Hybrid PET/MRI in Inflammatory Cardiac Diseases: A Systematic Review and Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Review
2.1.1. Search Strategy
2.1.2. Inclusion Criteria
- Original clinical studies (prospective or retrospective), case series, or case reports reporting the use of hybrid PET/MRI in patients with suspected or confirmed ICDs.
- Studies that included imaging-based diagnostic assessment and provided qualitative or quantitative results (e.g., diagnostic yield, imaging findings, or clinical impact).
- Studies with clearly described patient cohorts and imaging protocols.
2.1.3. Exclusion Criteria
- Reviews, editorials, conference abstracts without full text
- Studies using PET/CT or MRI alone
- Experimental animal studies or phantom studies
- Studies not focused on inflammatory cardiac disease
- Lack of specific data on inflammatory cardiac disease or mixed populations without separate analysis
2.1.4. Study Selection and Data Extraction
2.2. Institutional Experience
2.2.1. Study Design and Population
2.2.2. Examination Protocol
2.2.3. Image Analysis and Interpretation
- MRI-positive: presence of both LGE and myocardial edema
- MRI-negative: absence of both LGE and edema
- MRI-inconclusive: LGE present without concomitant edema
- PET-positive: focal increased [18F]FDG uptake in the myocardium
- PET-negative: absence of abnormal [18F]FDG uptake
- PET-inconclusive: diffuse, mild, or anatomically uncertain [18F]FDG uptake
- MRI-positive: clear evidence of vegetations or periannular abscesses
- MRI-negative: normal valve morphology without signs of infection
- MRI-inconclusive: nonspecific findings (e.g., mild valve thickening or ambiguous enhancement) or technically limited assessment (e.g., prosthetic valves, arrhythmias)
- PET-positive: focal increased [18F]FDG uptake in the valvular region
- PET-negative: no abnormal [18F]FDG uptake
- PET-inconclusive: diffuse, low-grade, or anatomically unclear [18F]FDG uptake
2.2.4. Statistical Analysis
3. Results
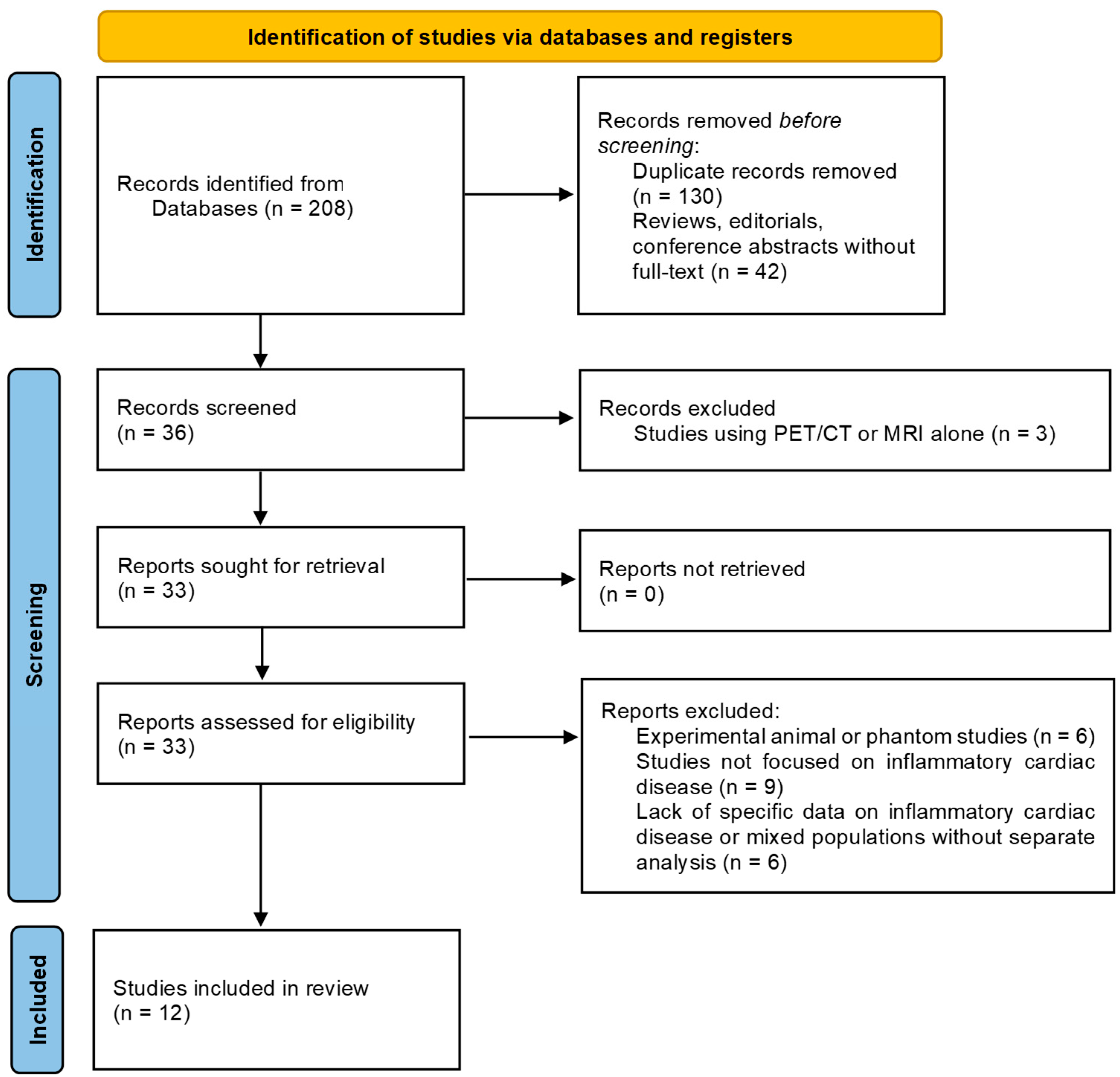
3.1. Systematic Literature Review
3.2. Institutional Experience
3.2.1. Study Population
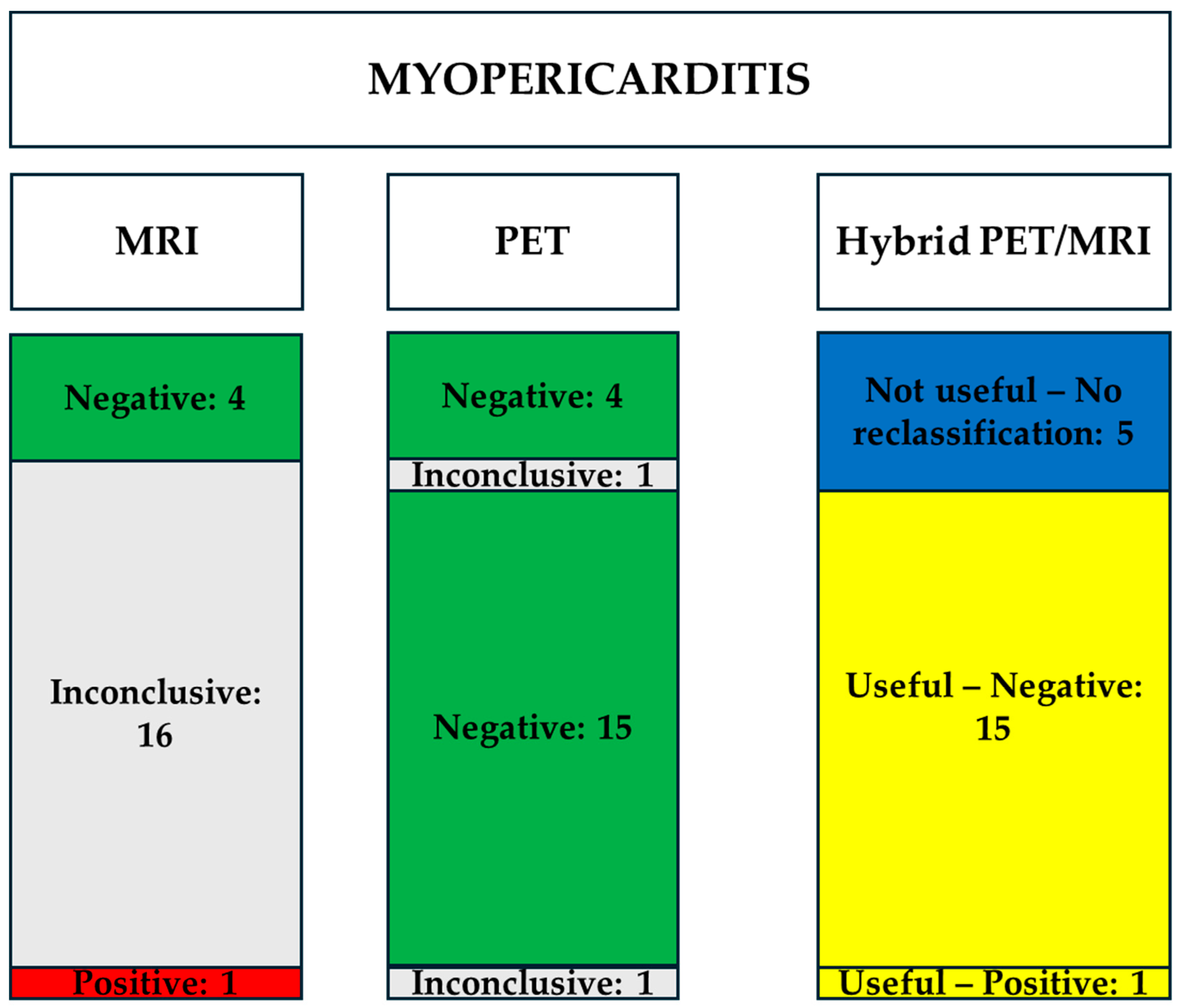
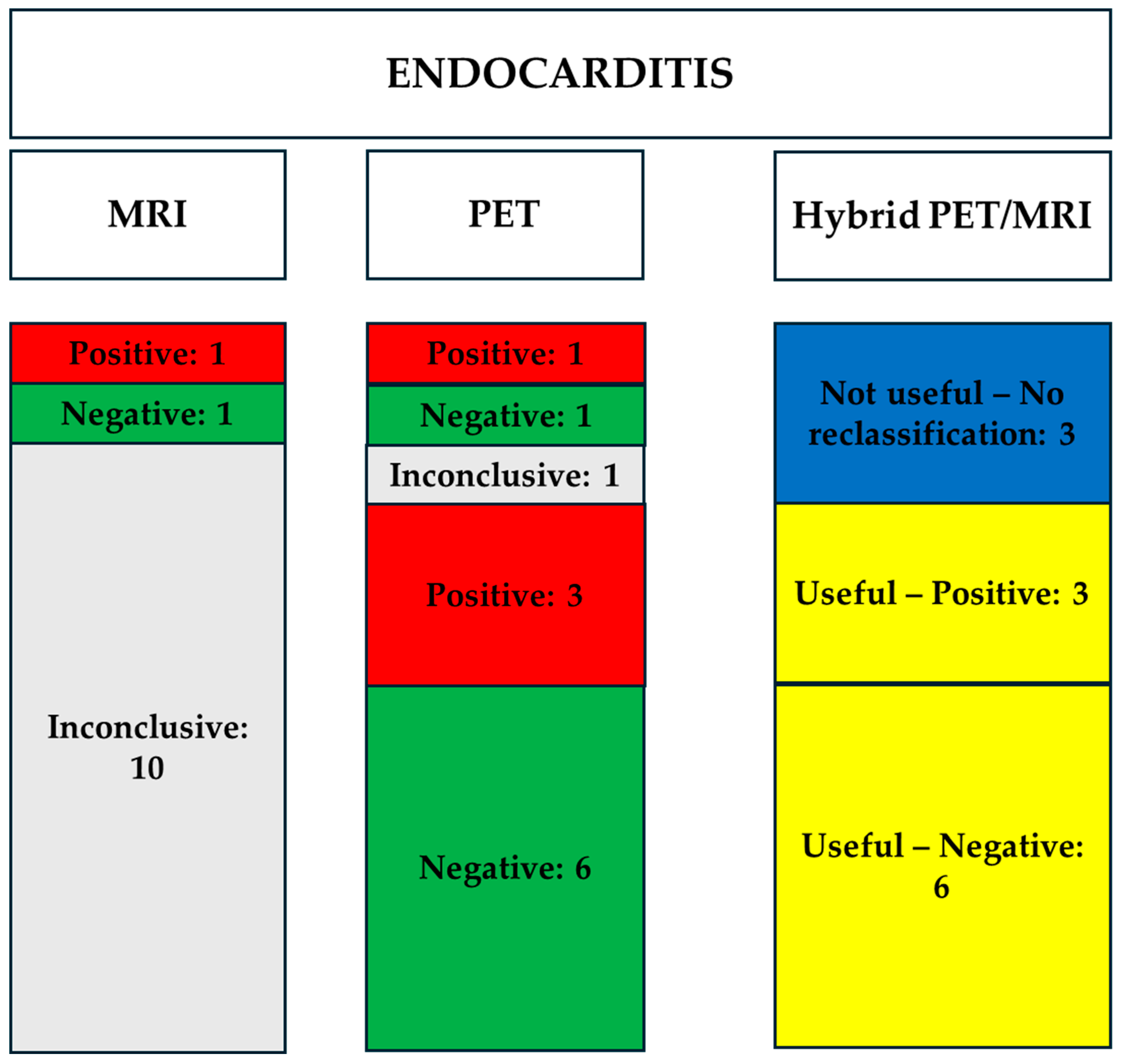
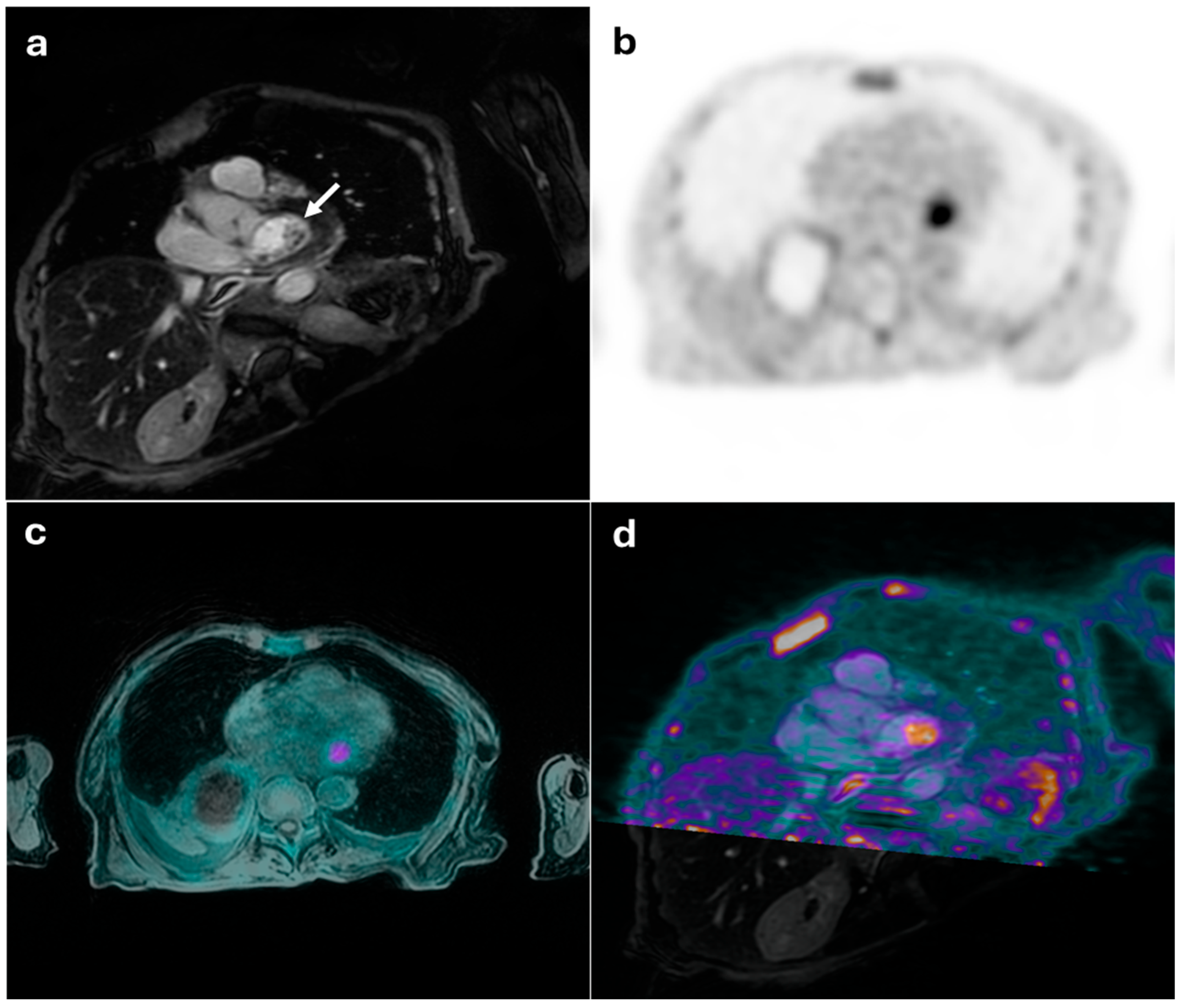
3.2.2. Images Analysis and Interpretation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirienko, M.; Erba, P.A.; Chiti, A.; Sollini, M. Hybrid PET/MRI in Infection and Inflammation: An Update About the Latest Available Literature Evidence. Semin. Nucl. Med. 2023, 53, 107–124. [Google Scholar] [CrossRef]
- Kohan, A.; Hanneman, K.; Mirshahvalad, S.A.; Afaq, A.; Mallak, N.; Metser, U.; Veit-Haibach, P. Current Applications of PET/MR: Part II: Clinical Applications II. Can. Assoc. Radiol. J. 2024, 75, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.; Scully, P.; Gordon, P.; Sado, D. Advances in the Diagnosis of Myocarditis in Idiopathic Inflammatory Myopathies: An Overview of Diagnostic Tests. Rheumatology 2024, 63, 1825–1836. [Google Scholar] [CrossRef]
- Arabi, H.; Zaidi, H. Recent Advances in Positron Emission Tomography/Magnetic Resonance Imaging Technology. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 503–515. [Google Scholar] [CrossRef]
- Rischpler, C.; Siebermair, J.; Kessler, L.; Quick, H.H.; Umutlu, L.; Rassaf, T.; Antoch, G.; Herrmann, K.; Nensa, F. Cardiac PET/MRI: Current Clinical Status and Future Perspectives. Semin. Nucl. Med. 2020, 50, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mirshahvalad, S.A.; Farag, A.; Thiessen, J.; Wong, R.; Veit-Haibach, P. Current Applications of PET/MR: Part I: Technical Basics and Preclinical/Clinical Applications. Can. Assoc. Radiol. J. 2024, 75, 815–825. [Google Scholar] [CrossRef]
- Argalia, G.; Fogante, M.; Schicchi, N.; Fringuelli, F.M.; Esposto Pirani, P.; Cottignoli, C.; Romagnolo, C.; Palucci, A.; Biscontini, G.; Balardi, L.; et al. Hybrid PET/MRI Imaging in Non-Ischemic Cardiovascular Disease. Clin. Transl. Imaging 2024, 12, 69–80. [Google Scholar] [CrossRef]
- Cardoso, R.; Leucker, T.M. Applications of PET-MR Imaging in Cardiovascular Disorders. PET Clin. 2020, 15, 509–520. [Google Scholar] [CrossRef]
- Sammartino, A.M.; Bonfioli, G.B.; Dondi, F.; Riccardi, M.; Bertagna, F.; Metra, M.; Vizzardi, E. Contemporary Role of Positron Emission Tomography (PET) in Endocarditis: A Narrative Review. J. Clin. Med. 2024, 13, 4124. [Google Scholar] [CrossRef]
- Lauriero, F.; Vita, C.V.; Perazzolo, A.; Sanseverino, G.; Moliterno, E.; Rovere, G.; Marano, R.; Natale, L. Acute Myocarditis and Inflammatory Cardiomyopathies: Insights From Cardiac Magnetic Resonance Findings. Echocardiography 2025, 42, e70099. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Ormerod, J.O.M.; Ziakas, A.; Neubauer, S.; Karamitsos, T.D. The Role of Cardiovascular Magnetic Resonance Imaging in Patients with Cardiac Arrhythmias. Rev. Cardiovasc. Med. 2023, 24, 252. [Google Scholar] [CrossRef] [PubMed]
- Lucinian, Y.A.; Martineau, P.; Abikhzer, G.; Harel, F.; Pelletier-Galarneau, M. Novel Tracers to Assess Myocardial Inflammation with Radionuclide Imaging. J. Nucl. Cardiol. 2024, 42, 102012. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, M.D.; Nahrendorf, M. Cardiovascular Molecular Imaging: The Road Ahead. J. Nucl. Med. 2012, 53, 673–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nikpanah, M.; Katal, S.; Christensen, T.Q.; Werner, T.J.; Hess, S.; Malayeri, A.A.; Gholamrezanezhad, A.; Alavi, A.; Saboury, B. Potential Applications of PET Scans, CT Scans, and MR Imaging in Inflammatory Diseases: Part II: Cardiopulmonary and Vascular Inflammation. PET Clin. 2020, 15, 559–576. [Google Scholar] [CrossRef]
- Schwaiger, M.; Kunze, K.; Rischpler, C.; Nekolla, S.G. PET/MR: Yet another Tesla? J. Nucl. Cardiol. 2017, 24, 1019–1031. [Google Scholar] [CrossRef]
- Rischpler, C.; Woodard, P.K. PET/MR Imaging in Cardiovascular Imaging. PET Clin. 2019, 14, 233–244. [Google Scholar] [CrossRef]
- Chen, W.; Jeudy, J. Assessment of Myocarditis: Cardiac MR, PET/CT, or PET/MR? Curr. Cardiol. Rep. 2019, 21, 76. [Google Scholar] [CrossRef]
- Chalian, H.; O’Donnell, J.K.; Bolen, M.; Rajiah, P. Incremental value of PET and MRI in the evaluation of cardiovascular abnormalities. Insights Imaging 2016, 7, 485–503. [Google Scholar] [CrossRef]
- Rischpler, C.; Nekolla, S.G. PET/MR Imaging in Heart Disease. PET Clin. 2016, 11, 465–477. [Google Scholar] [CrossRef]
- Rischpler, C.; Nekolla, S.G.; Kunze, K.P.; Schwaiger, M. PET/MRI of the heart. Semin. Nucl. Med. 2015, 45, 234–247. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, G.; Lin, J. PET/MR co-imaging in cardiovascular diseases: Current clinical applications and future development. Hell. J. Nucl. Med. 2024, 27, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Krumm, P.; Mangold, S.; Gatidis, S.; Nikolaou, K.; Nensa, F.; Bamberg, F.; la Fougère, C. Clinical use of cardiac PET/MRI: Current state-of-the-art and potential future applications. Jpn. J. Radiol. 2018, 36, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H. Cardiovascular PET/MR imaging: Quo Vadis? J. Nucl. Cardiol. 2017, 24, 1007–1018. [Google Scholar] [CrossRef]
- Farzaneh-Far, A.; Kwong, R.Y. Cardiovascular PET/MR: We need evidence, not hype. J. Nucl. Cardiol. 2017, 24, 1032–1035. [Google Scholar] [CrossRef]
- Abgral, R.; Dweck, M.R.; Trivieri, M.G.; Robson, P.M.; Karakatsanis, N.; Mani, V.; Padilla, M.; Miller, M.; Lala, A.; Sanz, J.; et al. Clinical Utility of Combined FDG-PET/MR to Assess Myocardial Disease. JACC Cardiovasc. Imaging 2017, 10, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Hanneman, K.; Kadoch, M.; Guo, H.H.; Jamali, M.; Quon, A.; Iagaru, A.; Herfkens, R. Initial Experience With Simultaneous 18F-FDG PET/MRI in the Evaluation of Cardiac Sarcoidosis and Myocarditis. Clin. Nucl. Med. 2017, 42, e328–e334. [Google Scholar] [CrossRef]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in Myocarditis: Comparison to CMR Using Integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Wisenberg, G.; Thiessen, J.D.; Pavlovsky, W.; Butler, J.; Wilk, B.; Prato, F.S. Same Day Comparison of PET/CT and PET/MR in Patients with Cardiac Sarcoidosis. J. Nucl. Cardiol. 2020, 27, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Vignale, D.; Peretto, G.; Busnardo, E.; Calcagno, C.; Campochiaro, C.; De Luca, G.; Sala, S.; Ferro, P.; Basso, C.; et al. Hybrid FDG-PET/MR or FDG-PET/CT to Detect Disease Activity in Patients With Persisting Arrhythmias After Myocarditis. JACC Cardiovasc Imaging 2021, 14, 288–292. [Google Scholar] [CrossRef]
- Barrio, P.; López-Melgar, B.; Fidalgo, A.; Romero-Castro, M.J.; Moreno-Arciniegas, A.; Field, C.; Garcerant, M.; Anmad Shihadeh, L.; Díaz-Antón, B.; Ruiz de Aguiar, S.; et al. Additional Value of Hybrid PET/MR Imaging versus MR or PET Performed Separately to Assess Cardiovascular Disease. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 303–311. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Y.; Liu, Q.; Shen, Y.; Zhu, H.; Dong, X.; Huang, J.; Lu, J.; Yin, Q. Myocarditis Related to Immune Checkpoint Inhibitors Treatment: Two Case Reports and Literature Review. Ann. Palliat. Med. 2021, 10, 8512–8517. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Ibrahim, E.-S.H.; Dudek, N.; Lu, J.C.; Kalia, V.; Runge, M.; Srinivasan, A.; Stojanovska, J.; Agarwal, P.P. Improving MR Image Quality in Patients with Metallic Implants. RadioGraphics 2021, 41, E126–E137. [Google Scholar] [CrossRef]
- Greulich, S.; Gatidis, S.; Gräni, C.; Blankstein, R.; Glatthaar, A.; Mezger, K.; Müller, K.A.L.; Castor, T.; Mahrholdt, H.; Häntschel, M.; et al. Hybrid Cardiac Magnetic Resonance/Fluorodeoxyglucose Positron Emission Tomography to Differentiate Active From Chronic Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2022, 15, 445–456. [Google Scholar] [CrossRef]
- Chau, O.W.; Islam, A.; Lock, M.; Yu, E.; Dinniwell, R.; Yaremko, B.; Brackstone, M.; Pavlosky, W.; Butler, J.; Biernaski, H.; et al. PET/MRI Assessment of Acute Cardiac Inflammation 1 Month After Left-Sided Breast Cancer Radiation Therapy. J. Nucl. Med. Technol. 2023, 51, 133–139. [Google Scholar] [CrossRef]
- Marschner, C.A.; Thavendiranathan, P.; Gustafson, D.; Howe, K.L.; Fish, J.E.; Iwanochko, R.M.; Wald, R.M.; Abdel-Qadir, H.; Epelman, S.; Cheung, A.M.; et al. Myocardial Inflammation on FDG PET/MRI and Clinical Outcomes in Symptomatic and Asymptomatic Participants after COVID-19 Vaccination. Radiol. Cardiothorac. Imaging 2023, 5, e220247. [Google Scholar] [CrossRef] [PubMed]
- Trivieri, M.G.; Devesa, A.; Robson, P.M.; Bose, S.; Cangut, B.; Liao, S.; Kaufman, A.; Pyzik, R.; Fauveau, V.; Wood, J.; et al. Prevalence of Persistent Cardiovascular and Pulmonary Abnormalities on PET/MRI and DECT Imaging in Long COVID Patients. J. Nucl. Med. 2025, 66, jnumed.124.268980. [Google Scholar] [CrossRef] [PubMed]
- Amsallem, M.; Saito, T.; Tada, Y.; Dash, R.; McConnell, M.V. Magnetic Resonance Imaging and Positron Emission Tomography Approaches to Imaging Vascular and Cardiac Inflammation. Circ. J. 2016, 80, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.; Fogante, M.; Tagliati, C.; Papiri, G. Cut-off point of CT-assessed epicardial adipose tissue volume for predicting worse clinical burden of SARS-CoV-2 pneumonia. Emerg Radiol. 2022, 29, 645–653. [Google Scholar] [CrossRef]
- Khalaf, S.; Al-Mallah, M.H. Fluorodeoxyglucose Applications in Cardiac PET: Viability, Inflammation, Infection, and Beyond. Methodist Debakey Cardiovasc. J. 2020, 16, 122–129. [Google Scholar] [CrossRef]
- Toner, Y.C.; Ghotbi, A.A.; Naidu, S.; Sakurai, K.; van Leent, M.M.T.; Jordan, S.; Ordikhani, F.; Amadori, L.; Sofias, A.M.; Fisher, E.L.; et al. Systematically Evaluating DOTATATE and FDG as PET Immuno-Imaging Tracers of Cardiovascular Inflammation. Sci. Rep. 2022, 12, 6185. [Google Scholar] [CrossRef]

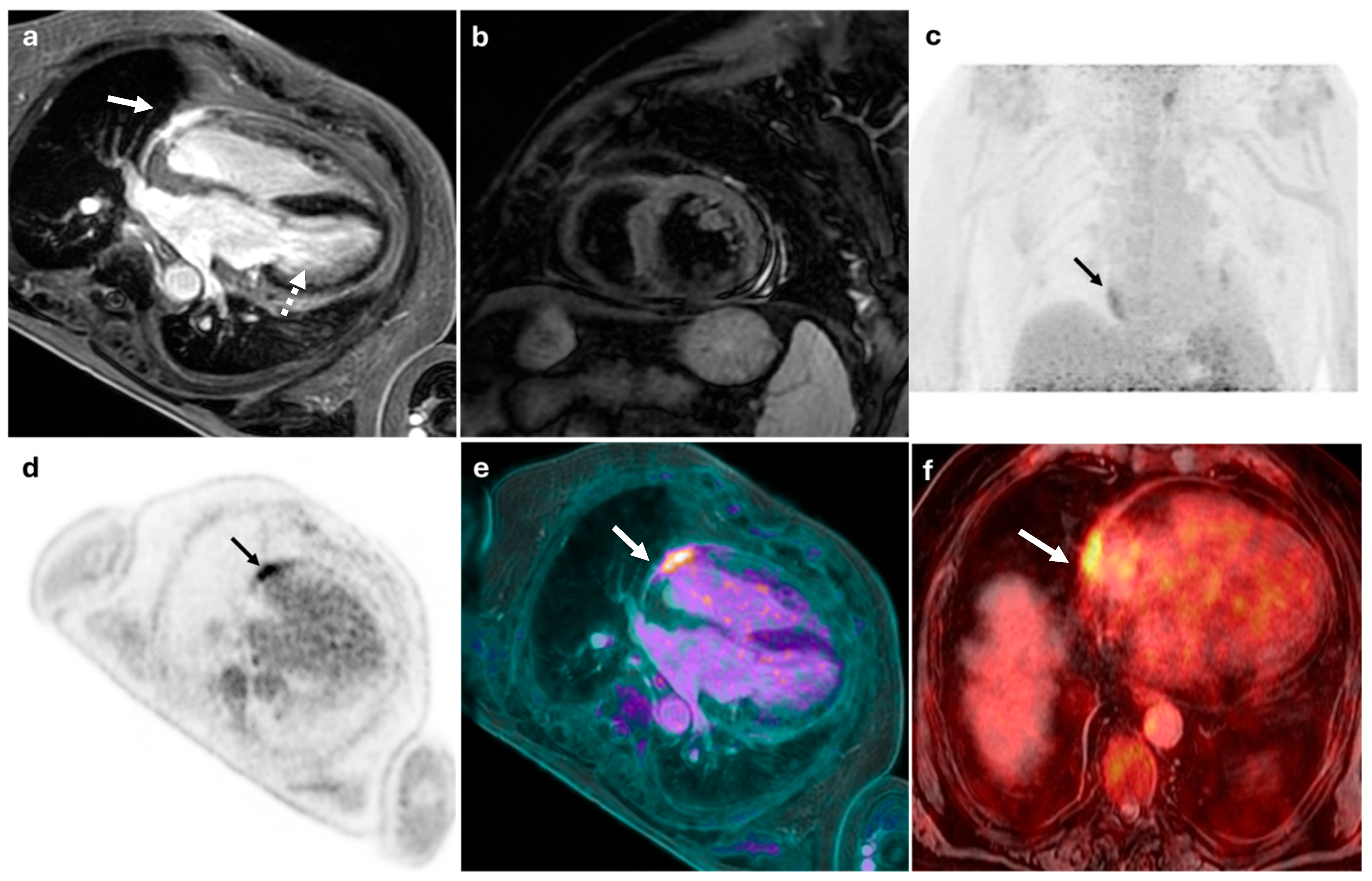
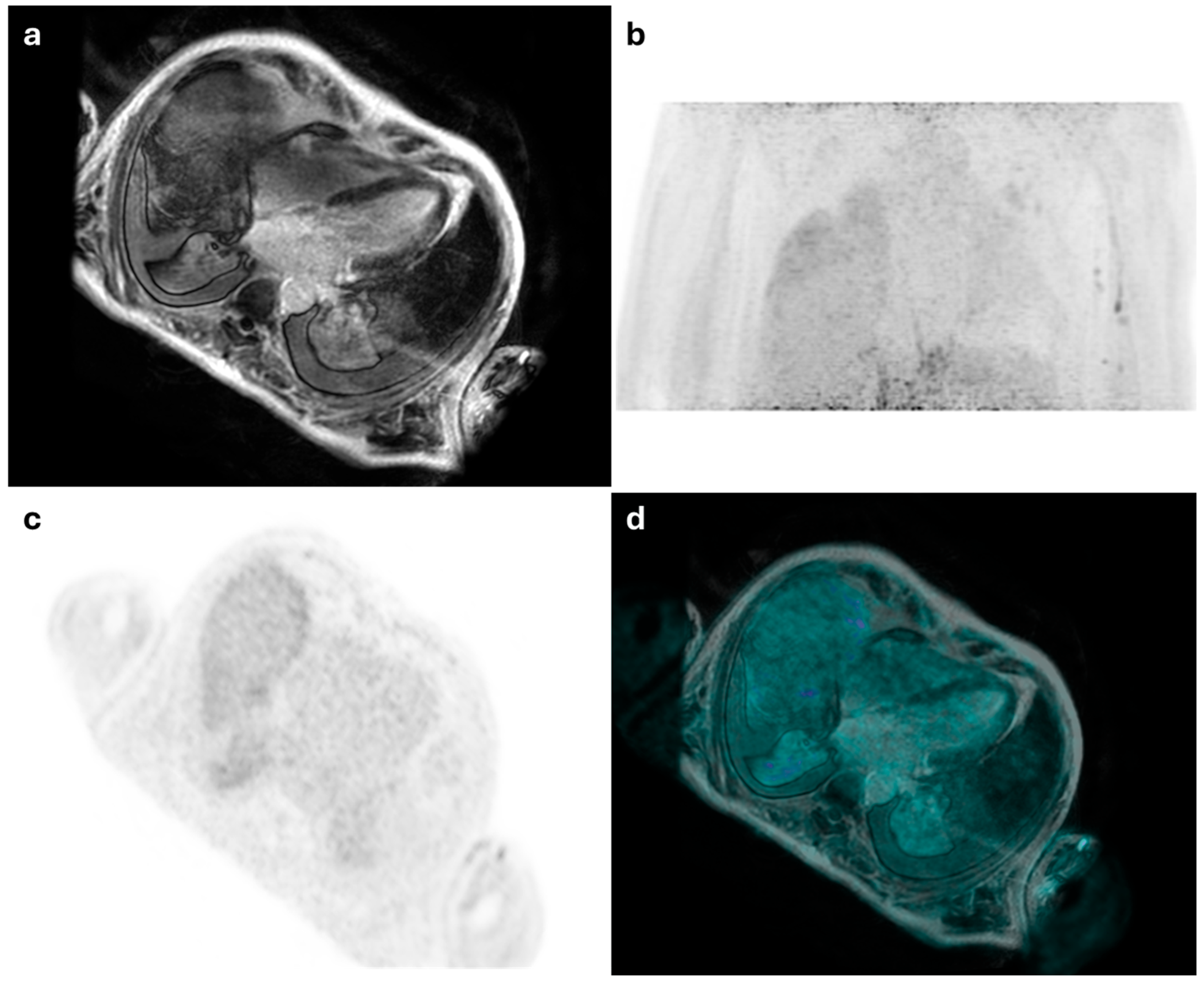
| Author—Year | Study Design | Number of Patients | Clinical Indication | Main Findings | Patients in Whom PET/MRI Added Diagnostic Value (n, %) |
|---|---|---|---|---|---|
| Abgral R et al., 2017 [25] | Case series | 3 | ICDs after myocardial injury | PET/MRI was useful for the detection and localization of ICDs | 3 (100%) |
| Hanneman K et al., 2017 [26] | P | 10 | ICDs in myocarditis and sarcoidosis | PET/MRI was feasible in the detection of ICDs in cardiac sarcoidosis and myocarditis | 0 (0%) |
| Nensa F et al., 2018 [27] | P | 55 | Myocarditis | [18F]FDG-PET was in good agreement with CMR findings | 0 (0%) |
| Wisenberg G et al., 2020 [28] | P | 10 | ICDs in sarcoidosis | PET/MRI provided further insights into the disease process | 10 (100%) |
| Palmisano A et al., 2021 [29] | Case series | 2 | Myocarditis in patients with persisting arrhythmias | PET/MRI detected active inflammation for scar in patients with persisting arrhythmias after myocarditis | 2 (100%) |
| Barrio P et al., 2021 [30] | P | 10 | Myocarditis and endocarditis | PET/MRI was useful and provided additional data | 7 (70%) |
| Chen Y et al., 2021 [31] | Case reports | 2 | Myocarditis in immunotherapy | PET/MRI was useful for the detection of ICDs | 2 (100%) |
| Lee CH et al., 2022 [32] | Case report | 1 | Myocarditis after a COVID-19 mRNA vaccination | PET/MRI clearly visualized focal myocarditis | 1 (100%) |
| Greulich S et al., 2022 [33] | P | 43 | ICDs in sarcoidosis | PET/MRI was useful for the detection of ICDs in cardiac sarcoidosis | 33 (76.7%) |
| Chau OW et al., 2023 [34] | P | 15 | ICDs after radiotherapy | PET/MRI was sensitive to detecting acute cardiac inflammatory response to RT | 15 (100%) |
| Marschner CA et al., 2023 [35] | P | 17 | ICDs after COVID-19 vaccination | PET/MRI showed evidence of myocardial inflammation after COVID-19 vaccination | 17 (100%) |
| Trivieri MG et al., 2025 [36] | P | 99 | ICDs after COVID-19 | PET/MRI was useful for the detection of ICDs | 56 (56.6%) |
| Variable | Total (n = 33) |
|---|---|
| Age (years, mean ± SD) | 57.4 ± 14.2 |
| Sex (M/F, n [%]) | 20 (61%)/13 (39%) |
| Weight (kg, mean ± SD) | 75.6 ± 12.5 |
| Height (cm, mean ± SD) | 170 ± 9 |
| Clinical presentations | Chest pain Fever of unknown origin Elevated inflammatory markers Cardiac dysfunction |
| 18F-FDG dose (MBq, mean ± SD) | 280.2 ± 30.5 |
| Radiation dose (mSv, mean ± SD) | 5.6 ± 1.2 |
| Parameter | Myo-Pericarditis (n = 21) | Endocarditis (n = 12) |
|---|---|---|
| MR Data | ||
| EDV Index (mL/m2) | 92 ± 18 | 85 ± 20 |
| ESV Index (mL/m2) | 45 ± 12 | 40 ± 15 |
| LVEF (%) | 55 ± 8 | 53 ± 10 |
| Edema | 1 (5%) | 0 (%) |
| Late Gadolinium Enhancement, n (%) | 17 (81%) | 3 (25%) |
| PET Data | ||
| SUVmax | 2.9 ± 1.7 | 4.7 ± 1.3 |
| Modality | Myopericarditis (n = 21) | Endocarditis (n = 12) |
|---|---|---|
| MRI | ||
| Positive | 1 (5%) | 1 (8%) |
| Negative, n (%) | 4 (19%) | 1 (8%) |
| Inconclusive, n (%) | 16 (76%) | 10 (84%) |
| PET | ||
| Positive | 0 (0%) | 4 (33%) |
| Negative, n (%) | 19 (90%) | 7 (58%) |
| Inconclusive, n (%) | 2 (10%) | 1 (9%) |
| Hybrid PET/MRI Useful | 16/21 (76%) | 9/12 (75%) |
| Patients with Suspected Myopericarditis | ||||
|---|---|---|---|---|
| MRI Results | PET Results | Hybrid PET/MRI Diagnostic Reclassification of the Inconclusive Cases | What Helped the Reclassification of Inconclusive Cases | |
| 1 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 2 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 3 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 4 | Negative | Negative | Not useful—No reclassification | |
| 5 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 6 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 7 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 8 | Negative | Negative | Not useful—No reclassification | |
| 9 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 10 | Inconclusive | Inconclusive | Not useful—No reclassification | |
| 11 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 12 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 13 | Negative | Negative | Not useful—No reclassification | |
| 14 | Positive | Inconclusive | Useful—Positive | MRI anatomically localizes the uptake in the pericardium, making the examination positive for pericarditis |
| 15 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 16 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 17 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 18 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 19 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 20 | Negative | Negative | Not useful—No reclassification | |
| 21 | Inconclusive | Negative | Negative | The lack of uptake in PET excludes the presence of active inflammation |
| Patients with Suspected Endocarditis | ||||
|---|---|---|---|---|
| MRI Results | PET Results | Hybrid PET/MRI Diagnostic Reclassification of the Inconclusive Cases | What Helped the Reclassification of Inconclusive Cases | |
| 1 | Inconclusive | Positive | Useful—Positive | The evidence of uptake in PET confirms the presence of active inflammation |
| 2 | Inconclusive | Positive | Useful—Positive | The evidence of uptake in PET confirms the presence of active inflammation |
| 3 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 4 | Positive | Positive | Not useful—No reclassification | |
| 5 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 6 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 7 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 8 | Inconclusive | Positive | Useful—Positive | The evidence of uptake in PET confirms the presence of active inflammation |
| 9 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 10 | Inconclusive | Inconclusive | Not useful—No reclassification | |
| 11 | Inconclusive | Negative | Useful—Negative | The lack of uptake in PET excludes the presence of active inflammation |
| 12 | Negative | Negative | Not useful—No reclassification | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogante, M.; Argalia, G.; Esposto Pirani, P.; Romagnolo, C.; Balardi, L.; Argalia, G.; Fringuelli, F.M.; Schicchi, N. Hybrid PET/MRI in Inflammatory Cardiac Diseases: A Systematic Review and Single-Center Experience. Diagnostics 2025, 15, 1670. https://doi.org/10.3390/diagnostics15131670
Fogante M, Argalia G, Esposto Pirani P, Romagnolo C, Balardi L, Argalia G, Fringuelli FM, Schicchi N. Hybrid PET/MRI in Inflammatory Cardiac Diseases: A Systematic Review and Single-Center Experience. Diagnostics. 2025; 15(13):1670. https://doi.org/10.3390/diagnostics15131670
Chicago/Turabian StyleFogante, Marco, Giulia Argalia, Paolo Esposto Pirani, Cinzia Romagnolo, Liliana Balardi, Giulio Argalia, Fabio Massimo Fringuelli, and Nicolò Schicchi. 2025. "Hybrid PET/MRI in Inflammatory Cardiac Diseases: A Systematic Review and Single-Center Experience" Diagnostics 15, no. 13: 1670. https://doi.org/10.3390/diagnostics15131670
APA StyleFogante, M., Argalia, G., Esposto Pirani, P., Romagnolo, C., Balardi, L., Argalia, G., Fringuelli, F. M., & Schicchi, N. (2025). Hybrid PET/MRI in Inflammatory Cardiac Diseases: A Systematic Review and Single-Center Experience. Diagnostics, 15(13), 1670. https://doi.org/10.3390/diagnostics15131670






