Utilizing Shear Wave Elastography for the Evaluation of Ocular Involvement in Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Ophthalmological Examination
2.3. Orbital SWE Examination
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asano, Y. The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies. J. Clin. Med. 2020, 9, 2687. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Mishra, D.; Singh, T.R.R. Epidemiology of ocular manifestations in autoimmune disease. Front. Immunol. 2021, 12, 744396. [Google Scholar] [CrossRef]
- Desbois, A.C.; Cacoub, P. Systemic sclerosis: An update in 2016. Autoimmun. Rev. 2016, 15, 417–426. [Google Scholar] [CrossRef]
- Lepri, G.; Orlandi, M.; Di Battista, M.; De Mattia, G.; Da Rio, M.; Codullo, V.; Guiducci, S.; Della Rossa, A. Systemic sclerosis: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 1911–1920. [Google Scholar] [CrossRef]
- Esen, E.; Tas, D.A.; Sizmaz, S.; Turk, I.; Unal, I.; Demircan, N. Evaluating Choroidal Characteristics in Systemic Sclerosis Using Enhanced Depth Imaging Optical Coherence Tomography. Ocul. Immunol. Inflamm. 2017, 25, 356–362. [Google Scholar] [CrossRef]
- Shenavandeh, S.; Afarid, M.; Hasanaghaei, T.; Nazarinia, M.A. Prevalence of retinal changes in patients with systemic sclerosis: The association between retinal vascular changes and nailfold capillaroscopic findings. Reumatologia 2021, 59, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Paczwa, K.; Rerych, M.; Romanowska-Próchnicka, K.; Różycki, R.; Gołębiewska, J. Ocular Manifestation in Systemic Sclerosis—A Literature Review. Life 2024, 14, 627. [Google Scholar] [CrossRef]
- Kök, M.; Ayan, A.; Küçük, M.F.; Erol, M.K.; Yaprak, L. Evaluation of the direct effects on retinal and choroidal microvascularity of systemic scleroderma. Microvasc. Res. 2021, 136, 104166. [Google Scholar] [CrossRef]
- Kreps, E.O.; Carton, C.; Cutolo, M.; Cutolo, C.A.; Vanhaecke, A.; Leroy, B.P.; Smith, V. Ocular involvement in systemic sclerosis: A systematic literature review, it’s not all scleroderma that meets the eye. Semin. Arthritis Rheum. 2019, 49, 119–125. [Google Scholar] [CrossRef]
- Zemanova, M. Shear wave elastography in ophthalmic diagnosis. J. Fr. Ophtalmol. 2019, 42, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.W.; Li, K.N.; Yi, A.J.; Wang, B.; Wei, Q.; Wu, G.G.; Dietrich, C.F. Ultrasound elastography. Endosc. Ultrasound 2022, 11, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Dong, Y.; Cui, X.-W.; Fink, M.; Jenssen, C.; Moeller, K.; Sandrin, L.; Tsuneyoshi, S.; Tanter, M. Ultrasound elastography: A brief clinical history of an evolving. 2024. Ultrasound Int. Open 2024, 10, a23786926. [Google Scholar] [PubMed]
- Detorakis, E.T.; Drakonaki, E.E.; Tsilimbaris, M.K.; Pallikaris, I.G.; Giarmenitis, S. Real-time ultrasound elastographic imaging of ocular and periocular tissues: A feasibility study. Ophthalmic Surg. Lasers Imaging Retin. 2010, 41, 135–141. [Google Scholar] [CrossRef]
- Dikici, A.S.; Mihmanli, I.; Kilic, F.; Ozkok, A.; Kuyumcu, G.; Sultan, P.; Samanci, C.; Yilmaz, M.H.; Rafiee, B.; Tamcelik, N. In vivo evaluation of the biomechanical properties of optic nerve and peripapillary structures by ultrasonic shear wave elastography in glaucoma. Iran. J. Radiol. 2016, 13, e36849. [Google Scholar] [CrossRef]
- İnal, M.; Tan, S.; Yumusak, E.M.; Şahan, M.H.; Alpua, M.; Örnek, K. Evaluation of the optic nerve using strain and shear wave elastography in patients with multiple sclerosis and healthy subjects. Med. Ultrason. 2017, 19, 39–44. [Google Scholar] [CrossRef]
- Batur, M.; Batur, A.; Çilingir, V.; Seven, E.; Çinal, A.; Bora, A.; Yaşar, T. Ultrasonic elastography evaluation in optic neuritis. Semin. Ophthalmol. 2018, 33, 237–241. [Google Scholar] [CrossRef]
- Singla, R.K.; Kadatz, M.; Rohling, R.; Nguan, C. Kidney ultrasound for nephrologists: A review. Kidney Med. 2022, 4, 100464. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Cappelli, L.; Wigley, F.M. Management of Raynaud Phenomenon and Digital Ulcers in scleroderma. Rheum. Dis. Clin. N. Am. 2015, 41, 419–438. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Gomes Bde, A.; Santhiago, M.R.; Magalhães, P.; Kara-Junior, N.; Azevedo, M.N.; Moraes, H.V., Jr. Ocular findings in patients with systemic sclerosis. Clinics 2011, 66, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Can, M.E.; Unal, Ö.; Kars, M.E.; Erten, S.; Can, G.D.; Duru, N.; Cagil, N. An assessment of ocular elasticity using real time ultrasound and ocular response analyzer in active or remission rheumatoid arthritis. Int. Ophthalmol. 2019, 39, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Özen, Ö.; Özer, M.A.; Tosun, A.; Özen, S. Evaluation of the optic nerve and scleral-choroidal-retinal layer with ultrasound elastography in glaucoma and physiological optic nerve head cupping. Med. Ultrason. 2018, 1, 76–79. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, C.-H. Study of the optic nerve in patients with type 2 diabetic retinopathy by shear-wave elastography. Clin. Radiol. 2024, 79, e574–e581. [Google Scholar] [CrossRef]
- Santiago, S.; Enwereji, N.; Jiang, C.; Durrani, K.; Chaudhry, S.; Lu, J. Ocular and eyelid involvement in collagen vascular diseases. Part II. Dermatomyositis, scleroderma, and sarcoidosis. Clin. Dermatol. 2024, 42, 9–16. [Google Scholar] [CrossRef]
- Helm, L.A.; Clebak, K.T.; Foulke, G.; Helm, M. Autoimmune Skin Conditions: Systemic Sclerosis. FP Essent. 2023, 526, 18–24. [Google Scholar] [PubMed]
- Raciborska, A.; Pieklarz, B.; Gińdzieńska-Sieśkiewicz, E.; Zonenberg, A.; Kowal-Bielecka, O.; Konopińska, J.; Dmuchowska, D.A.; Gi, E. Assessment of interocular symmetry of choroidal vascularity index and thickness in patients with systemic sclerosis: A prospective study. Front. Med. 2025, 11, 1513679. [Google Scholar] [CrossRef]
- Carlà, M.M.; Gambini, G.; Caporossi, T.; Giannuzzi, F.; Boselli, F.; Crincoli, E.; Ripa, M.; Rizzo, S. Ocular Involvement in Systemic Sclerosis: Updated Review and New Insights on Microvascular Impairment. Ocul. Immunol. Inflamm. 2024, 32, 2209–2216. [Google Scholar] [CrossRef]
- Waszczykowska, A.; Goś, R.; Waszczykowska, E.; Dziankowska-Bartkowiak, B.; Jurowski, P. Prevalence of ocular manifestations in systemic sclerosis patients. Arch. Med. Sci. 2013, 9, 1107–1113. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Gualtierotti, R.; Pierro, L.; Del Turco, C.; Miserocchi, E.; Schioppo, T.; Meroni, P.L.; Gagliardi, M.; Modorati, G.; Querques, G. Choroidal impairment and macular thinning in patients with systemic sclerosis: The acute study. Microvasc. Res. 2015, 97, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Rothe, M.; Klapa, S.; Lange, T.; Prasuhn, M.; Grisanti, S.; Riemekasten, G.; Humrich, J. Evaluation of choroidal substructure perfusion in patients affected by systemic sclerosis: An optical coherence tomography angiography study. Scand. J. Rheumatol. 2020, 49, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, N.; Lahme, L.; Braasch, S.; Rosenberger, F.; Eter, N.; Ehrchen, J.; Alnawaiseh, M. Altered ocular microvasculature in patients with systemic sclerosis and very early disease of systemic sclerosis using optical coherence tomography angiography. Sci. Rep. 2022, 12, 10990. [Google Scholar] [CrossRef]
- Szucs, G.; Szekanecz, Z.; Aszalos, Z.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; Kemeny-Beke, A. A wide spectrum of ocular manifestations signify patients with systemic sclerosis. Ocul. Immunol. Inflamm. 2021, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Unal, O.; Cay, N.; Yulek, F.; Taslipinar, A.G.; Bozkurt, S.; Gumus, M. Real-time ultrasound elastographic features of primary open angle glaucoma. Ultrasound Q. 2016, 32, 333–337. [Google Scholar] [CrossRef]
- Agladioglu, K.; Pekel, G.; Altintas Kasikci, S.; Yagci, R.; Kiroglu, Y. An evaluation of ocular elasticity using real-time ultrasound elastography in primary open-angle glaucoma. Br. J. Radiol. 2016, 89, 20150429. [Google Scholar] [CrossRef]
- Hekimoğlu, A.; Ergun, O.; Dogan, A.Ş.; Ergun, Ş.B.; Acar, M.; Gürdal, C.; Hekimoğlu, B. Shear wave elastography findings in glaucoma patients. Kocatepe Tıp Derg. 2023, 24, 1–7. [Google Scholar] [CrossRef]
- Srinivasan, A.; Milman, T.; Lane, K.A.; Bilyk, J.R. Pathology of the orbit: Inflammations and infections. In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Bahn, R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef]
- Karesh, J.W.; On, A.V.; Hirschbein, M.J. Noninfectious orbital inflammatory disease. In Du Ane’s Ophthalmology, 15th ed.; Tasman, W., Jaeger, E.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 245–296. [Google Scholar]
- Tobe, L.A.; Harris, A.; Hussain, R.M.; Eckert, G.; Huck, A.; Park, J.; Egan, P.; Kim, N.J.; Siesky, B. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br. J. Ophthalmol. 2015, 99, 609–612. [Google Scholar] [CrossRef]
- Unsworth, S.P.; Heisel, C.J.; Tingle, C.F.; Rajesh, N.; Kish, P.E.; Kahana, A. Retinoic acid potentiates orbital tissues for inflammation through NF-κB and MCP-1. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef] [PubMed]
- Gontarz-Nowak, K.; Szychlińska, M.; Matuszewski, W.; Stefanowicz-Rutkowska, M.; Bandurska-Stankiewicz, E. Current knowledge on Graves’ orbitopathy. J. Clin. Med. 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Perelas, A.; Silver, R.M.; Arrossi, A.; Highland, K.B. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 2020, 8, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, J.G.; Denton, C.P.; Grünig, E.; Bonderman, D.; Distler, O.; Khanna, D.; Müller-Ladner, U.; E Pope, J.; Vonk, M.C.; Doelberg, M.; et al. DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann. Rheum. Dis. 2014, 73, 1340–1349. [Google Scholar] [CrossRef]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers. 2015, 1, 15002. [Google Scholar] [CrossRef]
- Morrisroe, K.; Stevens, W.; Sahhar, J.; Ngian, G.-S.; Ferdowsi, N.; Hill, C.L.; Roddy, J.; Walker, J.; Proudman, S.; Nikpour, M. Digital ulcers in systemic sclerosis: Their epidemiology, clinical characteristics, and associated clinical and economic burden. Arthritis Res. Ther. 2019, 21, 299. [Google Scholar] [CrossRef]
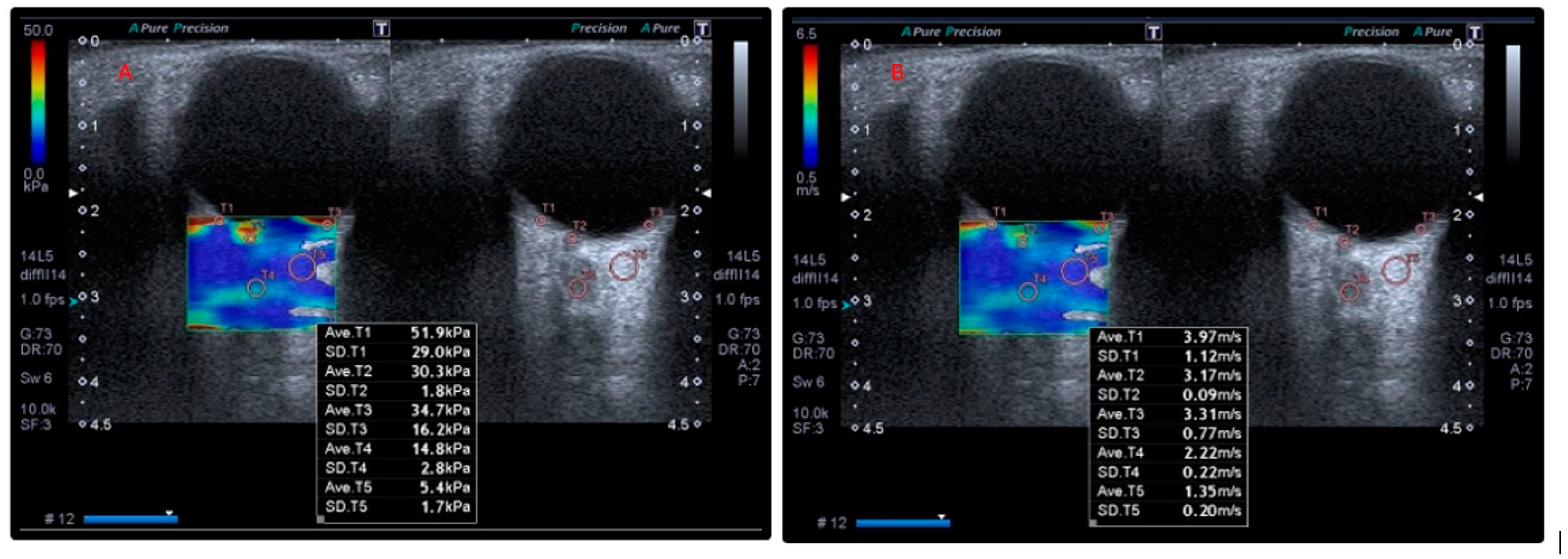
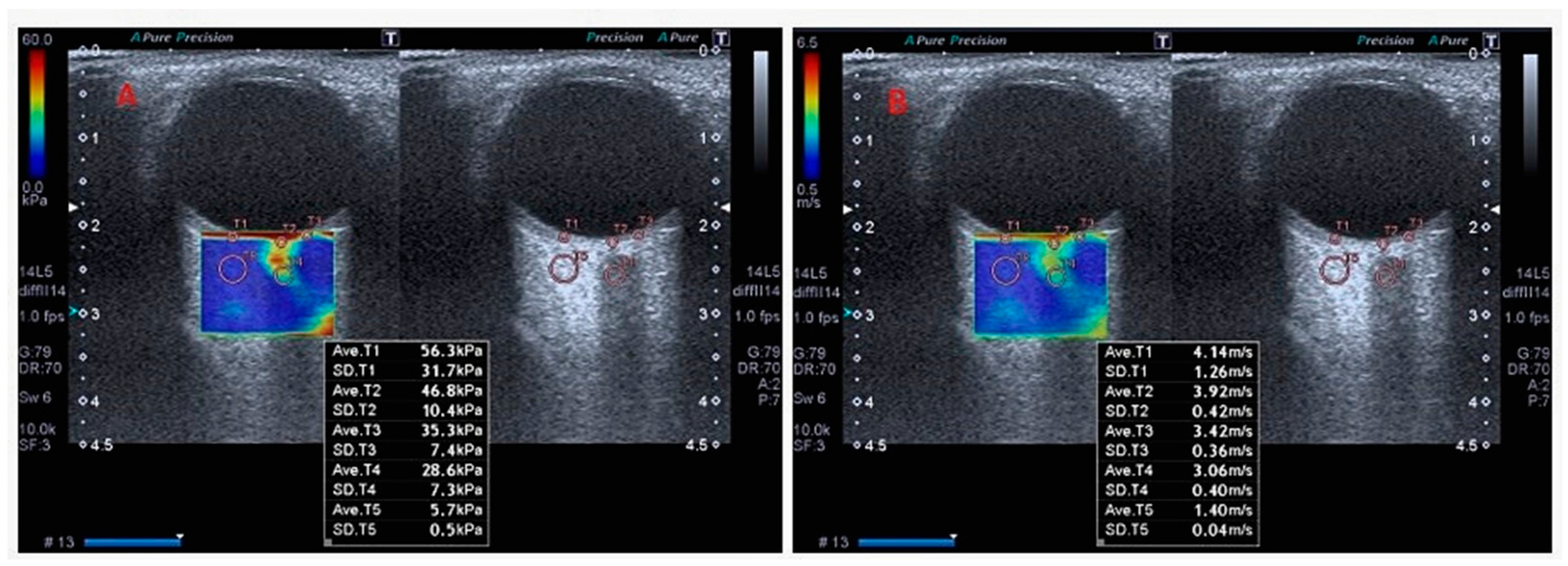
| Parameters | Patients | Control | t/x2 | p * | ||
|---|---|---|---|---|---|---|
| ± S | Lower-Upper | ± SD | Lower-Upper | |||
| Age, years | 51.21 ± 13.06 | 21–71 | 50.37 ± 14.37 | 20–70 | 0.44 | 0.515 |
| BMI, kg/m2 | 25.86 ± 3.58 | 19.81–34.21 | 25.54 ± 3.18 | 21.22–32.05 | 0.36 | 0.717 |
| FVC | 90.34 ± 12.52 | 62–100 | - | - | - | |
| DLCO | 91.00 ± 13.52 | 60–102 | - | - | - | - |
| Post-first RP disease duration, month | 41.31 ± 27.99 | 12–140 | - | - | - | - |
| Post-non-Raynaud’s symptoms disease duration, month | 28.34 ± 26.71 | 4–128 | - | - | - | - |
| Gender | Female = 27 | Male = 2 | Female = 26 | Male = 4 | 0.67 | 0.413 |
| Disease Type | Diffuse = 22 | Limited = 7 | - | - | - | - |
| PAD | Yes = 1 | None = 28 | - | - | - | - |
| ILD | Yes = 5 | None = 24 | - | - | - | - |
| DU | Yes = 16 | None = 9 | - | - | - | - |
| Antibody Type | anti-Scl-70 = 8 | Anti centromere = 21 | - | - | - | - |
| Reflux | Yes = 15 | None = 14 | - | - | - | - |
| PHT | Yes = 6 | None = 23 | - | - | - | - |
| Measurement Variables | Mean Value (Mean) | Mean Standard Deviation (SD) | Mean Coefficient of Variation (CV, %) |
|---|---|---|---|
| Elasticity RCS | 94.34 | 1.31 | 1.42 |
| Elasticity OD | 60.57 | 0.22 | 0.49 |
| Elasticity ON | 19.22 | 0.16 | 1.02 |
| Elasticity RBF | 7.39 | 0.10 | 1.50 |
| Velocity RCS | 5.72 | 0.05 | 0.84 |
| Velocity OD | 4.32 | 0.03 | 0.68 |
| Velocity ON | 2.56 | 0.02 | 0.68 |
| Velocity RBF | 1.51 | 0.01 | 0.68 |
| Elastography Parameters | Subgroup | N | ± SD | t | p * | D |
|---|---|---|---|---|---|---|
| Elasticity RCS | Patient | 29 | 91.62 ± 24.34 | 1.36 | 0.179 | - |
| Control | 30 | 102.50 ± 35.83 | ||||
| Elasticity OD | Patient | 29 | 60.37 ± 35.59 | 0.06 | 0.956 | - |
| Control | 30 | 60.88 ± 35.18 | ||||
| Elasticity ON | Patient | 29 | 19.67 ± 9.27 | 0.39 | 0.698 | - |
| Control | 30 | 18.86 ± 6.39 | ||||
| Elasticity RBF | Patient | 29 | 8.78 ± 2.75 | 4.74 | <0.001 * | 1.23 |
| Control | 30 | 6.08 ± 1.48 | ||||
| Velocity RCS | Patient | 29 | 5.61 ± 0.95 | 0.62 | 0.538 | - |
| Control | 30 | 5.78 ± 1.15 | ||||
| Velocity OD | Patient | 29 | 4.31 ± 1.44 | 0.04 | 0.967 | - |
| Control | 30 | 4.32 ± 1.40 | ||||
| Velocity ON | Patient | 29 | 2.66 ± 0.63 | 1.44 | 0.156 | - |
| Control | 30 | 2.46 ± 0.41 | ||||
| Velocity RBF | Patient | 29 | 1.60 ± 0.37 | 2.13 | 0.037 * | 0.53 |
| Control | 30 | 1.44 ± 0.21 |
| Elastography Parameters | Subgroup | N | ± SD | t | p * |
|---|---|---|---|---|---|
| Elasticity RCS | Diffuse | 22 | 91.10 ± 24.07 | 0.20 | 0.841 |
| Limited | 7 | 93.27 ± 27.09 | |||
| Elasticity OD | Diffuse | 22 | 61.09 ± 35.09 | 0.19 | 0.852 |
| Limited | 7 | 58.13 ± 39.89 | |||
| Elasticity ON | Diffuse | 22 | 19.36 ± 9.97 | 0.31 | 0.762 |
| Limited | 7 | 20.61 ± 7.15 | |||
| Elasticity RBF | Diffuse | 22 | 8.56 ± 1.91 | 0.76 | 0.456 |
| Limited | 7 | 9.47 ± 4.66 | |||
| Velocity RCS | Diffuse | 22 | 5.57 ± 0.96 | 0.37 | 0.718 |
| Limited | 7 | 5.73 ± 0.97 | |||
| Velocity OD | Diffuse | 22 | 4.45 ± 1.54 | 0.97 | 0.340 |
| Limited | 7 | 3.84 ± 1.07 | |||
| Velocity ON | Diffuse | 22 | 2.69 ± 0.69 | 0.45 | 0.657 |
| Limited | 7 | 2.56 ± 0.44 | |||
| Velocity RBF | Diffuse | 22 | 1.60 ± 0.40 | 0.04 | 0.972 |
| Limited | 7 | 1.61 ± 0.28 |
| Scale | FVC | DLCO | Post-First RP Disease Duration | Post-Non-Raynaud’s Symptoms Disease Duration |
|---|---|---|---|---|
| Elasticity RCS | −0.30 | −0.22 | 0.22 | 0.15 |
| Elasticity OD | −0.22 | −0.18 | −0.01 | −0.06 |
| Elasticity ON | −0.12 | −0.09 | −0.06 | −0.04 |
| Elasticity RBF | 0.02 | 0.05 | −0.10 | −0.15 |
| Velocity RCS | −0.33 | −0.28 | −0.05 | −0.11 |
| Velocity OD | −0.12 | −0.06 | −0.13 | −0.11 |
| Velocity ON | 0.03 | 0.04 | −0.20 | −0.15 |
| Velocity RBF | −0.06 | 0.01 | −0.28 | −0.32 |
| Elastography Parameters | Subgroup | N | ± SD | T | p * | d |
|---|---|---|---|---|---|---|
| Elasticity RCS | Yes | 15 | 95.77 ± 27.19 | 0.95 | 0.352 | - |
| None | 14 | 87.18 ± 20.95 | ||||
| Elasticity OD | Yes | 15 | 76.00 ± 36.88 | 2.71 | 0.012 * | 1.01 |
| None | 14 | 43.63 ± 26.06 | ||||
| Elasticity ON | Yes | 15 | 21.43 ± 7.66 | 1.06 | 0.298 | - |
| None | 14 | 17.78 ± 10.69 | ||||
| Elasticity RBF | Yes | 15 | 8.72 ± 3.29 | 0.13 | 0.901 | - |
| None | 14 | 8.85 ± 2.15 | ||||
| Velocity RCS | Yes | 15 | 5.80 ± 0.99 | 1.13 | 0.267 | - |
| None | 14 | 5.40 ± 0.89 | ||||
| Velocity OD | Yes | 15 | 4.88 ± 1.43 | 2.42 | 0.023 * | 0.90 |
| None | 14 | 3.69 ± 1.22 | ||||
| Velocity ON | Yes | 15 | 2.60 ± 0.50 | 0.49 | 0.627 | - |
| None | 14 | 2.72 ± 0.77 | ||||
| Velocity RBF | Yes | 15 | 1.54 ± 0.27 | 0.90 | 0.375 | - |
| None | 14 | 1.67 ± 0.45 |
| Elastography Parameters | Subgroup | N | ± SD | T | p * | D |
|---|---|---|---|---|---|---|
| Elasticity RCS | Yes | 16 | 104.41 ± 21.01 | 3.83 | 0.001 * | 0.87 |
| None | 13 | 75.88 ± 18.56 | ||||
| Elasticity OD | Yes | 16 | 71.04 ± 39.39 | 1.87 | 0.072 | |
| None | 13 | 47.24 ± 26.02 | ||||
| Elasticity ON | Yes | 16 | 22.23 ± 10.16 | 1.71 | 0.099 | - |
| None | 13 | 16.51 ± 7.19 | ||||
| Elasticity RBF | Yes | 16 | 8.98 ± 3.11 | 0.43 | 0.674 | - |
| None | 13 | 8.54 ± 2.32 | ||||
| Velocity RCS | Yes | 16 | 6.11 ± 0.77 | 3.87 | 0.001 * | 0.88 |
| None | 13 | 4.99 ± 0.78 | ||||
| Velocity OD | Yes | 16 | 4.68 ± 1.50 | 1.53 | 0.137 | - |
| None | 13 | 3.86 ± 1.29 | ||||
| Velocity ON | Yes | 16 | 2.83 ± 0.67 | 1.70 | 0.101 | - |
| None | 13 | 2.44 ± 0.53 | ||||
| Velocity RBF | Yes | 16 | 1.64 ± 0.32 | 0.55 | 0.56 | - |
| None | 13 | 1.56 ± 0.42 |
| Risk Factor | AUC (%95) | Cut Off | p * | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Elasticity RCS | 0.427 (0.279–0.577) | 94.25 | 0.336 | 44.8 | 43.3 |
| Elasticity OD | 0.483 (0.332–0.633) | 56.10 | 0.820 | 51.0 | 50.0 |
| Elasticity ON | 0.508 (0.358–0.658) | 18.75 | 0.915 | 52.0 | 53.0 |
| Elasticity RBF | 0.870 (0.782–0.952) | 7.35 | <0.001 * | 79.0 | 77.0 |
| Velocity RCS | 0.462 (0.313–0.611) | 5.67 | 0.617 | 48.0 | 50.0 |
| Velocity OD | 0.482 (0.332–0.632) | 4.37 | 0.814 | 52.0 | 50.0 |
| Velocity ON | 0.582 (0.434–0.730) | 2.50 | 0.278 | 59.0 | 57.0 |
| Velocity RBF | 0.623 (0.479–0.767) | 1.51 | 0.105 | 59.0 | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kök, M.; Ayan, A.; Arayici, M.E.; Ülgen, S. Utilizing Shear Wave Elastography for the Evaluation of Ocular Involvement in Systemic Sclerosis. Diagnostics 2025, 15, 1227. https://doi.org/10.3390/diagnostics15101227
Kök M, Ayan A, Arayici ME, Ülgen S. Utilizing Shear Wave Elastography for the Evaluation of Ocular Involvement in Systemic Sclerosis. Diagnostics. 2025; 15(10):1227. https://doi.org/10.3390/diagnostics15101227
Chicago/Turabian StyleKök, Mehmet, Ayşe Ayan, Mehmet Emin Arayici, and Sinan Ülgen. 2025. "Utilizing Shear Wave Elastography for the Evaluation of Ocular Involvement in Systemic Sclerosis" Diagnostics 15, no. 10: 1227. https://doi.org/10.3390/diagnostics15101227
APA StyleKök, M., Ayan, A., Arayici, M. E., & Ülgen, S. (2025). Utilizing Shear Wave Elastography for the Evaluation of Ocular Involvement in Systemic Sclerosis. Diagnostics, 15(10), 1227. https://doi.org/10.3390/diagnostics15101227






