NF-κB as an Inflammatory Biomarker in Thin Endometrium: Predictive Value for Live Birth in Recurrent Implantation Failure
Abstract
1. Introduction
2. Materials and Methods
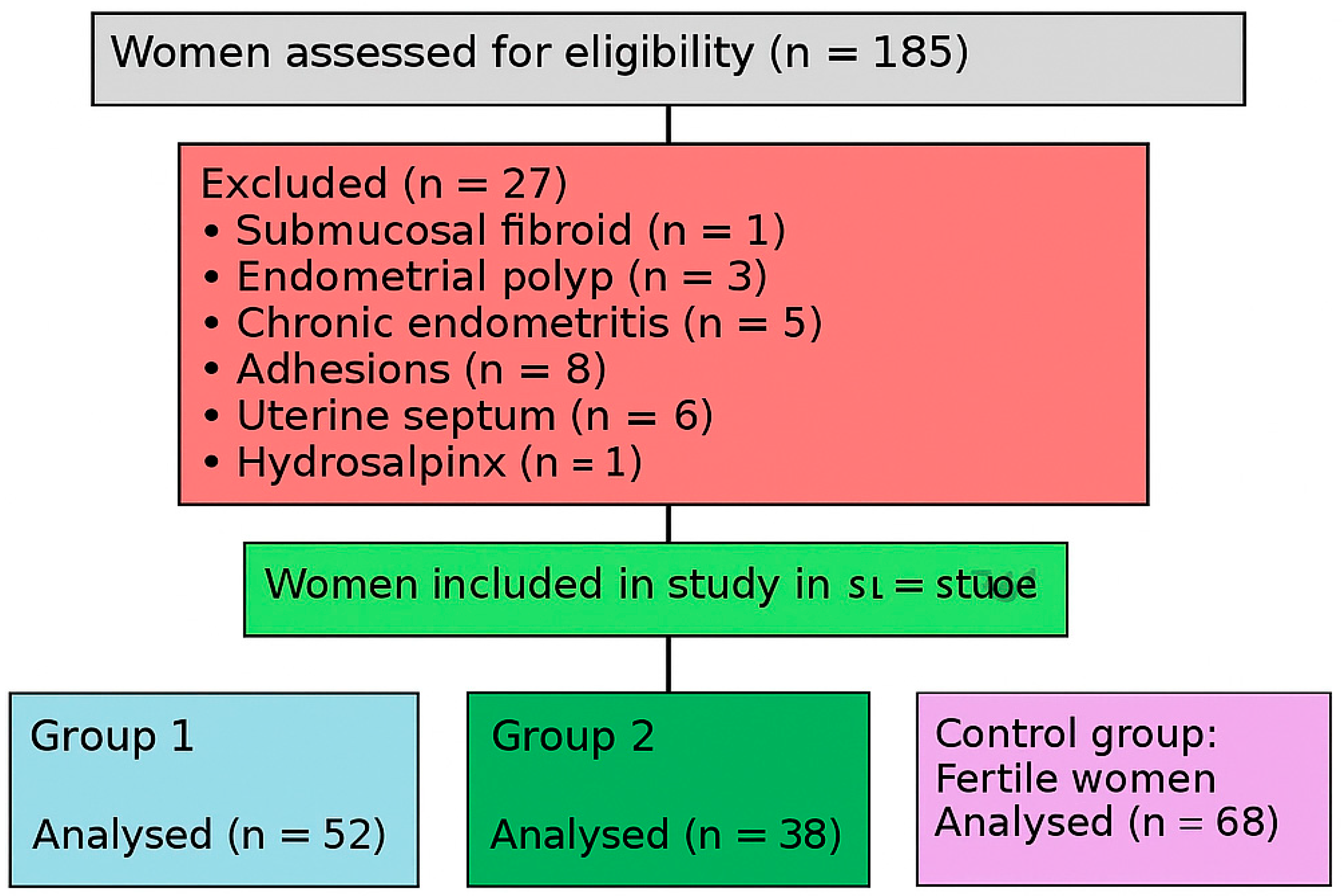
2.1. Study Design and Participants
- Group 1 (n = 52): Patients with RIF and thin endometrium (EMT ≤ 7 mm).
- Group 2 (n = 38): Patients with RIF and normal endometrium (EMT > 7 mm).
- Control Group (n = 68): Fertile women with proven fertility.
- A.
- A portion was preserved in phosphate-buffered saline (PBS) and stored at –80 °C for subsequent NF-κB protein analysis using ELISA.
- B.
- The remaining tissue was fixed in 10% formalin for immunohistochemical analysis.
2.2. Clinical and Laboratory Assessments
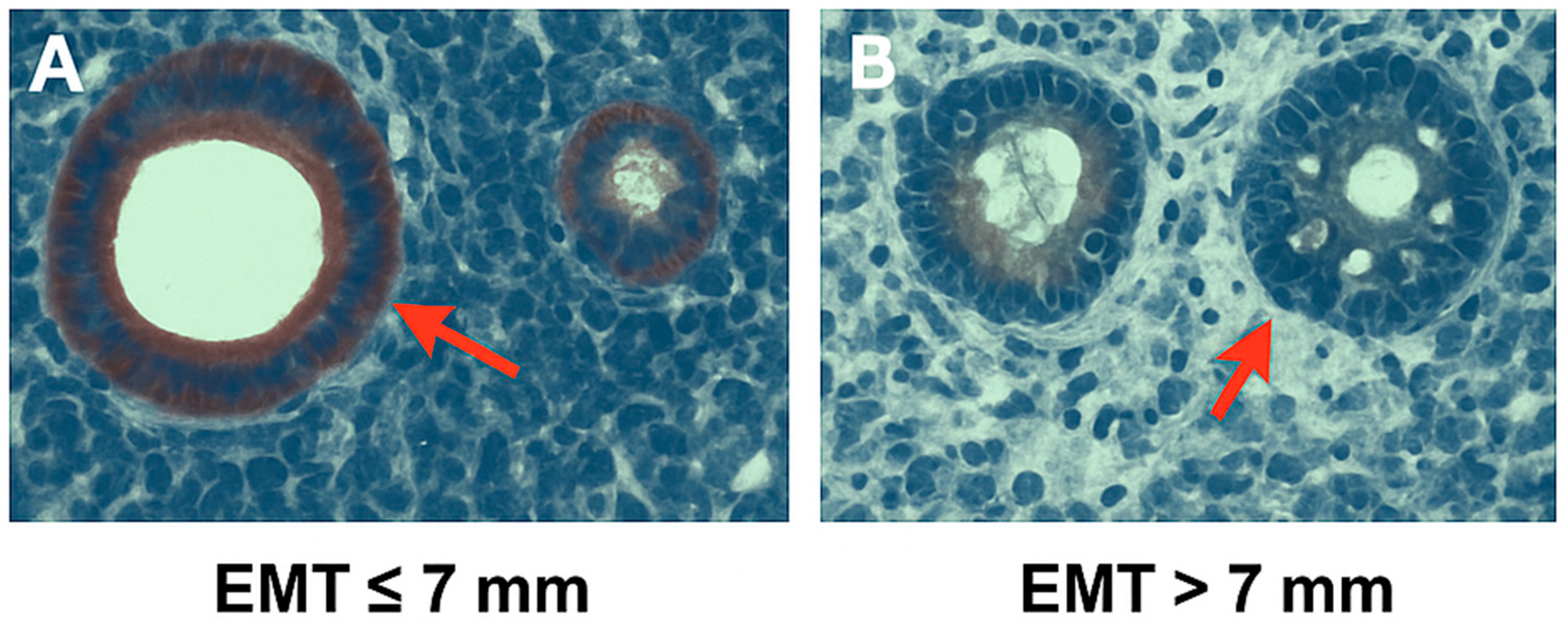
2.3. Endometrial NF-κB Expression Analysis (Immunohistochemistry)
2.4. Endometrial NF-κB Protein Measurement via ELISA
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Gao, X.; Li, Y. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 2023, 13, 1061766. [Google Scholar] [CrossRef]
- ESHRE Working Group on Recurrent Implantation Failure. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open. 2023, 2023, hoad023. [Google Scholar] [CrossRef]
- Macklon, N. Resolving recurrent implantation failure. Reprod. Biomed. Online 2025, 50, 104827. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, L.; Lv, X.; Yuan, Y. Comparative efficacy of intrauterine infusion treatments for recurrent implantation failure: A network meta-analysis of randomized controlled trials. J. Assist. Reprod. Genet. 2025, 42, 345–356. [Google Scholar] [CrossRef]
- Liu, K.E.; Hartman, M.; Mahutte, N.; Luo, Z.C. Association of endometrial thickness with live birth rates among women undergoing fresh and frozen embryo transfer. Front. Cell Dev. Biol. 2025, 13, 1530953. [Google Scholar]
- Wu, J.; Huang, J.; Dong, J.; Xiao, X.; Li, M.; Wang, X. The thicker the endometrium, the better the neonatal outcomes? Hum. Reprod. Open. 2023, 2023, hoad028. [Google Scholar]
- Wang, J.; Xia, F.; Zhou, Y.; Wei, X.; Zhuang, Y.; Huang, Y. Ultrasound-assessed endometrial receptivity measures for the prediction of pregnancy outcomes in IVF-ET: A meta-analysis. Exp. Ther. Med. 2023, 25, 12152. [Google Scholar]
- Sharqawi, M.; Shalom-Paz, E. Does endometrial thickness or compaction impact the success of frozen embryo transfer cycles? J. Clin. Med. 2024, 13, 7254. [Google Scholar]
- Yuan, X.; Saravelos, S.H.; Wang, Q.; Xu, Y.; Li, T.C.; Zhou, C. Endometrial thickness as a predictor of pregnancy outcomes in 10 787 fresh IVF-ICSI cycles. Reprod. Biomed. Online 2016, 33, 197–205. [Google Scholar] [CrossRef]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Seyhan, A.; Urman, B. Effect of the endometrial thickness on the live birth rate: Insights from 959 single euploid frozen embryo transfers without a cutoff for thickness. Fertil. Steril. 2023, 120, 91–98. [Google Scholar]
- Lu, Y.; Cherouveim, P.; Jiang, V.; Dimitriadis, I.; James, K.E.; Bormann, C.; Souter, I. Comparison of endometrial thickness in the same patients undergoing ovulation induction with clomiphene citrate or gonadotropins: A retrospective self-controlled study. Front. Endocrinol. 2024, 15, 1414481. [Google Scholar] [CrossRef]
- Qi, M.; Jin, Y.; Si, L.; Fu, H.; Shi, X.; Liu, Y.; Wang, Y.; Guo, R. Evaluation of the effects of estrogens on endometrial cancer cells of different grades. J. Biochem. Toxicol. 2025, 39, e70129. [Google Scholar] [CrossRef]
- Evans, J.; Salamonsen, L.A. The menstrual endometrium: From physiology to future treatments. Front. Reprod. Health 2021, 3, 794352. [Google Scholar] [CrossRef]
- Bo, C.; Wang, Y. Angiogenesis signaling in endometriosis: Molecules, diagnosis and potential therapeutic targets. Mol. Med. Rep. 2024, 29, 13167. [Google Scholar] [CrossRef]
- Maybin, J.A.; Murray, A.A.; Saunders, P.T.K.; Hirani, N.; Carmeliet, P.; Critchley, H.O.D. Hypoxia and hypoxia-inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 2018, 9, 2956. [Google Scholar] [CrossRef]
- Kim, S.H.; Ihm, H.J.; Oh, Y.S.; Chae, H.D.; Kim, C.; Kang, B.M. Increased nuclear expression of NF-κB p65 subunit in the eutopic endometrium and ovarian endometriomas of women with advanced-stage endometriosis. Am. J. Reprod. Immunol. 2013, 70, 497–508. [Google Scholar] [CrossRef]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and immune dysfunction in endometriosis. Endocr. Rev. 2021, 42, 314–344. [Google Scholar] [CrossRef]
- Lédée, N.; Petitbarat, M.; Chevrier, L.; Vitoux, D.; Vezmar, K.; Rahmati, M.; Dubanchet, S.; Gahéry, H.; Bensussan, A.; Chaouat, G. Uterine immune profiling may help women with repeated unexplained embryo implantation failure after IVF. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salker, M.S.; Christian, M.; Steel, J.H.; Nautiyal, J.; Lavery, S.; Trew, G.; Webster, Z.; Al-Sabbagh, M.; Puchchakayala, G.; Föller, M.; et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat. Med. 2011, 17, 1509–1513. [Google Scholar] [CrossRef]
- Walker, E.R.; McGrane, M.; Aplin, J.D.; Brison, D.R.; Ruane, P.T. A systematic review of transcriptomic studies of the human endometrium reveals inconsistently reported differentially expressed genes. Reprod. Fertil. 2023, 4, RAF-22-0115. [Google Scholar] [CrossRef]
- Moneva-Sakelarieva, M.; Kobakova, Y.; Konstantinov, S.; Momekov, G.; Ivanova, S.; Atanasova, V.; Chaneva, M.; Tododrov, R.; Bashev, N.; Atanasov, P. The role of the transcription factor NF-κB in the pathogenesis of inflammation and carcinogenesis. Modulation capabilities. Pharmacia 2025, 72, 1–13. [Google Scholar] [CrossRef]
- Chen, C.; Luo, F.; Liu, X.; Lu, L.; Xu, H.; Yang, Q.; Xue, J.; Shi, L.; Li, J.; Zhang, A.; et al. NF-κB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett. 2017, 388, 21–33. [Google Scholar] [CrossRef]
- González-Ramos, R.; Defrère, S.; Devoto, L. Nuclear factor–kappaB: A main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil. Steril. 2012, 98, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Teng, X. New advances in the treatment of thin endometrium. Front. Endocrinol. 2024, 15, 1269382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, M.; Li, X.; Huang, H.; Sun, Y. Gene profiling reveals the role of inflammation, abnormal uterine muscle contraction and vascularity in recurrent implantation failure. Front. Genet. 2023, 14, 1108805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezaei, M.; Moghoofei, M. The role of viral infection in implantation failure: Direct and indirect effects. Reprod. Biol. Endocrinol. 2024, 22, 142. [Google Scholar]
- Zhang, Q.; Yang, D.; Han, X.; Wang, Y.; Sun, Z.; Wang, J.; Zhao, X.; Li, Y.; Liu, X.; Xu, H.; et al. Role of NF-κB signaling pathway in endometriosis: New insights and future therapeutic strategies. Front. Immunol. 2021, 12, 702842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ersahin, A.; Acet, M.; Acet, T.; Yavuz, Y. Disturbed endometrial NF-κB expression in women with recurrent implantation failure. J. Assist. Reprod. Genet. 2016, 33, 1299–1304. [Google Scholar]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant expression of DNA methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil. Steril. 2007, 87, 24–32. [Google Scholar] [CrossRef] [PubMed]
- van Mourik, M.S.M.; Macklon, N.S.; Heijnen, C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009, 85, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Chin, P.Y.; Glynn, D.J.; Thompson, J.G. Peri-conceptual cytokines—Setting the trajectory for embryo implantation, pregnancy and beyond. Am. J. Reprod. Immunol. 2011, 66 (Suppl. S1), 2–10. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Zdrojkowski, Ł.; Jasiński, T.; Ferreira-Dias, G.; Pawliński, B.; Domino, M. The Role of NF-κB in Endometrial Diseases in Humans and Animals: A Review. Int. J. Mol. Sci. 2023, 24, 2901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paul, D.; Agrawal, R.; Iqbal, M.A. An overview of endometriosis and molecular target-based therapeutic approach. Middle East. Fertil. Soc. J. 2025, 30, 6. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. Inflammation and implantation. Am. J. Reprod. Immunol. 2010, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Şahin, L.; Ulaş, M. Hysteroscopic polypectomy decreases NF-κB1 expression in the mid-secretory endometrium of women with endometrial polyp. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). 2022 Assisted Reproductive Technology Fertility Clinic Success Rates Report; US Department of Health and Human Services: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/art/success-rates/index.html (accessed on 4 July 2025).
- Society for Assisted Reproductive Technology (SART). 2023 National Summary Report; SART: Birmingham, AL, USA, 2024; Available online: https://www.sartcorsonline.com/rptcsr_publicmultyear.aspx (accessed on 4 July 2025).
- Fan, J.; Zhang, M.; Wu, H.; Ye, Z.; Wang, L. Estrogen Promotes Endometrial Cancer Development by Modulating ZNF626, SLK, and RFWD3 Gene Expression and Inducing Immune Inflammatory Changes. Biomedicines 2025, 13, 498. [Google Scholar] [CrossRef]
- Gullo, G.; Carlomagno, G.; Unfer, V.; D’Anna, R. Myo-inositol: From induction of ovulation to menopausal disorder management. Minerva Ginecol. 2015, 67, 485–486. [Google Scholar]
| Variable | Group 1 (n = 52) | Group 2 (n = 38) | Control (n = 68) | p-Value |
|---|---|---|---|---|
| Age (years) | 28.76 ± 6.70 | 29.21 ± 8.32 | 30.12 ± 7.40 | 0.34 |
| BMI (kg/m2) | 22.33 ± 5.11 | 22.41 ± 5.40 | 22.62 ± 5.20 | 0.11 |
| Duration of infertility (years) | 3.43 ± 2.30 | 3.76 ± 2.87 | – | – |
| Failed IVF attempts (n) | 3.22 ± 1.44 | 3.02 ± 1.09 | – | – |
| Estradiol (pg/mL) | 35.6 ± 6.12 | 34.9 ± 5.41 | 45.7 ± 4.80 | 0.66 |
| LH (mIU/mL) | 4.95 ± 2.30 | 5.11 ± 3.22 | 5.47 ± 2.90 | 0.41 |
| FSH (mIU/mL) | 5.13 ± 3.01 | 5.22 ± 3.05 | 5.36 ± 2.85 | 0.39 |
| AMH (ng/mL) | 2.23 ± 1.47 | 1.86 ± 1.31 | 2.47 ± 0.94 | 0.1164 |
| TSH (uIU/mL) | 2.47 ± 0.64 | 2.70 ± 0.70 | 1.00 ± 0.31 | 0.1266 |
| Antral Follicle Count | 9.32 ± 5.09 | 11.64 ± 7.67 | – | – |
| Stimulation Duration (days) | 10.16 ± 1.83 | 11.82 ± 3.19 | – | – |
| Total MII Oocyte Count | 6.00 ± 4.03 | 7.96 ± 5.91 | – | – |
| High-Quality Embryo Count | 3.42 ± 2.17 | 3.79 ± 2.51 | – | – |
| Transferred Embryo Count | 1.11 ± 0.32 | 1.04 ± 0.19 | – | – |
| EMT (mm) | 4.32 ± 1.28 | 9.21 ± 3.42 | 9.45 ± 2.06 | 0.0000 * |
| NF-κB Level | 11.28 ± 5.60 | 4.90 ± 2.34 | 3.76 ± 0.41 | 0.0017 * |
| NF-κB Histoscore | 1.20 ± 0.54 | 0.55 ± 0.81 | 0.95 ± 0.45 | 0.0478 * |
| Outcome Type | Group 1 (n = 52) | Group 2 (n = 38) | Control (n = 68) | p-Value |
|---|---|---|---|---|
| Biochemical pregnancy | 6 (11.5%) | 2 (5.3%) | 3 (4.4%) | 0.1454 |
| Clinical pregnancy | 5 (9.6%) | 12 (31.6%) | 24 (35.3%) | 0.0314 * |
| Abortus | 3 (5.8%) | 3 (7.9%) | 5 (7.4%) | 0.9849 |
| Live birth | 2 (3.8%) | 9 (23.7%) | 19 (27.9%) | 0.0141 * |
| Non pregnant | 41 (78.8%) | 24 (63.2%) | 41 (60.3%) | 0.0035 * |
| Variable | With Live Birth (n = 30) | Without Live Birth (n = 22) | p-Value |
|---|---|---|---|
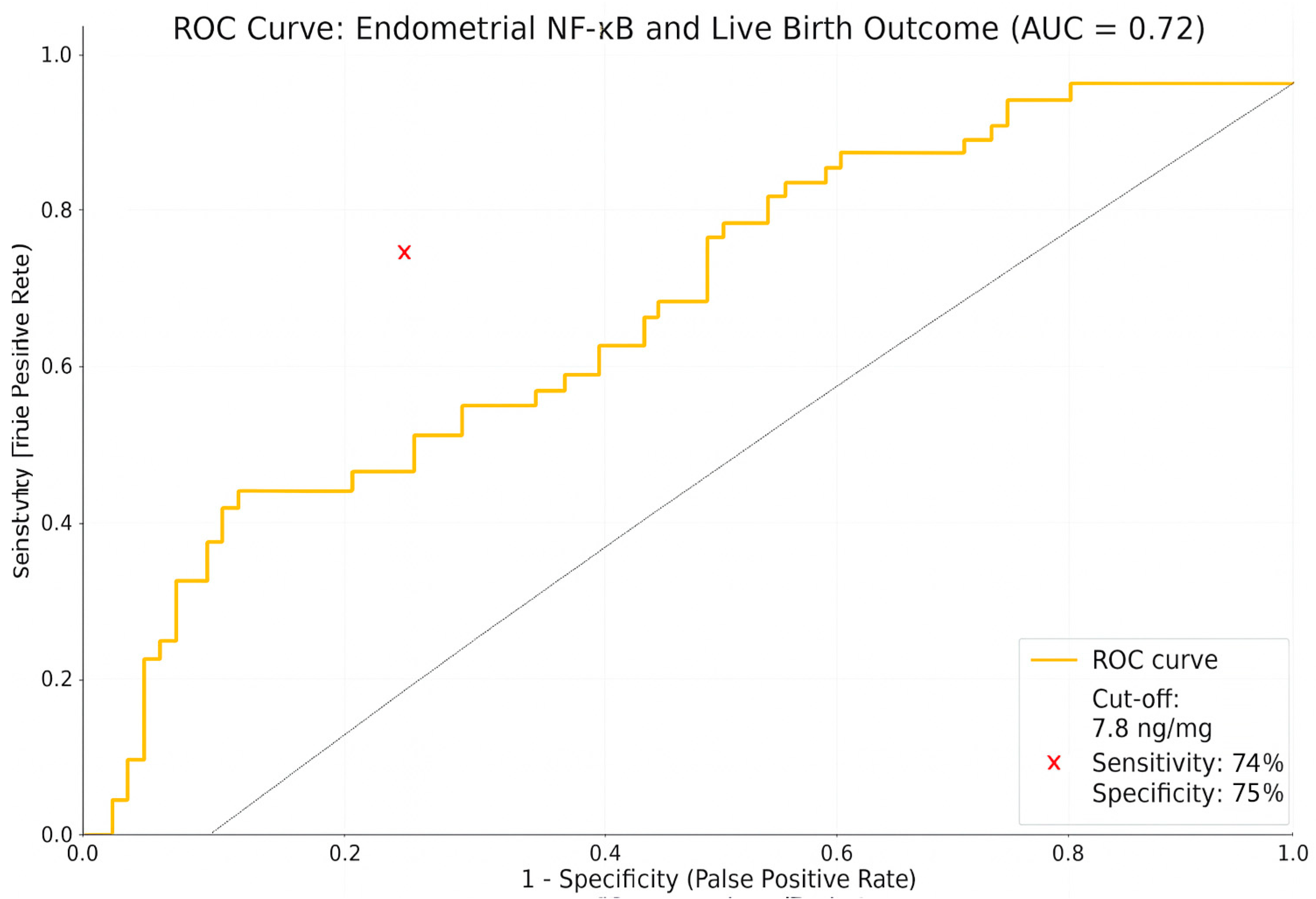
| NF-κB (ng/mg) | 4.90 ± 2.34 | 11.28 ± 5.60 | 0.0004 * |
| Age (years) | 29.21 ± 8.32 | 28.76 ± 6.70 | 0.340 |
| BMI (kg/m2) | 22.41 ± 5.40 | 22.33 ± 5.11 | 0.110 |
| FSH (mIU/mL) | 5.22 ± 3.05 | 5.13 ± 3.01 | 0.390 |
| LH (mIU/mL) | 5.11 ± 3.22 | 4.95 ± 2.30 | 0.410 |
| E2 (pg/mL) | 34.9 ± 5.41 | 35.6 ± 6.12 | 0.660 |
| AMH (ng/mL) | 1.86 ± 1.31 | 2.23 ± 1.47 | 0.1164 |
| TSH (uIU/mL) | 2.70 ± 0.70 | 2.47 ± 0.64 | 0.1266 |
| EMT (mm) | 9.21 ± 3.42 | 4.32 ± 1.28 | 0.0000 * |
| Variable | Coefficient (B) | Standard Error | p-Value | Odds Ratio (exp(B)) | 95% CI for B | 95% CI for OR |
|---|---|---|---|---|---|---|
| NF-κB level | −0.204 | 0.106 | 0.045 * | 0.815 | −0.413–0.005 | 0.66–1.00 |
| EMT | 0.164 | 0.134 | 0.222 | 1.178 | −0.099–0.427 | 0.91–1.53 |
| AMH (ng/mL) | 0.423 | 0.386 | 0.272 | 1.527 | −0.333–1.179 | 0.72–3.25 |
| Age | −0.031 | 0.103 | 0.766 | 0.970 | −0.233–0.172 | 0.79–1.19 |
| BMI | −0.204 | 0.123 | 0.099 | 0.816 | −0.445–0.038 | 0.64–1.04 |
| FSH (mIU/mL) | 0.322 | 0.213 | 0.130 | 1.380 | −0.095–0.739 | 0.91–2.09 |
| LH (mIU/mL) | −0.290 | 0.151 | 0.055 | 0.748 | −0.587–0.007 | 0.56–1.01 |
| E2 (pg/mL) | −0.001 | 0.013 | 0.917 | 0.999 | −0.027–0.024 | 0.97–1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalı, Z.; Karlı, P.; Tanılır, F.; Kırıcı, P.; Ege, S. NF-κB as an Inflammatory Biomarker in Thin Endometrium: Predictive Value for Live Birth in Recurrent Implantation Failure. Diagnostics 2025, 15, 1762. https://doi.org/10.3390/diagnostics15141762
Kalı Z, Karlı P, Tanılır F, Kırıcı P, Ege S. NF-κB as an Inflammatory Biomarker in Thin Endometrium: Predictive Value for Live Birth in Recurrent Implantation Failure. Diagnostics. 2025; 15(14):1762. https://doi.org/10.3390/diagnostics15141762
Chicago/Turabian StyleKalı, Zercan, Pervin Karlı, Fatma Tanılır, Pınar Kırıcı, and Serhat Ege. 2025. "NF-κB as an Inflammatory Biomarker in Thin Endometrium: Predictive Value for Live Birth in Recurrent Implantation Failure" Diagnostics 15, no. 14: 1762. https://doi.org/10.3390/diagnostics15141762
APA StyleKalı, Z., Karlı, P., Tanılır, F., Kırıcı, P., & Ege, S. (2025). NF-κB as an Inflammatory Biomarker in Thin Endometrium: Predictive Value for Live Birth in Recurrent Implantation Failure. Diagnostics, 15(14), 1762. https://doi.org/10.3390/diagnostics15141762







