Structural Retinal and Optic Nerve Changes in Prostate Cancer Patients Receiving Androgen Receptor Pathway Inhibitors: An OCT-Based In Vivo Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ophthalmic Examination
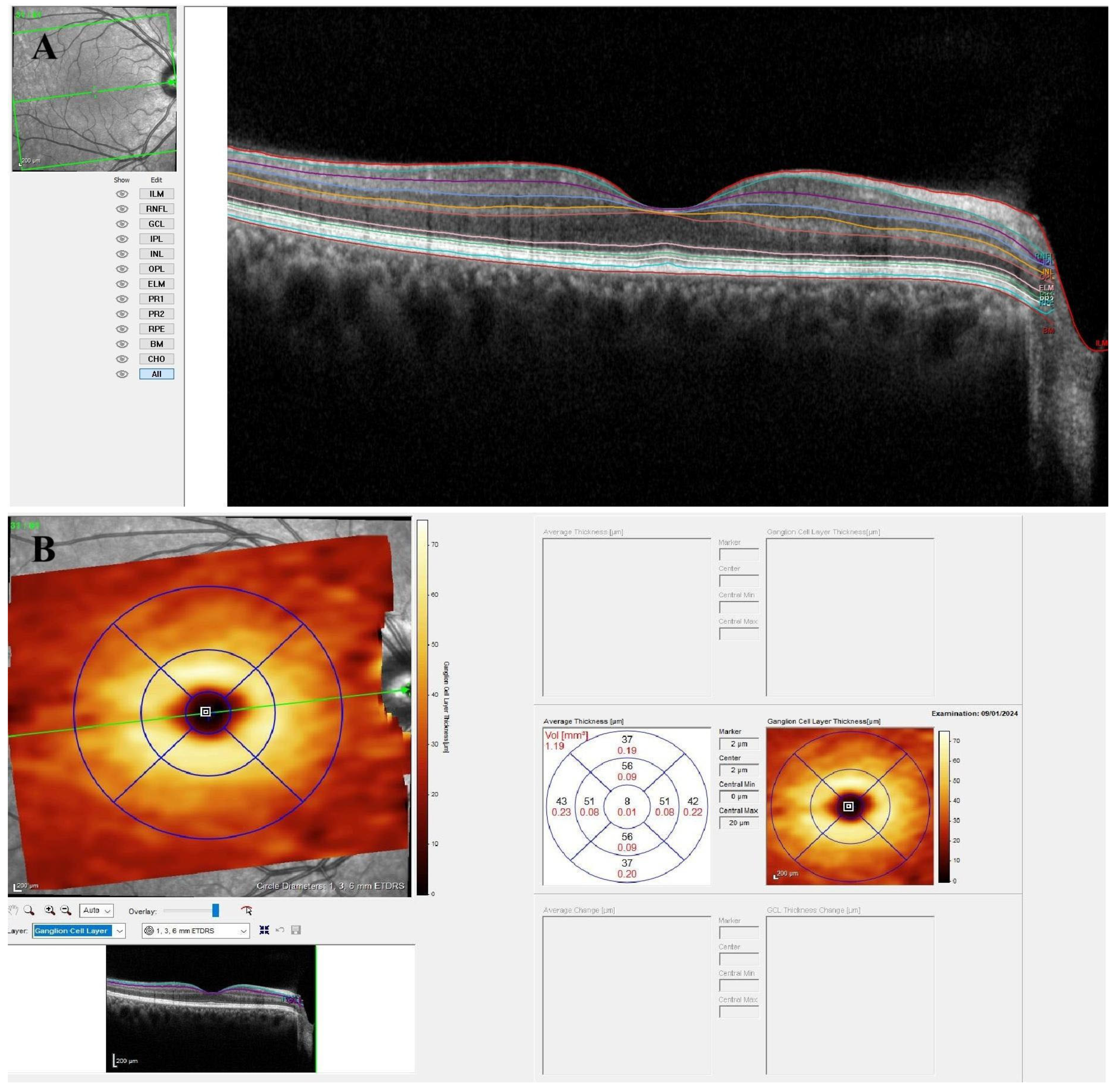
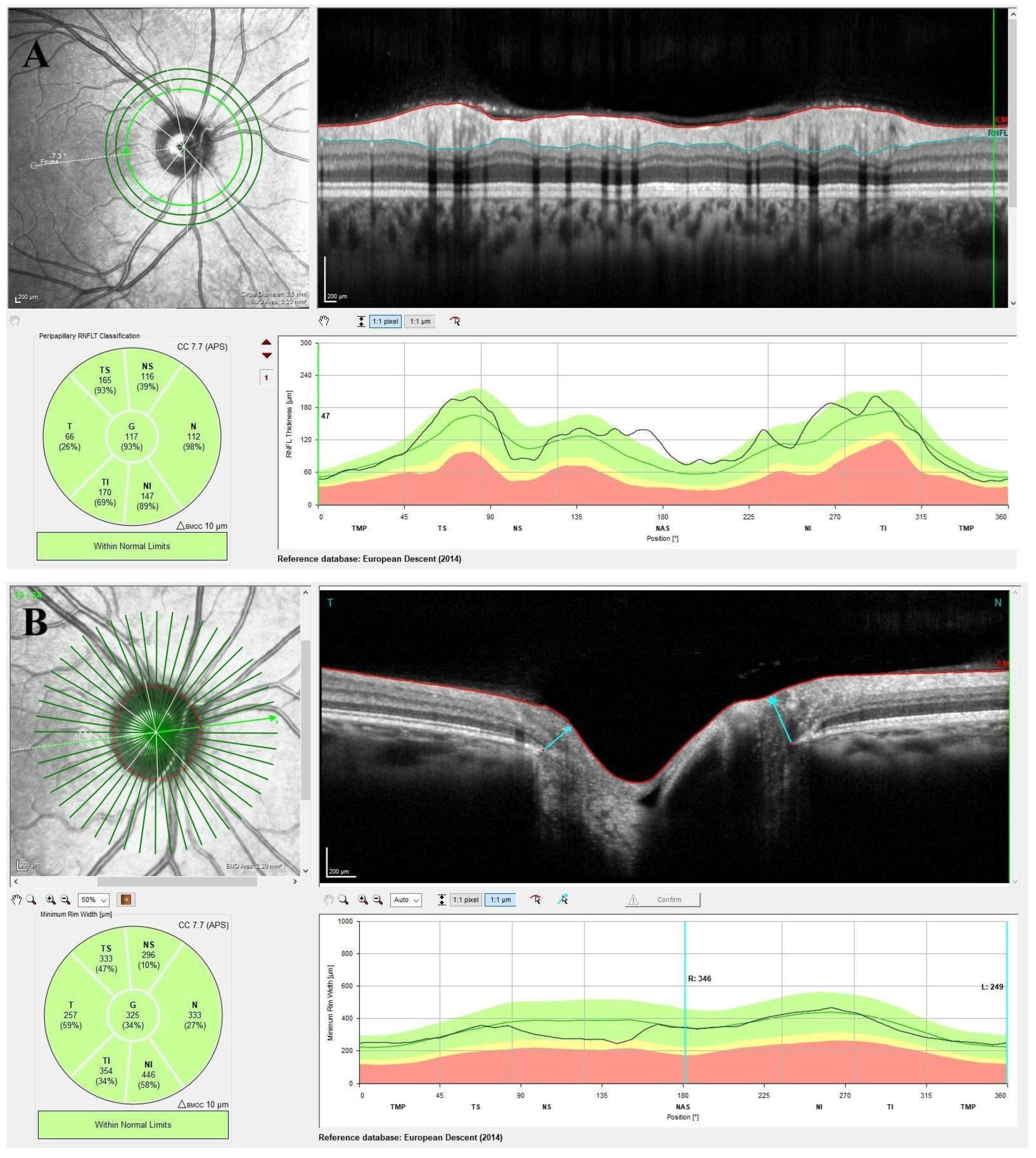
2.3. Optical Coherence Tomography Imaging
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Goddard, K.A.B.; Feuer, E.J.; Mandelblatt, J.S.; Meza, R.; Holford, T.R.; Jeon, J.; Lansdorp-Vogelaar, I.; Gulati, R.; Stout, N.K.; Howlader, N.; et al. Estimation of Cancer Deaths Averted from Prevention, Screening, and Treatment Efforts, 1975–2020. JAMA Oncol. 2025, 11, 162–167. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2022, 20, 1288–1298. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thakur, A.; Roy, A.; Ghosh, A.; Chhabra, M.; Banerjee, S. Abiraterone acetate in the treatment of prostate cancer. Biomed. Pharmacother. 2018, 101, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef]
- Rocha, E.M.; Wickham, L.A.; Ada Silveira, L.; Krenzer, K.L.; Yu, F.-S.; Toda, I.; Sullivan, B.D.; ASullivan, D. Identification of androgen receptor protein and 5alpha -reductase mRNA in human ocular tissues. Br. J. Ophthalmol. 2000, 84, 76–84. [Google Scholar] [CrossRef]
- Wickham, L.A.; Gao, J.; Toda, I.; Rocha, E.M.; Ono, M.; Sullivan, D.A. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 2000, 78, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Johar, K.; Nagpal, K.; Vasavada, A.R. Sex hormone receptors in the human eye. Surv. Ophthalmol. 2005, 50, 274–284. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Alibhai, S.M.H.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef]
- Leong, D.P.; Cirne, F.; Pinthus, J.H. Cardiovascular Risk in Prostate Cancer. Cardiol. Clin. 2025, 43, 83–91. [Google Scholar] [CrossRef]
- Pilon, D.; Behl, A.S.; AEllis, L.; Robitaille, M.-N.; Lefebvre, P.; Dawson, N.A. Assessment of Real-World Central Nervous System Events in Patients with Advanced Prostate Cancer Using Abiraterone Acetate, Bicalutamide, Enzalutamide, or Chemotherapy. Am. Health Drug Benefits 2017, 10, 143–153. [Google Scholar]
- Cao, B.; Kim, M.; Reizine, N.M.; Moreira, D.M. Adverse Events and Androgen Receptor Signaling Inhibitors in the Treatment of Prostate Cancer: A Systematic Review and Multivariate Network Meta-analysis. Eur. Urol. Oncol. 2023, 6, 237–250. [Google Scholar] [CrossRef]
- Slovin, S.; Clark, W.; Carles, J.; Krivoshik, A.; Park, J.W.; Wang, F.; George, D. Seizure rates in enzalutamide-treated men with metastatic castration-resistant prostate cancer and risk of seizure: The UPWARD study. JAMA Oncol. 2018, 4, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Whang, Y.E. Posterior reversible encephalopathy syndrome induced by enzalutamide in a patient with castration-resistant prostate cancer. Investig. New Drugs 2015, 33, 751–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozaltun, O.A.; Koz, O.G.; Yarangumeli, A.A. Evaluation of macula ganglion cell analysis and retinal nerve fiber layer thickness in preperimetric glaucoma, early stage glaucoma and healthy individuals. Photodiagnosis Photodyn. Ther. 2025, 52, 104495. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Cui, F.; Xu, Z.; Dong, N.; Li, C.; Shi, W.-Q. The Ocular Surface Characteristics in Prostate Cancer Patients Treated with Androgen Deprivation Therapy. Dis. Markers 2021, 2021, 5390195. [Google Scholar] [CrossRef]
- Chien, H.-W.; Lin, C.-W.; Lee, C.-Y.; Huang, J.-Y.; Yang, S.-F.; Wang, K. The use of androgen deprivation therapy for prostate cancer and its effect on the subsequent dry eye disease: A population-based cohort study. Int. J. Med. Sci. 2022, 19, 1103–1109. [Google Scholar] [CrossRef]
- Agapova, O.A.; Kaufman, P.L.; Hernandez, M.R. Androgen receptor and NFkB expression in human normal and glaucomatous optic nerve head astrocytes in vitro and in experimental glaucoma. Exp. Eye Res. 2006, 82, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Shiromani, S.; Bhatnagar, K.R.; Singh, P.; Suman, S.; Meena, S.; Parveen, S. A study of retinal changes in women with polycystic ovarian syndrome. Indian J. Ophthalmol. 2022, 70, 3591–3595. [Google Scholar] [CrossRef]
- Alpogan, O.; Donmez, E.E.; Balık, A.Ö.; Vural, F.; Kaplan, G. Effects of testosterone on intraocular pressure, thicknesses of retinal nerve fiber layer, ganglion cell complex, macula and on ocular blood flow in female-to-male transgender persons. Int. Ophthalmol. 2021, 41, 3651–3661. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Galindez, S.M.; Keightley, A.; Koulen, P. Differential distribution of steroid hormone signaling networks in the human choroid-retinal pigment epithelial complex. BMC Ophthalmol. 2022, 22, 406. [Google Scholar] [CrossRef] [PubMed]
| Patient Group (n = 80) | Control Group (n = 80) | p | |
|---|---|---|---|
| Age (years) | 70 (62–75.75) | 70 (60–72) | 0.136 a |
| BCVA (logMAR) | 0.1 (0.1–0.3) | 0.1 (0.1–0.3) | 0.300 a |
| IOP | 14 (13–14.75) | 13.5 (11–15.75) | 0.079 a |
| CCT | 534.5 (526–541.75) | 531 (525–539) | 0.179 a |
| Lens status | 0.054 b | ||
| Phakic | 40 (50.0%) | 53 (66.3%) | |
| Pseudophakic | 40 (50.0%) | 27 (33.7%) | |
| Treatment characteristics | Abiraterone: 14 (17.5%), Enzalutamide: 66 (82.5%) | - |
| Patient Group (n = 80) | Control Group (n = 80) | p a | |
|---|---|---|---|
| Macular nerve fiber layer thickness (mNFL), µm | |||
| mNFL outer superior | 22 (16–37.75) | 29 (24–36) | 0.003 |
| mNFL outer inferior | 24 (19.25–43.75) | 29 (24.25–34.75) | 0.432 |
| mNFL outer temporal | 19 (17–21) | 19 (17–21.75) | 0.624 |
| mNFL outer nasal | 19 (18–21) | 28.5 (16–59) | 0.058 |
| mNFL inner superior | 30 (24–32) | 64 (55–74) | <0.001 |
| mNFL inner inferior | 27 (22–32.75) | 32 (25.25–35) | 0.003 |
| mNFL inner temporal | 18 (16–20) | 31 (30–33) | <0.001 |
| mNFL inner nasal | 24 (19.25–34) | 26 (24.25–27.75) | 0.176 |
| mNFL central | 17 (14–18) | 29 (26–32) | <0.001 |
| Ganglion cell layer thickness (GCL), µm | |||
| GCL outer superior | 30 (25–48) | 48 (41–54) | <0.001 |
| GCL outer inferior | 27 (23–52) | 45 (41–48) | <0.001 |
| GCL outer temporal | 28.5 (22.25–35) | 41 (34–46) | <0.001 |
| GCL outer nasal | 33 (29–37.75) | 47 (44–51) | <0.001 |
| GCL inner superior | 44 (39.25–48.75) | 52.5 (44.25–56) | <0.001 |
| GCL inner inferior | 45 (39–49) | 52 (43.5–56) | <0.001 |
| GCL inner temporal | 40 (33.25–48) | 44.5 (40–48.75) | 0.011 |
| GCL inner nasal | 45 (40.25–50) | 45.5 (40–51) | 0.973 |
| GCL central | 36.5 (24–41.75) | 45 (40–48) | <0.001 |
| Inner plexiform layer thickness (IPL), µm | |||
| IPL outer superior | 26 (22–42) | 38 (33–40) | <0.001 |
| IPL outer inferior | 27.5 (20–41.75) | 34.5 (32–37) | 0.059 |
| IPL outer temporal | 31.5 (27–36) | 33 (29–35) | 0.762 |
| IPL outer nasal | 28 (24–34) | 34 (29–35) | <0.001 |
| IPL inner superior | 34 (30–36) | 41 (35–44) | <0.001 |
| IPL inner inferior | 33 (30–34) | 41 (38–44) | <0.001 |
| IPL inner temporal | 34 (29.25–36) | 36 (32.25–41) | <0.001 |
| IPL inner nasal | 32 (28–38) | 36 (32–42) | <0.001 |
| IPL central | 32.5 (26.25–37) | 39 (37–40.75) | <0.001 |
| Inner nuclear layer thickness (INL), µm | |||
| INL outer superior | 33 (27–39) | 35 (32–40) | <0.001 |
| INL outer inferior | 33 (32–35) | 33.5 (26–39) | <0.001 |
| INL outer temporal | 30 (28–33.75) | 37 (35–38) | <0.001 |
| INL outer nasal | 32 (30–35) | 36.5 (32–38) | <0.001 |
| INL inner superior | 34 (32–35) | 42 (38–45) | <0.001 |
| INL inner inferior | 32 (31–34) | 41 (38–44) | <0.001 |
| INL inner temporal | 33 (31.25–35) | 36 (33–41) | <0.001 |
| INL inner nasal | 32 (31–34) | 39 (35–43) | <0.001 |
| INL central | 33 (32–35) | 35 (28.5–40) | 0.016 |
| Outer plexiform layer thickness (OPL), µm | |||
| OPL outer superior | 26.5 (24–29.75) | 29 (28–31) | 0.160 |
| OPL outer inferior | 26 (24–29) | 27 (25–29) | 0.142 |
| OPL outer temporal | 26 (25–28) | 27 (25.25–29) | <0.001 |
| OPL outer nasal | 28 (25–29) | 28 (26.25–31) | <0.001 |
| OPL inner superior | 27 (25–28) | 31 (29–37) | <0.001 |
| OPL inner inferior | 26 (25–28) | 31 (28–36.75) | <0.001 |
| OPL inner temporal | 27 (25–29) | 31 (27–35) | <0.001 |
| OPL inner nasal | 28 (25–29) | 31 (28–35) | <0.001 |
| OPL central | 27 (25–28) | 33.5 (28–40.75) | <0.001 |
| Outer nuclear layer thickness (ONL), µm | |||
| ONL outer superior | 60 (50.25–70.25) | 68 (65–69) | <0.001 |
| ONL outer inferior | 55 (47–70) | 58 (55–61.75) | 0.199 |
| ONL outer temporal | 53 (48–60) | 59 (55–63) | <0.001 |
| ONL outer nasal | 58 (53.25–62) | 59 (46.25–74) | 0.827 |
| ONL inner superior | 59 (55–61) | 65.5 (55–75.75) | <0.001 |
| ONL inner inferior | 58 (55–60.75) | 70 (60–77) | <0.001 |
| ONL inner temporal | 59 (55–62) | 69.5 (60–79.75) | <0.001 |
| ONL inner nasal | 58 (55–63) | 64 (56.25–73.75) | 0.001 |
| ONL central | 59 (55–62) | 85.5 (75.25–95) | <0.001 |
| Retina pigment epithelium thickness (RPE), µm | |||
| RPE outer superior | 13 (12–14) | 16 (15–17) | <0.001 |
| RPE outer inferior | 12 (11–13) | 15 (14–16) | <0.001 |
| RPE outer temporal | 12 (11–13) | 15 (15–16) | <0.001 |
| RPE outer nasal | 13 (11–15) | 15 (15–16) | <0.001 |
| RPE inner superior | 14 (13–15) | 16 (15–16.75) | <0.001 |
| RPE inner inferior | 14 (13–15) | 16 (15–17) | <0.001 |
| RPE inner temporal | 14 (13–15) | 16 (15–17) | <0.001 |
| RPE inner nasal | 13.5 (13–15) | 16 (15–16) | <0.001 |
| RPE central | 15 (14–16) | 16 (15–17) | 0.064 |
| Patient Group (n = 80) | Control Group (n = 80) | p a | |
|---|---|---|---|
| Peripapillary retinal nerve fiber layer thickness (pRNFL), µm | |||
| pRNFL-TS | 126 (119–137.5) | 128 (116–143) | 0.777 |
| pRNFL-NS | 106.5 (95–120) | 120 (102.5–139.5) | <0.001 |
| pRNFL-N | 78.5 (68.5–95) | 86.5 (79–89) | 0.160 |
| pRNFL-NI | 120.5 (104.25–134.25) | 132 (122–135) | 0.005 |
| pRNFL-TI | 135 (124.25–138) | 137 (123.25–155.5) | 0.047 |
| pRNFL-T | 75 (67.25–86.5) | 85 (79–89) | 0.001 |
| pRNFL-G | 103 (97.25–110) | 117 (112–121) | <0.001 |
| Minimum rim width (MRW), µm | |||
| MRW-TS | 320 (260–374.25) | 397 (355–425) | <0.001 |
| MRW-NS | 356.5 (265.75–407.75) | 421 (353–521) | <0.001 |
| MRW-N | 324.5 (266.5–396.5) | 410.50(344.50–433.00) | <0.001 |
| MRW-NI | 403.5 (357.75–452.5) | 469.00(409.00–522.00) | <0.001 |
| MRW-TI | 352 (287.5–411) | 421.00(325.00–449.00) | <0.001 |
| MRW-T | 249.5 (219.5–345.75) | 250.00(195.00–285.00) | 0.035 |
| MRW-G | 338.5 (285.75–365.75) | 388.5 (316.25–424.75) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakkal Temi, Y.; Yılmaz Tuğan, B.; Çıtakkul, İ.; Baydar, E.; Karaca, G.; Balcı, S.; Çabuk, D.; Kefeli, U.; Yüksel, N.; Uygun, K. Structural Retinal and Optic Nerve Changes in Prostate Cancer Patients Receiving Androgen Receptor Pathway Inhibitors: An OCT-Based In Vivo Analysis. Diagnostics 2025, 15, 1682. https://doi.org/10.3390/diagnostics15131682
Bakkal Temi Y, Yılmaz Tuğan B, Çıtakkul İ, Baydar E, Karaca G, Balcı S, Çabuk D, Kefeli U, Yüksel N, Uygun K. Structural Retinal and Optic Nerve Changes in Prostate Cancer Patients Receiving Androgen Receptor Pathway Inhibitors: An OCT-Based In Vivo Analysis. Diagnostics. 2025; 15(13):1682. https://doi.org/10.3390/diagnostics15131682
Chicago/Turabian StyleBakkal Temi, Yasemin, Büşra Yılmaz Tuğan, İlkay Çıtakkul, Ece Baydar, Gözde Karaca, Sibel Balcı, Devrim Çabuk, Umut Kefeli, Nurşen Yüksel, and Kazım Uygun. 2025. "Structural Retinal and Optic Nerve Changes in Prostate Cancer Patients Receiving Androgen Receptor Pathway Inhibitors: An OCT-Based In Vivo Analysis" Diagnostics 15, no. 13: 1682. https://doi.org/10.3390/diagnostics15131682
APA StyleBakkal Temi, Y., Yılmaz Tuğan, B., Çıtakkul, İ., Baydar, E., Karaca, G., Balcı, S., Çabuk, D., Kefeli, U., Yüksel, N., & Uygun, K. (2025). Structural Retinal and Optic Nerve Changes in Prostate Cancer Patients Receiving Androgen Receptor Pathway Inhibitors: An OCT-Based In Vivo Analysis. Diagnostics, 15(13), 1682. https://doi.org/10.3390/diagnostics15131682





