Proinflammatory Cytokines in Women with PCOS in Atypical Pathogen Infections
Abstract
1. Introduction
1.1. Atypical Infections
1.2. Cytokines Response
2. Materials and Methods
2.1. Patient Group
2.2. Material
2.3. Methods
3. Results
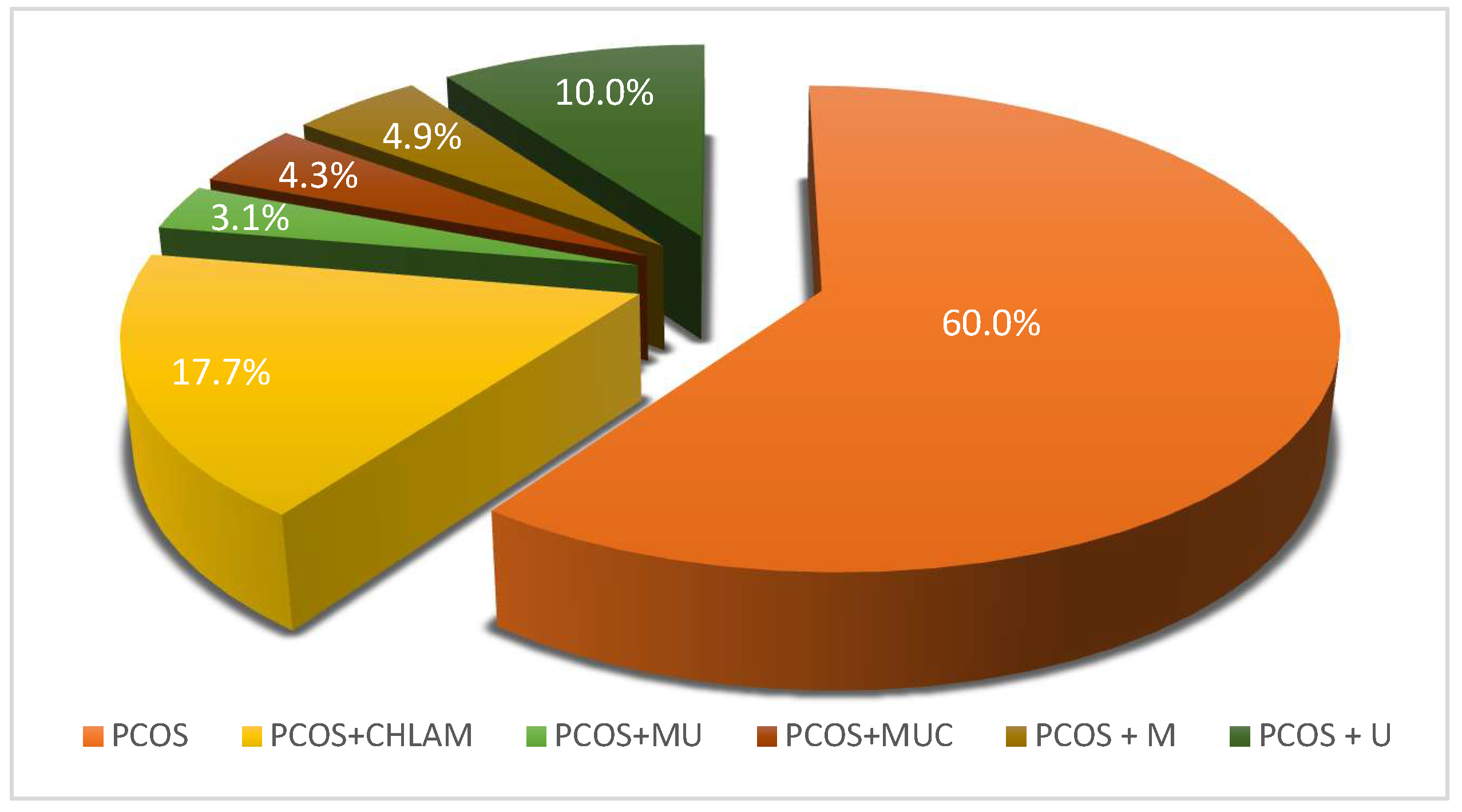
3.1. Assessment of the Prevalence of Atypical Pathogen Infections in a Study Population of Women with PCOS
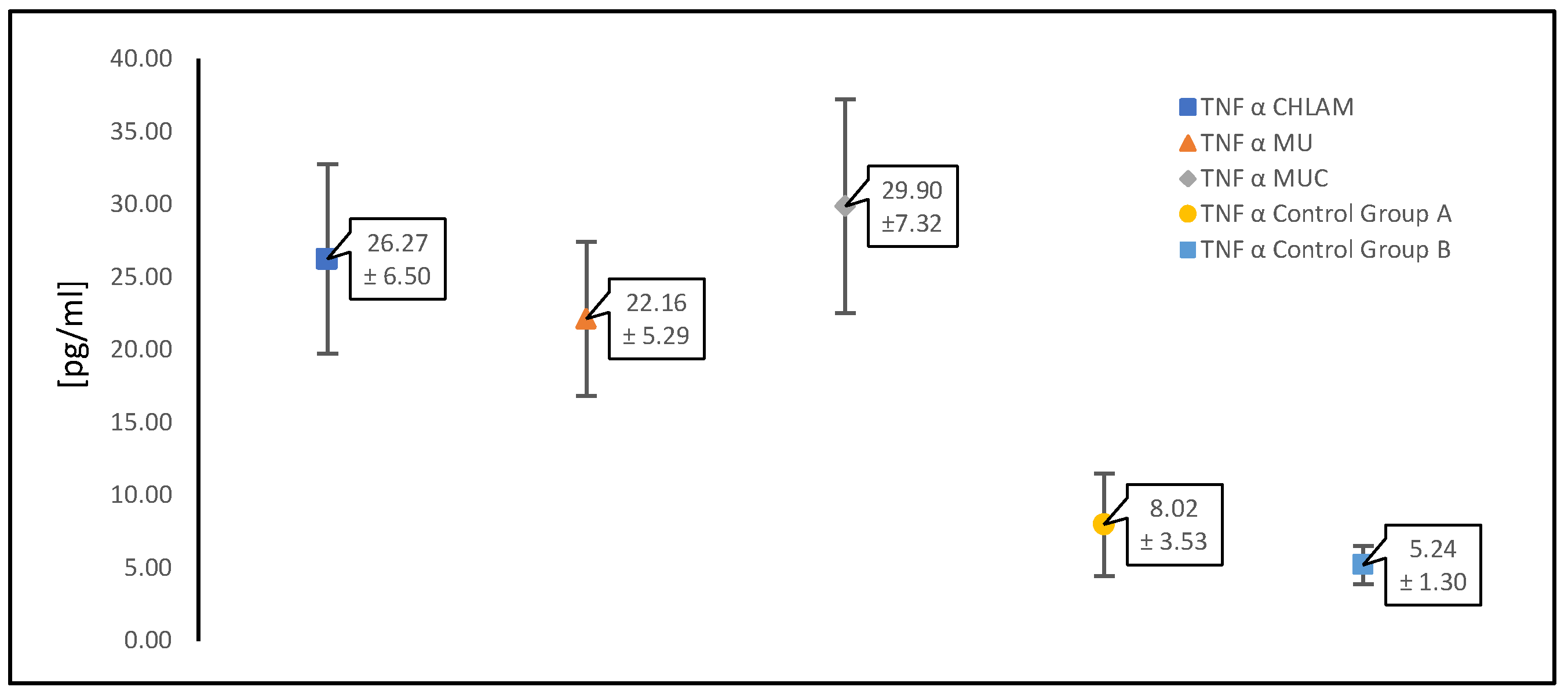
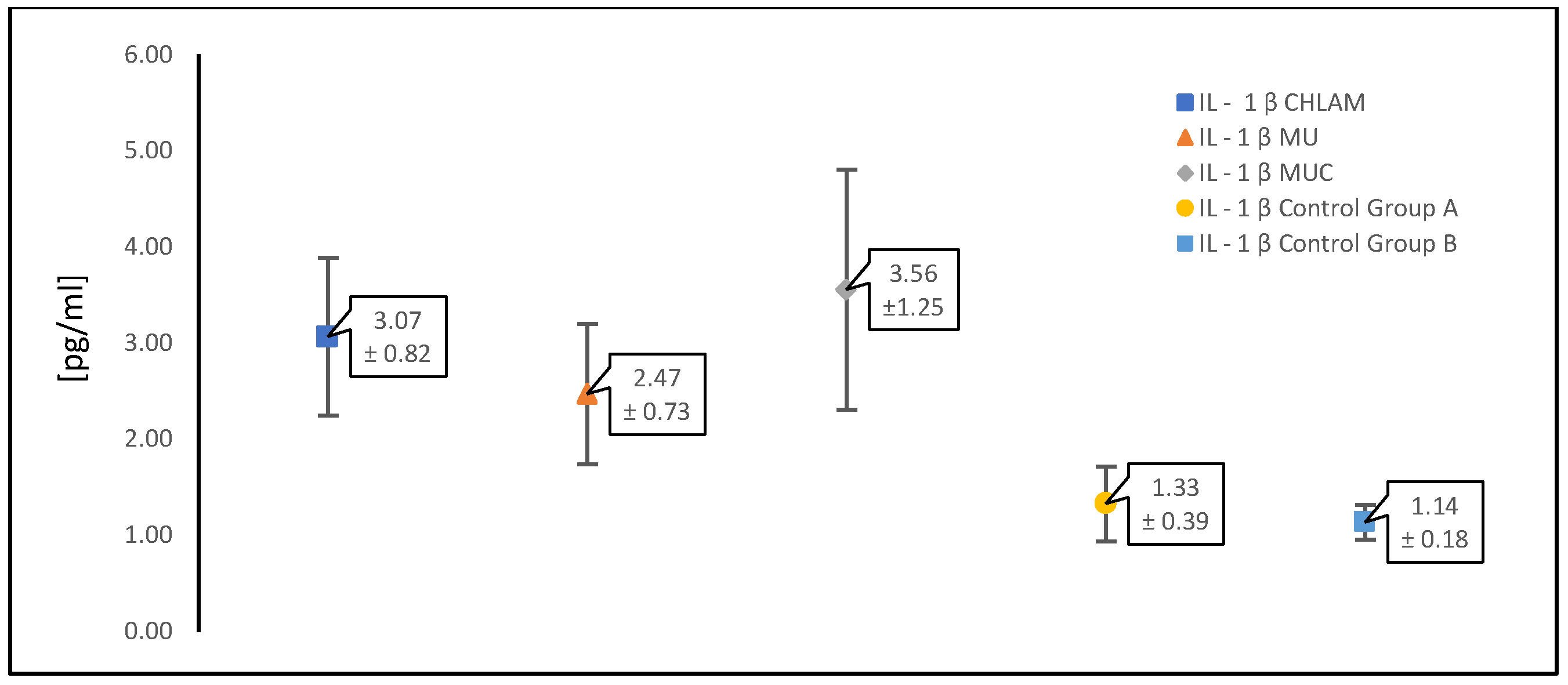
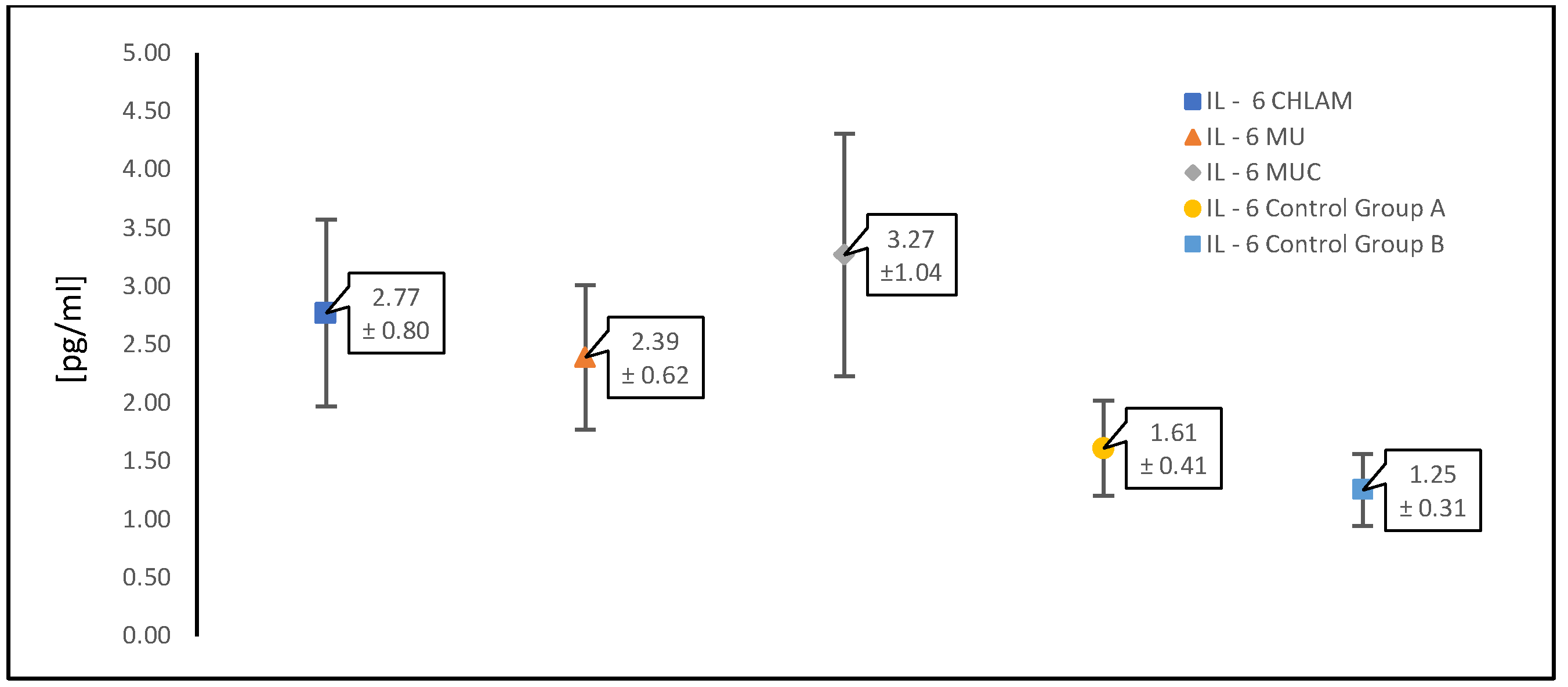
3.2. Evaluation of Cytokine (TNF-α, IL-1β and IL-6) Levels in a Study Population of Women with PCOS
| CHLAM (n = 87) | MU (n = 88) | MUC (n = 21) | Control Group A | Control Group B | |
|---|---|---|---|---|---|
| TNF α [pg/mL] | 26.27 ± 6.50 | 22.16 ± 5.29 | 29.90 ± 7.32 | 8.02 ± 3.53 | 5.24 ± 1.30 |
| IL-1 β [pg/mL] | 3.07 ± 0.82 | 2.47 ± 0.73 | 3.56 ± 1.25 | 1.33 ± 0.39 | 1.14 ± 0.18 |
| IL-6 [pg/mL] | 2.77 ± 0.80 | 2.39 ± 0.62 | 3.27 ± 1.04 | 1.61 ± 0.41 | 1.25 ± 0.31 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R. Polycystic ovary syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pan, J.; Liu, Y.; Meng, Q.; Lv, P.; Qu, F.; Ding, G.-L.; Klausen, C.; Leung, P.C.; Chan, H.C. Alternative splicing of the androgen receptor in polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Rodríguez-Tabernero, L.; Robles, A.; Muñoz, E.; Martínez, F.; Landeras, J.; García-Velasco, J.; Fontes, J.; Álvarez, M.; Álvarez, C. Polycystic ovary syndrome throughout a woman’s life. J. Assist. Reprod. Genet. 2018, 35, 25–39. [Google Scholar] [CrossRef]
- Hong, X.; Qin, P.; Huang, K.; Ding, X.; Ma, J.; Xuan, Y.; Zhu, X.; Peng, D.; Wang, B. Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin. Endocrinol. 2020, 93, 52–60. [Google Scholar] [CrossRef]
- Tu, Y.; Zheng, G.; Ding, G.; Wu, Y.; Xi, J.; Ge, Y.; Gu, H.; Wang, Y.; Sheng, J.; Liu, X. Comparative analysis of lower genital tract microbiome between PCOS and healthy women. Front. Physiol. 2020, 11, 1108. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of vaginal microbiota dysbiosis in gynecological diseases and the potential interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Yang, J.; Zhang, X.; Chen, Y.; Feng, R.; Qian, Y. Changes in vaginal microbiome diversity in women with polycystic ovary syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 755741. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef]
- Torcia, M.G. Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int. J. Mol. Sci. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef] [PubMed]
- Rostamtabar, M.; Esmaeilzadeh, S.; Tourani, M.; Rahmani, A.; Baee, M.; Shirafkan, F.; Saleki, K.; Mirzababayi, S.S.; Ebrahimpour, S.; Nouri, H.R. Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J. Cell. Physiol. 2021, 236, 824–838. [Google Scholar] [CrossRef]
- Luan, Y.-Y.; Zhang, L.; Peng, Y.-Q.; Li, Y.-Y.; Liu, R.-X.; Yin, C.-H. Immune regulation in polycystic ovary syndrome. Clin. Chim. Acta 2022, 531, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.S.; Temple-Smith, M.; Guy, R.; Donovan, B.; Braat, S.; Law, M.; Gunn, J.; Regan, D.; Vaisey, A.; Bulfone, L. Population effectiveness of opportunistic chlamydia testing in primary care in Australia: A cluster-randomised controlled trial. Lancet 2018, 392, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chalmydia. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/chlamydia (accessed on 5 May 2025).
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar]
- Rodrigues, R.; Silva, A.R.; Sousa, C.; Vale, N. Addressing Challenges in Chlamydia trachomatis Detection: A Comparative Review of Diagnostic Methods. Medicina 2024, 60, 1236. [Google Scholar] [CrossRef]
- Meyer, T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms 2016, 4, 25. [Google Scholar] [CrossRef]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Price, M.J.; Ades, A.; Soldan, K.; Welton, N.J.; Macleod, J.; Simms, I.; DeAngelis, D.; Turner, K.M.; Horner, P.J. The natural history of Chlamydia trachomatis infection in women: A multi-parameter evidence synthesis. Health Technol. Assess. 2016, 20, 1–250. [Google Scholar] [CrossRef]
- Gupta, R.S.; Sawnani, S.; Adeolu, M.; Alnajar, S.; Oren, A. Phylogenetic framework for the phylum Tenericutes based on genome sequence data: Proposal for the creation of a new order Mycoplasmoidales ord. nov., containing two new families Mycoplasmoidaceae fam. nov. and Metamycoplasmataceae fam. nov. harbouring Eperythrozoon, Ureaplasma and five novel genera. Antonie Van Leeuwenhoek 2018, 111, 1583–1630. [Google Scholar] [PubMed]
- Ariel, I.; Singer, D. Placental Pathologies–Intrauterine Infections; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Cunningham, S.A.; Mandrekar, J.N.; Rosenblatt, J.E.; Patel, R. Rapid PCR detection of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum. Int. J. Bacteriol. 2013, 2013, 168742. [Google Scholar] [CrossRef]
- Tam, P.C.; Alexander, B.D.; Lee, M.J.; Hardie, R.G.; Reynolds, J.M.; Haney, J.C.; Waites, K.B.; Perfect, J.R.; Baker, A.W. Donor-derived Mycoplasma and Ureaplasma infections in lung transplant recipients: A prospective study of donor and recipient respiratory tract screening and recipient outcomes. Am. J. Transplant. 2024, 24, 2258–2268. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Min, J.-W.; Kim, Y.-S. Positive culture rate and antimicrobial susceptibilities of Mycoplasma hominis and Ureaplasma urealyticum. Obstet. Gynecol. Sci. 2019, 62, 127–133. [Google Scholar] [CrossRef]
- Rottem, S. Interaction of mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Girón, J.A.; Lange, M.; Baseman, J.B. Adherence, fibronectin binding, and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect. Immun. 1996, 64, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Vallely, L.M.; Egli-Gany, D.; Pomat, W.; Homer, C.S.; Guy, R.; Wand, H.; Silver, B.; Rumbold, A.R.; Kaldor, J.M.; Low, N. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: A systematic review and meta-analysis protocol. BMJ Open 2018, 8, e024175. [Google Scholar] [CrossRef]
- Wren, B.W. Microbial genome analysis: Insights into virulence, host adaptation and evolution. Nat. Rev. Genet. 2000, 1, 30–39. [Google Scholar] [CrossRef]
- Chambaud, I.; Wróblewski, H.; Blanchard, A. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 1999, 7, 493–499. [Google Scholar] [CrossRef]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef]
- Wise, K.S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993, 1, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Pobeguts, O.V.; Galaymina, M.A.; Sikamov, K.V.; Urazaeva, D.R.; Avshalumov, A.S.; Mikhailycheva, M.V.; Babenko, V.V.; Smirnov, I.P.; Gorbachev, A.Y. Unraveling the adaptive strategies of Mycoplasma hominis through proteogenomic profiling of clinical isolates. Front. Cell. Infect. Microbiol. 2024, 14, 1398706. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.; Talkington, D. New developments in human diseases due to mycoplasmas. In Mycoplasmas: Molecular Biology, Pathogenicity, and Strategies for Control; CRC Press: Oxford, UK, 2005. [Google Scholar]
- Donders, G.G.; Ruban, K.; Bellen, G.; Petricevic, L. Mycoplasma/Ureaplasma infection in pregnancy: To screen or not to screen. J. Perinat. Med. 2017, 45, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Cooney, L.G.; Stanic, A.K. Immune dysfunction in polycystic ovary syndrome. Immunohorizons 2023, 7, 323–332. [Google Scholar] [CrossRef]
- Le Thuc, O.; Blondeau, N.; Nahon, J.L.; Rovère, C. The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Ann. N. Y. Acad. Sci. 2015, 1351, 127–140. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Rodriguez-Garcia, M.; Shen, Z.; Patel, M.V. Regulation of mucosal immunity in the female reproductive tract: The role of sex hormones in immune protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 2014, 72, 236–258. [Google Scholar] [CrossRef]
- Xiang, W.; Yu, N.; Lei, A.; Li, X.; Tan, S.; Huang, L.; Zhou, Z. Insights into host cell cytokines in Chlamydia infection. Front. Immunol. 2021, 12, 639834. [Google Scholar] [CrossRef]
- Buckner, L.R.; Lewis, M.E.; Greene, S.J.; Foster, T.P.; Quayle, A.J. Chlamydia trachomatis infection results in a modest pro-inflammatory cytokine response and a decrease in T cell chemokine secretion in human polarized endocervical epithelial cells. Cytokine 2013, 63, 151–165. [Google Scholar] [CrossRef]
- Noda-Nicolau, N.M.; Tantengco, O.A.G.; Polettini, J.; Silva, M.C.; Bento, G.F.; Cursino, G.C.; Marconi, C.; Lamont, R.F.; Taylor, B.D.; Silva, M.G. Genital mycoplasmas and biomarkers of inflammation and their association with spontaneous preterm birth and preterm prelabor rupture of membranes: A systematic review and meta-analysis. Front. Microbiol. 2022, 13, 859732. [Google Scholar] [CrossRef]
- Silwedel, C.; Speer, C.P.; Haarmann, A.; Fehrholz, M.; Claus, H.; Schlegel, N.; Glaser, K. Ureaplasma species modulate cytokine and chemokine responses in human brain microvascular endothelial cells. Int. J. Mol. Sci. 2019, 20, 3583. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Poku, V.O. Maternal mortality: The role of mycoplasma hominis and its impact on neonatal health. Health Sci. Rev. 2022, 4, 100036. [Google Scholar] [CrossRef]
- Honkila, M.; Renko, M.; Pokka, T.; Wikström, E.; Uhari, M.; Tapiainen, T. Symptoms, signs and long-term prognosis of vertically transmitted Chlamydia trachomatis infections. Pediatr. Infect. Dis. J. 2018, 37, 930–933. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.D.; Escarcega-Tame, M.A.; López-Hurtado, M.; Flores-Salazar, V.R.; Escobedo-Guerra, M.R.; Giono-Cerezo, S.; Guerra-Infante, F.M. Identification of Chlamydia trachomatis genotypes in newborns with respiratory distress. An. Pediatría 2023, 98, 436–445. [Google Scholar] [CrossRef]
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, J.S. Prevalence and antimicrobial susceptibility of Mycoplasma hominis and Ureaplasma species in nonpregnant female patients in South Korea indicate an increasing trend of pristinamycin-resistant isolates. Antimicrob. Agents Chemother. 2020, 64, 10-1128. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Li, R.-H.; Zheng, L.-Q.; Shang, X.-H. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in female outpatients, 2009–2013. J. Microbiol. Immunol. Infect. 2016, 49, 359–362. [Google Scholar] [CrossRef]
- Esen, B.; Gozalan, A.; Sevindi, D.F.; Demirbas, A.; Onde, U.; Erkayran, U.; Karakoc, A.E.; Hasçiçek, A.M.; Ergün, Y.; Adiloglu, A.K. Ureaplasma urealyticum: Presence among sexually transmitted diseases. Jpn. J. Infect. Dis. 2017, 70, 75–79. [Google Scholar] [CrossRef]
- Cutoiu, A.; Boda, D. Prevalence of Ureaplasma urealyticum, Mycoplasma hominis and Chlamydia trachomatis in symptomatic and asymptomatic patients. Biomed. Rep. 2023, 19, 74. [Google Scholar] [CrossRef]
- Piscopo, R.C.; Guimarães, R.V.; Ueno, J.; Ikeda, F.; Jarmy-Di Bella, Z.I.; Girão, M.J.; Samama, M. Increased prevalence of endocervical Mycoplasma and Ureaplasma colonization in infertile women with tubal factor. JBRA Assist. Reprod. 2020, 24, 152. [Google Scholar] [CrossRef]
- Paavonen, J. Chlamydia trachomatis infections of the female genital tract: State of the art. Ann. Med. 2012, 44, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Xin, N.; Tong, X.-N.; Wang, J.-Y.; Liu, Z.-W. Prevalence and antibiotic susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Xi’an, China. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1941–1947. [Google Scholar] [CrossRef]
- Doroftei, B.; Ilie, O.-D.; Armeanu, T.; Anton, E.; Scripcariu, I.; Maftei, R. The prevalence of Ureaplasma urealyticum and Mycoplasma hominis infections in infertile patients in the Northeast Region of Romania. Medicina 2021, 57, 211. [Google Scholar] [CrossRef]
- Taylor-Robinson, D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res. Microbiol. 2017, 168, 875–881. [Google Scholar] [CrossRef]
- Sleha, R.; Boštíková, V.; Hampl, R.; Salavec, M.; Halada, P.; Štěpán, M.; Novotná, Š.; Kukla, R.; Slehová, E.; Kacerovský, M. Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in women undergoing an initial infertility evaluation. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Pro Epidemiol. A Mikrobiol. Ceske Lek. Spol. JE Purkyne 2016, 65, 232–237. [Google Scholar]
- Graspeuntner, S.; Bohlmann, M.K.; Gillmann, K.; Speer, R.; Kuenzel, S.; Mark, H.; Hoellen, F.; Lettau, R.; Griesinger, G.; König, I.R. Microbiota-based analysis reveals specific bacterial traits and a novel strategy for the diagnosis of infectious infertility. PLoS ONE 2018, 13, e0191047. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between vaginal microbiome and female health: A review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.; Morré, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.-J.S.; Beckers, N.; Broekmans, F. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host–pathogen interactions during female genital tract infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef]
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis—A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.B.; Arruda, J.T.; Approbato, M.S.; García-Zapata, M.T.A. Chlamydia trachomatis and Neisseria gonorrhoeae infection: Factors associated with infertility in women treated at a human reproduction public service. Rev. Bras. Ginecol. E Obs. 2014, 36, 353–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morin-Papunen, L.C.; Duleba, A.J.; Bloigu, A.; Järvelin, M.-R.; Saikku, P.; Pouta, A. Chlamydia antibodies and self-reported symptoms of oligo-amenorrhea and hirsutism: A new etiologic factor in polycystic ovary syndrome? Fertil. Steril. 2010, 94, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis. 2010, 201, S134–S155. [Google Scholar] [CrossRef]
- Davies, B.; Turner, K.M.; Leung, S.; Yu, B.N.; Frølund, M.; Benfield, T.; Blanchard, J.; Westh, H.; Study, D.C.; Ward, H. Comparison of the population excess fraction of Chlamydia trachomatis infection on pelvic inflammatory disease at 12-months in the presence and absence of chlamydia testing and treatment: Systematic review and retrospective cohort analysis. PLoS ONE 2017, 12, e0171551. [Google Scholar] [CrossRef]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually transmitted diseases and infertility. Am. J. Obstet. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef]
- Chudzicka-Strugała, I.; Gołębiewska, I.; Banaszewska, B.; Trzciński, M.; Brudecki, G.; Elamin, W.; Zwoździak, B. Bacterial Vaginosis (BV) and Vaginal Microbiome Disorders in Women Suffering from Polycystic Ovary Syndrome (PCOS). Diagnostics 2024, 14, 404. [Google Scholar] [CrossRef]
- Thackray, V.G. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Zhou, F.; Li, Y.; Wu, Q.; He, H.; Zhang, Y.; Ma, C.; Ding, J.; Hua, K. Gut and vaginal microbiomes in PCOS: Implications for women’s health. Front. Endocrinol. 2022, 13, 808508. [Google Scholar] [CrossRef]
- Pereira, M.P.; Jones, S.; Costin, J.M.; Pereira, M.; Costin, J. Association of Polycystic Ovarian Syndrome (PCOS) With Vaginal Microbiome Dysbiosis: A Scoping Review. Cureus 2024, 16, e62611. [Google Scholar] [CrossRef]
- Peelen, M.J.; Luef, B.M.; Lamont, R.F.; de Milliano, I.; Jensen, J.S.; Limpens, J.; Hajenius, P.J.; Jørgensen, J.S.; Menon, R.; PREBIC Biomarker Working Group. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta 2019, 79, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; Van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal microbiota and reproductive health: The virtue of simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e611–611.e619. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.-M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE 2013, 8, e63514. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Brotman, R.; Gajer, P.; Fadrosh, D.; Mahurkar, A.; White, O.; Terplan, M.; Bavoil, P.; Forney, L.; Ravel, J. O05. 4 Association Between Chlamydia Trachomatis Genital Infection and the Vaginal Microbiome. Sex. Transm. Infect. 2013, 89, A35. [Google Scholar] [CrossRef][Green Version]
- Van Der Veer, C.; Bruisten, S.M.; Van Der Helm, J.J.; De Vries, H.J.; Van Houdt, R. The cervicovaginal microbiota in women notified for Chlamydia trachomatis infection: A case-control study at the sexually transmitted infection outpatient clinic in Amsterdam, The Netherlands. Clin. Infect. Dis. 2017, 64, 24–31. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Lewis, F.M.; Bernstein, K.T.; Aral, S.O. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Porpora, M.G.; Recine, N.; Latino, M.A.; Sessa, R. HPV/Chlamydia trachomatis co-infection: Metagenomic analysis of cervical microbiota in asymptomatic women. New Microbiol 2018, 41, 34–41. [Google Scholar]
- Hong, X.; Qin, P.; Yin, J.; Shi, Y.; Xuan, Y.; Chen, Z.; Zhou, X.; Yu, H.; Peng, D.; Wang, B. Clinical manifestations of polycystic ovary syndrome and associations with the vaginal microbiome: A cross-sectional based exploratory study. Front. Endocrinol. 2021, 12, 662725. [Google Scholar] [CrossRef]
- Salah, R.M.; Allam, A.M.; Magdy, A.M.; Mohamed, A.S. Bacterial vaginosis and infertility: Cause or association? Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O. Mechanistic insights into immune suppression and evasion in bacterial vaginosis. Curr. Microbiol. 2022, 79, 84. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J.; Muruzábal, F.J.; Burrell, M.A. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E827–E847. [Google Scholar] [CrossRef]
- Grunfeld, C.; Feingold, K.R. The metabolic effects of tumor necrosis factor and other cytokines. Biotherapy 1991, 3, 143–158. [Google Scholar] [CrossRef]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15, 120. [Google Scholar] [CrossRef]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF therapy in Crohn’s disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J. Crohn’s Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic low grade inflammation in pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Amato, G.; Conte, M.; Mazziotti, G.; Lalli, E.; Vitolo, G.; Tucker, A.T.; Bellastella, A.; Carella, C.; Izzo, A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet. Gynecol. 2003, 101, 1177–1182. [Google Scholar]
- Oróstica Arévalo, M.L.; García, P.; Vera, C.; García, V.; Romero Osses, C.; Vega Blanco, M.M. Effect of TNF-alpha on molecules related to the Iinsulin action in endometrial cells exposed to hyperandrogenic and hyperinsulinic conditions characteristics of polycystic ovary syndrome. Reprod. Sci. 2018, 25, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Choi, J.-W.; Lee, K.-J.; Shin, J.-S.; Baek, K.-H. The promoter-1031 (T/C) polymorphism in tumor necrosis factor-alpha associated with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2011, 9, 131. [Google Scholar] [CrossRef]
- Silva, J.R.; Lima, F.E.; Souza, A.L.; Silva, A.W. Interleukin-1β and TNF-α systems in ovarian follicles and their roles during follicular development, oocyte maturation and ovulation. Zygote 2020, 28, 270–277. [Google Scholar] [CrossRef]
- Lv, J.; Shan, X.; Yang, H.; Wen, Y.; Zhang, X.; Chen, H.; Li, H.; Tian, D.; Wang, C.C.; Zhang, R. Single cell proteomics profiling reveals that embryo-secreted TNF-α plays a critical role during embryo implantation to the endometrium. Reprod. Sci. 2022, 29, 1608–1617. [Google Scholar] [CrossRef]
- Salama, K.M.; Alloush, M.K. Are the cytokines TNF alpha and IL 1Beta early predictors of embryo implantation? Cross sectional study. J. Reprod. Immunol. 2020, 137, 102618. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola, K.; Xystra, P.; Pantou, A.; Kokkali, G.; Pappas, A.; Lambropoulou, M.; Sfakianoudis, K. The role of interleukins in recurrent implantation failure: A comprehensive review of the literature. Int. J. Mol. Sci. 2022, 23, 2198. [Google Scholar] [CrossRef]
- Kniotek, M.; Zych, M.; Roszczyk, A.; Szafarowska, M.; Jerzak, M.M. Decreased production of TNF-α and IL-6 inflammatory cytokines in non-pregnant idiopathic RPL women immunomodulatory effect of sildenafil citrate on the cellular response of idiopathic RPL women. J. Clin. Med. 2021, 10, 3115. [Google Scholar] [CrossRef] [PubMed]
- Ha, L.-X.; Li, W.-X.; Du, Y.-D.; Yuan, Y.-Y.; Qu, X.-X. Tumor Necrosis Factor Alpha Level in the Uterine Fluid of Patients with Polycystic Ovary Syndrome and Its Correlation with Clinical Parameters. J. Inflamm. Res. 2022, 15, 6015–6020. [Google Scholar] [CrossRef] [PubMed]
- Thathapudi, S.; Kodati, V.; Erukkambattu, J.; Katragadda, A.; Addepally, U.; Hasan, Q. Tumor necrosis factor-alpha and polycystic ovarian syndrome: A clinical, biochemical, and molecular genetic study. Genet. Test. Mol. Biomark. 2014, 18, 605–609. [Google Scholar] [CrossRef]
- Hu, C.; Pang, B.; Ma, Z.; Yi, H. Immunophenotypic profiles in polycystic ovary syndrome. Mediat. Inflamm. 2020, 2020, 5894768. [Google Scholar] [CrossRef]
- Martoriati, A.; Caillaud, M.; Goudet, G.; Gérard, N. Inhibition of in vitro maturation of equine oocytes by interleukin 1ß via specific IL-1 receptors. Reproduction 2003, 126, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, F.Z.; Naghizadeh, M.M.; Masoumi, M. Polycystic ovary syndrome and circulating inflammatory markers. Int. J. Reprod. Biomed. 2017, 15, 375. [Google Scholar] [CrossRef]
- Alissa, E.M.; Algarni, S.A.; Khaffji, A.J.; Al Mansouri, N.M. Role of inflammatory markers in polycystic ovaries syndrome: In relation to insulin resistance. J. Obstet. Gynaecol. Res. 2021, 47, 1409–1415. [Google Scholar] [CrossRef]
- Fouani, F.Z.; Fadaei, R.; Moradi, N.; Zandieh, Z.; Ansaripour, S.; Yekaninejad, M.S.; Vatannejad, A.; Mahmoudi, M. Circulating levels of Meteorin-like protein in polycystic ovary syndrome: A case-control study. PLoS ONE 2020, 15, e0231943. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.-D.; Amer, S. The role of chronic inflammation in polycystic ovarian syndrome—A systematic review and meta-analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Mazloomi, S.; Barartabar, Z.; Pilehvari, S. The association between increment of interleukin-1 and interleukin-6 in women with polycystic ovary syndrome and body mass index. J. Reprod. Infertil. 2023, 24, 26. [Google Scholar] [CrossRef]
- Rothermel, C.; Schachter, J.; Lavrich, P.; Lipsitz, E.; Francus, T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect. Immun. 1989, 57, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Gervassi, A.; Alderson, M.R.; Suchland, R.; Maisonneuve, J.F.; Grabstein, K.H.; Probst, P. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect. Immun. 2004, 72, 7231–7239. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Mekasha, S.; Mavrogiorgos, N.; Fitzgerald, K.A.; Lien, E.; Ingalls, R.R. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J. Immunol. 2010, 184, 5743–5754. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial intracellular survival strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef]
- Faris, R.; Andersen, S.E.; McCullough, A.; Gourronc, F.; Klingelhutz, A.J.; Weber, M.M. Chlamydia trachomatis serovars drive differential production of proinflammatory cytokines and chemokines depending on the type of cell infected. Front. Cell. Infect. Microbiol. 2019, 9, 399. [Google Scholar] [CrossRef]
- Jendro, M.C.; Raum, E.; Schnarr, S.; Köhler, L.; Zeidler, H.; Kuipers, J.G.; Martin, M. Cytokine profile in serum and synovial fluid of arthritis patients with Chlamydia trachomatis infection. Rheumatol. Int. 2005, 25, 37–41. [Google Scholar] [CrossRef]
- Kamalakaran, S.; Chaganty, B.K.; Gupta, R.; Guentzel, M.N.; Chambers, J.P.; Murthy, A.K.; Arulanandam, B.P. Vaginal chlamydial clearance following primary or secondary infection in mice occurs independently of TNF-α. Front. Cell. Infect. Microbiol. 2013, 3, 11. [Google Scholar] [CrossRef]
- Pal, S.; de la Maza, L.M. Mechanism of T-cell mediated protection in newborn mice against a Chlamydia infection. Microbes Infect. 2013, 15, 607–614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagarajan, U.M.; Sikes, J.; Prantner, D.; Andrews, C.W., Jr.; Frazer, L.; Goodwin, A.; Snowden, J.N.; Darville, T. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect. Immun. 2011, 79, 486–498. [Google Scholar] [CrossRef]
- Rasmussen, S.J.; Eckmann, L.; Quayle, A.J.; Shen, L.; Zhang, Y.-X.; Anderson, D.J.; Fierer, J.; Stephens, R.S.; Kagnoff, M.F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 1997, 99, 77–87. [Google Scholar] [CrossRef]
- Malinverni, R. The role of cytokines in chlamydial infections. Curr. Opin. Infect. Dis. 1996, 9, 150–155. [Google Scholar] [CrossRef]
- Tan, M.; Bavoil, P. Intracellular Pathogens I: Chlamydiales; American Society for Microbiology Press: Washington, DC, USA, 2012; Volume 1. [Google Scholar]
- Strieter, R.; Kunkel, S.; Showell, H.; Remick, D.; Phan, S.; Ward, P.; Marks, R. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-α, LPS, and IL-1β. Science 1989, 243, 1467–1469. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Igietseme, J.U.; Omosun, Y.; Stuchlik, O.; Reed, M.S.; Partin, J.; He, Q.; Joseph, K.; Ellerson, D.; Bollweg, B.; George, Z. Role of epithelial-mesenchyme transition in Chlamydia pathogenesis. PLoS ONE 2015, 10, e0145198. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Sun, X.; Tian, Q.; Wang, L.; Xue, M.; Zhong, G. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract. Microbes Infect. 2017, 19, 536–545. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Bua, A.; Cannas, S.; Zanetti, S.; Molicotti, P. Levels of different cytokines in women and men with asymptomatic genital infection caused by Chlamydia. J. Infect. Dev. Ctries. 2019, 13, 847–850. [Google Scholar] [CrossRef]
- Maini, R.; Taylor, P.; Szechinski, J.; Pavelka, K.; Bröll, J.; Balint, G.; Emery, P.; Raemen, F.; Petersen, J.; Smolen, J. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006, 54, 2817–2829. [Google Scholar] [CrossRef]
- Prasad, P.; Singh, N.; Das, B.; Raisuddin, S.; Dudeja, M.; Rastogi, S. Differential expression of circulating Th1/Th2/Th17 cytokines in serum of Chlamydia trachomatis-infected women undergoing incomplete spontaneous abortion. Microb. Pathog. 2017, 110, 152–158. [Google Scholar] [CrossRef]
- Poston, T.B.; Lee, D.A.E.; Darville, T.; Zhong, W.; Dong, L.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Sempowski, G.D.; Zheng, X. Cervical cytokines associated with Chlamydia trachomatis susceptibility and protection. J. Infect. Dis. 2019, 220, 330–339. [Google Scholar] [CrossRef]
- Smith, J.S.; Bosetti, C.; MUnoz, N.; Herrero, R.; Bosch, F.X.; Eluf-Neto, J.; Meijer, C.J.; Van Den Brule, A.J.; Franceschi, S.; Peeling, R.W. Chlamydia trachomatis and invasive cervical cancer: A pooled analysis of the IARC multicentric case-control study. Int. J. Cancer 2004, 111, 431–439. [Google Scholar] [CrossRef]
- Lugo, L.Z.A.; Puga, M.A.M.; Jacob, C.M.B.; Padovani, C.T.J.; Nocetti, M.C.; Tupiná, M.S.; Pina, A.F.S.; de Freitas, J.N.M.; Ferreira, A.M.T.; Fernandes, C.E.d.S. Cytokine profiling of samples positive for Chlamydia trachomatis and Human papillomavirus. PLoS ONE 2023, 18, e0279390. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K. New insights into Chlamydia pathogenesis: Role of leukemia inhibitory factor. Front. Cell. Infect. Microbiol. 2022, 12, 1029178. [Google Scholar] [CrossRef]
- Edwards, C.J. IL-6 inhibition and infection: Treating patients with tocilizumab. Rheumatology 2012, 51, 769–770. [Google Scholar] [CrossRef][Green Version]
- Hvid, M.; Baczynska, A.; Deleuran, B.; Fedder, J.; Knudsen, H.J.; Christiansen, G.; Birkelund, S. Interleukin-1 is the initiator of fallopian tube destruction during Chlamydia trachomatis infection. Cell. Microbiol. 2007, 9, 2795–2803. [Google Scholar] [CrossRef]
- Marconi, C.; Santos-Greatti, M.M.; Parada, C.M.; Pontes, A.; Pontes, A.G.; Giraldo, P.C.; Donders, G.G.; Da Silva, M.G. Cervicovaginal levels of proinflammatory cytokines are increased during chlamydial infection in bacterial vaginosis but not in lactobacilli-dominated flora. J. Low. Genit. Tract Dis. 2014, 18, 261–265. [Google Scholar] [CrossRef]
- Shimizu, T.; Kida, Y.; Kuwano, K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-κB through TLR1, TLR2, and TLR6. J. Immunol. 2005, 175, 4641–4646. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Y.; You, X. Subversion of the immune response by human pathogenic mycoplasmas. Front. Microbiol. 2019, 10, 1934. [Google Scholar] [CrossRef]
- Amorim, A.; Lino, V.; Marques, L.; Martins, D.; Braga Junior, A.; Campos, G.; Oliveira, C.; Boccardo, E.; Timenetsky, J. Mycoplasma hominis Causes DNA Damage and Cell Death in Primary Human Keratinocytes. Microorganisms 2022, 10, 1962. [Google Scholar] [CrossRef]
- Ashshi, A.M.; Batwa, S.A.; Kutbi, S.Y.; Malibary, F.A.; Batwa, M.; Refaat, B. Prevalence of 7 sexually transmitted organisms by multiplex real-time PCR in Fallopian tube specimens collected from Saudi women with and without ectopic pregnancy. BMC Infect. Dis. 2015, 15, 569. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Djahanbakhch, O.; Saridogan, E.; Lyons, R. The effect of interleukin-6 on ciliary beat frequency in the human fallopian tube. Fertil. Steril. 2008, 90, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yoshioka, H.; Yoshida, S.; Iwabe, T.; Onohara, Y.; Tanikawa, M.; Terakawa, N. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am. J. Obstet. Gynecol. 1997, 176, 593–597. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice: Endometriosis. N. Engl. J. Med. 2010, 362, 2389. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.J.; Kim, D.J.; Lee, J.Y.; Park, J.H.; Kim, J.-S.; Han, J.W.; Kim, B.C.; Kim, C.J.; Lee, S.K. Ureaplasma urealyticum infection contributes to the development of pelvic endometriosis through toll-like receptor 2. Front. Immunol. 2019, 10, 2373. [Google Scholar] [CrossRef] [PubMed]
- Lobão, T.N.; Campos, G.; Selis, N.; Amorim, A.T.; Souza, S.; Mafra, S.; Pereira, L.; Dos Santos, D.; Figueiredo, T.; Marques, L. Ureaplasma urealyticum and U. parvum in sexually active women attending public health clinics in Brazil. Epidemiol. Infect. 2017, 145, 2341–2351. [Google Scholar] [CrossRef]
- Benedetti, F.; Curreli, S.; Zella, D. Mycoplasmas–host interaction: Mechanisms of inflammation and association with cellular transformation. Microorganisms 2020, 8, 1351. [Google Scholar] [CrossRef]
- Zariffard, M.R.; Novak, R.M.; Lurain, N.; Sha, B.E.; Graham, P.; Spear, G.T. Induction of tumor necrosis factor–α secretion and toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J. Infect. Dis. 2005, 191, 1913–1921. [Google Scholar] [CrossRef]
- Marconi, C.; de Andrade Ramos, B.R.; Peraçoli, J.C.; Donders, G.G.; Da Silva, M.G. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am. J. Reprod. Immunol. 2011, 65, 549–556. [Google Scholar] [CrossRef]
- Zhang, S.; Lo, S.-C. Effect of mycoplasmas on apoptosis of 32D cells is species-dependent. Curr. Microbiol. 2007, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Oróstica, L.; Astorga, I.; Plaza-Parrochia, F.; Vera, C.; Garcia, V.; Carvajal, R.; Gabler, F.; Romero, C.; Vega, M. Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int. J. Obes. 2016, 40, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Massaro, G.; Scaravilli, G.; Simeone, S.; Capuano, S.; Pastore, E.; Forte, A.; Parisi, P.; Ferraiolo, A.; Costanzo, A.; Balbi, C. Interleukin-6 and Mycoplasma hominis as markers of preterm birth and related brain damage: Our experience. J. Matern.-Fetal Neonatal Med. 2009, 22, 1063–1067. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Menon, R. Breaking down the barrier: The role of cervical infection and inflammation in preterm birth. Front. Glob. Women’s Health 2022, 2, 777643. [Google Scholar] [CrossRef] [PubMed]
- Altonen, B.L.; Arreglado, T.M.; Leroux, O.; Murray-Ramcharan, M.; Engdahl, R. Characteristics, comorbidities and survival analysis of young adults hospitalized with COVID-19 in New York City. PLoS ONE 2020, 15, e0243343. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Kannampuzha, S.; Murali, R.; Namachivayam, A.; Ganesan, R.; Dey, A.; Babu, A.; Renu, K.; Vellingiri, B. The implication of mechanistic approaches and the role of the microbiome in polycystic ovary syndrome (PCOS): A review. Metabolites 2023, 13, 129. [Google Scholar] [CrossRef]
- Kim, C.-H.; Moon, J.-W.; Moon, S.Y. The effect of interleukin 6 on controlled ovarian stimulation results and IVF outcome in infertile women with adenomyosis undergoing IVF. Fertil. Steril. 2019, 112, e187. [Google Scholar] [CrossRef]
- Graham, A.M.; Rasmussen, J.M.; Rudolph, M.D.; Heim, C.M.; Gilmore, J.H.; Styner, M.; Potkin, S.G.; Entringer, S.; Wadhwa, P.D.; Fair, D.A. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry 2018, 83, 109–119. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): A pilot study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- Banaszewska, B.; Siakowska, M.; Chudzicka-Strugala, I.; Chang, R.J.; Pawelczyk, L.; Zwozdziak, B.; Spaczynski, R.; Duleba, A.J. Elevation of markers of endotoxemia in women with polycystic ovary syndrome. Hum. Reprod. 2020, 35, 2303–2311. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Cardoso, N.S.; Ribeiro, V.B.; Dutra, S.G.V.; Ferriani, R.A.; Gastaldi, A.C.; Araújo, J.E.d.; Souza, H.C.D.d. Polycystic ovary syndrome associated with increased adiposity interferes with serum levels of TNF-alpha and IL-6 differently from leptin and adiponectin. Arch. Endocrinol. Metab. 2020, 64, 4–10. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.R.; Marshall, J.C. Polycystic ovary syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef]
- Niu, Z.; Ye, Y.; Xia, L.; Feng, Y.; Zhang, A. Follicular fluid cytokine composition and oocyte quality of polycystic ovary syndrome patients with metabolic syndrome undergoing in vitro fertilization. Cytokine 2017, 91, 180–186. [Google Scholar] [CrossRef]
- Prabhu, Y.D.; Borthakur, A.; Subeka, A.; Vellingiri, B.; Gopalakrishnan, A.V. Increased pro-inflammatory cytokines in ovary and effect of γ-linolenic acid on adipose tissue inflammation in a polycystic ovary syndrome model. J. Reprod. Immunol. 2021, 146, 103345. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, H.; Zeng, Y.; You, X.; Deng, Z.; Yu, M.; Zhu, C. Mycoplasma genitalium lipoproteins induce human monocytic cell expression of proinflammatory cytokines and apoptosis by activating nuclear factor κB. Mediat. Inflamm. 2008, 2008, 195427. [Google Scholar] [CrossRef]
- Ye, G.-Y.; Wang, K.-Y.; Gui, Q.-D.; Wang, M. Ureaplasma urealyticum-derived lipid-associated membrane proteins introduce IL-6, IL-8, and TNF-α cytokines into human amniotic epithelial cells via Toll-like receptor 2. J. Zhejiang Univ. Sci. B 2018, 19, 654. [Google Scholar] [CrossRef]
| TNF-α | Mean Concentration Values ± SD | p |
|---|---|---|
| CHLAM | 26.27 ± 6.50 | <0.05 |
| CONTROL WITH PCOS (GR.A) | 8.02 ± 3.53 | |
| MU | 22.16 ± 5.29 | <0.05 |
| CONTROL WITH PCOS (GR.A) | 8.02 ± 3.53 | |
| MUC | 29.90 ± 7.32 | <0.05 |
| CONTROL WITH PCOS (GR.A) | 8.02 ± 3.53 | |
| CHLAM | 26.27 ± 6.50 | <0.05 |
| MU | 22.16 ± 5.29 | |
| CHLAM | 26.27 ± 6.50 | >0.05 |
| MUC | 29.90 ± 7.32 | |
| MU | 22.16 ± 5.29 | <0.05 |
| MUC | 29.90 ± 7.32 | |
| CONTROL WITH PCOS (GR.A) | 8.02 ± 3.53 | <0.05 |
| CONTROL WITHOUT PCOS (GR.B) | 5.24 ± 1.30 |
| IL-1β | Mean Concentration Values ± SD | p |
|---|---|---|
| CHLAM | 3.07 ± 0.82 | <0.05 |
| control with PCOS (gr. A) | 1.33 ± 0.39 | |
| MU | 2.47 ± 0.73 | <0.05 |
| control with PCOS (gr. A) | 1.33 ± 0.39 | |
| MUC | 3.56 ± 1.25 | <0.05 |
| control with PCOS (gr. A) | 1.33 ± 0.39 | |
| CHLAM | 3.07 ± 0.82 | <0.05 |
| MU | 2.47 ± 0.73 | |
| CHLAM | 3.07 ± 0.82 | >0.05 |
| MUC | 3.56 ± 1.25 | |
| MU | 2.47 ± 0.73 | <0.05 |
| MUC | 3.56 ± 1.25 | |
| control with PCOS (gr. A) | 1.33 ± 0.39 | <0.05 |
| control without PCOS (gr. B) | 1.14 ± 0.18 |
| IL-6 | Mean Concentration Values ± SD | p |
|---|---|---|
| CHLAM | 2.77 ± 0.80 | <0.05 |
| CONTROL WITH PCOS (GR.A) | 1.61 ± 0.41 | |
| MU | 2.39 ± 0.62 | <0.05 |
| CONTROL WITH PCOS (GR.A) | 1.61 ± 0.41 | |
| MUC | 3.27 ± 1.04 | <0.05 |
| CONTROL WITH PCOS (GR.A) | 1.61 ± 0.41 | |
| CHLAM | 2.77 ± 0.80 | <0.05 |
| MU | 2.39 ± 0.62 | |
| CHLAM | 2.77 ± 0.80 | >0.05 |
| MUC | 3.27 ± 1.04 | |
| MU | 2.39 ± 0.62 | <0.05 |
| MUC | 3.27 ± 1.04 | |
| CONTROL WITH PCOS (GR.A) | 1.61 ± 0.41 | <0.05 |
| CONTROL WITHOUT PCOS (GR.B) | 1.25 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudzicka-Strugała, I.; Gołębiewska, I.; Brudecki, G.; Elamin, W.; Banaszewska, B.; Chudzicka-Adamczak, M.; Strugała, D.; Zwoździak, B. Proinflammatory Cytokines in Women with PCOS in Atypical Pathogen Infections. Diagnostics 2025, 15, 1669. https://doi.org/10.3390/diagnostics15131669
Chudzicka-Strugała I, Gołębiewska I, Brudecki G, Elamin W, Banaszewska B, Chudzicka-Adamczak M, Strugała D, Zwoździak B. Proinflammatory Cytokines in Women with PCOS in Atypical Pathogen Infections. Diagnostics. 2025; 15(13):1669. https://doi.org/10.3390/diagnostics15131669
Chicago/Turabian StyleChudzicka-Strugała, Izabela, Iwona Gołębiewska, Grzegorz Brudecki, Wael Elamin, Beata Banaszewska, Marta Chudzicka-Adamczak, Dominik Strugała, and Barbara Zwoździak. 2025. "Proinflammatory Cytokines in Women with PCOS in Atypical Pathogen Infections" Diagnostics 15, no. 13: 1669. https://doi.org/10.3390/diagnostics15131669
APA StyleChudzicka-Strugała, I., Gołębiewska, I., Brudecki, G., Elamin, W., Banaszewska, B., Chudzicka-Adamczak, M., Strugała, D., & Zwoździak, B. (2025). Proinflammatory Cytokines in Women with PCOS in Atypical Pathogen Infections. Diagnostics, 15(13), 1669. https://doi.org/10.3390/diagnostics15131669






