bruchfeld, A.; Mastroianni Kirsztajn, G.; Stone, J.H. Diagnosis and management of ANCA-associated vasculitis. Lancet 2024, 403, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Cotch, M.F.; Hoffman, G.S.; Yerg, D.E.; Kaufman, G.I.; Targonski, P.; Kaslow, R.A. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996, 39, 87–92. [Google Scholar] [CrossRef]
- Hewins, P.; Tervaert, J.W.; Savage, C.O.; Kallenberg, C.G. Is Wegener’s granulomatosis an autoimmune disease? Curr. Opin. Rheumatol. 2000, 12, 3–10. [Google Scholar] [CrossRef]
- Lyons, P.A.; Rayner, T.F.; Trivedi, S.; Holle, J.U.; Watts, R.A.; Jayne, D.R.W.; Baslund, B.; Brenchley, P.; Bruchfeld, A.; Chaudhry, A.N.; et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012, 367, 214–223. [Google Scholar] [CrossRef]
- Holme, S.S.; Moen, J.M.; Kilian, K.; Haukeland, H.; Molberg, Ø.; Eggesbø, H.B. Development of CT-based methods for longitudinal analyses of paranasal sinus osteitis in granulomatosis with polyangiitis. BMC Med. Imaging 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- D’Anza, B.; Langford, C.A.; Sindwani, R. Sinonasal imaging findings in granulomatosis with polyangiitis (Wegener granulomatosis): A systematic review. Am. J. Rhinol. Allergy 2017, 31, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Pietris, J.; Bacchi, S.; Chan, W.O.; Psaltis, A.J.; Selva, D.; Lim, W.Y. Imaging Features of Invasive Fungal Rhinosinusitis: A Systematic Review. Can. Assoc. Radiol. J. 2024, 75, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Mizuta, K.; Aoki, M.; Hara, A.; Matsuo, M. Imaging Characteristics of Malignant Sinonasal Tumors. J. Clin. Med. 2017, 6, 116. [Google Scholar] [CrossRef]
- Lamprecht, P.; Kabelitz, D. T-cells in ANCA-associated vasculitis. Z. Rheumatol. 2011, 70, 698–700. [Google Scholar] [CrossRef]
- Fokkens, W.J. EPOS2020: A major step forward. Rhinology 2020, 58, 1. [Google Scholar] [CrossRef]
- Tateyama, K.; Umemoto, S.; Iwano, S.; Hirano, T.; Suzuki, M. Sinonasal manifestations of granulomatosis with polyangiitis: A retrospective analysis. Auris Nasus Larynx 2024, 51, 625–630. [Google Scholar] [CrossRef]
- Coordes, A.; Loose, S.M.; Hofmann, V.M.; Hamilton, G.S., 3rd; Riedel, F.; Menger, D.J.; Albers, A.E. Saddle nose deformity and septal perforation in granulomatosis with polyangiitis. Clin. Otolaryngol. 2018, 43, 291–299. [Google Scholar] [CrossRef]
- Begum, S.; Srinivasan, S.; Kathirvelu, S.; Vaithy, A. Limited granulomatosis with polyangiitis presenting as an isolated lung lesion. Indian J. Pathol. Microbiol. 2020, 63, 611–614. [Google Scholar] [CrossRef]
- Thornton, M.A.; O’Sullivan, T.J. Otological Wegeners granulomatosis: A diagnostic dilemma. Clin. Otolaryngol. Allied Sci. 2000, 25, 433–434. [Google Scholar] [CrossRef]
- Lutalo, P.M.K.; D’Cruz, D.P. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis). J. Autoimmun. 2014, 48–49, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Jaimes, J.; Rojas-Figueroa, V.H.; Corcuera, R.; Arenas, J.; García-Reynoso, J. Wegener’s granulomatosis and differential diagnosis of mucosal leishmaniasis. Clin. Case Rep. 2021, 9, e04280. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Dechartres, A.; Girard, C.; Jouneau, S.; Kahn, J.-E.; Dhote, R.; Lazaro, E.; Cabane, J.; Papo, T.; Schleinitz, N.; et al. Granulomatosis with polyangiitis: Endoscopic management of tracheobronchial stenosis: Results from a multicentre experience. Rheumatology 2015, 54, 1852–1857. [Google Scholar] [CrossRef]
- Fauci, A.S.; Haynes, B.F.; Katz, P.; Wolff, S.M. Wegener’s granulomatosis: Prospective clinical and therapeutic experience with 85 patients for 21 years. Ann. Intern. Med. 1983, 98, 76–85. [Google Scholar] [CrossRef]
- Yates, M.; Watts, R.A.; Bajema, I.M.; Cid, M.C.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.U.; Laudien, M.; Little, M.A.; et al. EULAR/ERA-EDTA Recommendations for the Management of ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2016, 75, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.M.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef]
- Jayne, D.R.W.; Merkel, P.A.; Schall, T.J.; Bekker, P. Avacopan for the Treatment of ANCA-Associated Vasculitis. N. Engl. J. Med. 2016, 384, 599–609. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ling, Y.; Huang, Y.; Zhao, L.; Lou, Z.; Fan, G.; Xue, J. An Unveiling of the Misdiagnosis of Granulomatosis with Polyangiitis as Acute Sinusitis: A Case Report. Diagnostics 2025, 15, 2218. https://doi.org/10.3390/diagnostics15172218
Wang Q, Ling Y, Huang Y, Zhao L, Lou Z, Fan G, Xue J. An Unveiling of the Misdiagnosis of Granulomatosis with Polyangiitis as Acute Sinusitis: A Case Report. Diagnostics. 2025; 15(17):2218. https://doi.org/10.3390/diagnostics15172218
Chicago/Turabian StyleWang, Qi, Yi Ling, Yangyiyi Huang, Lijing Zhao, Zhewei Lou, Guokang Fan, and Jing Xue. 2025. "An Unveiling of the Misdiagnosis of Granulomatosis with Polyangiitis as Acute Sinusitis: A Case Report" Diagnostics 15, no. 17: 2218. https://doi.org/10.3390/diagnostics15172218
APA StyleWang, Q., Ling, Y., Huang, Y., Zhao, L., Lou, Z., Fan, G., & Xue, J. (2025). An Unveiling of the Misdiagnosis of Granulomatosis with Polyangiitis as Acute Sinusitis: A Case Report. Diagnostics, 15(17), 2218. https://doi.org/10.3390/diagnostics15172218





