Diagnostic Value of T2 Mapping in Sacroiliitis Associated with Spondyloarthropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Protocol
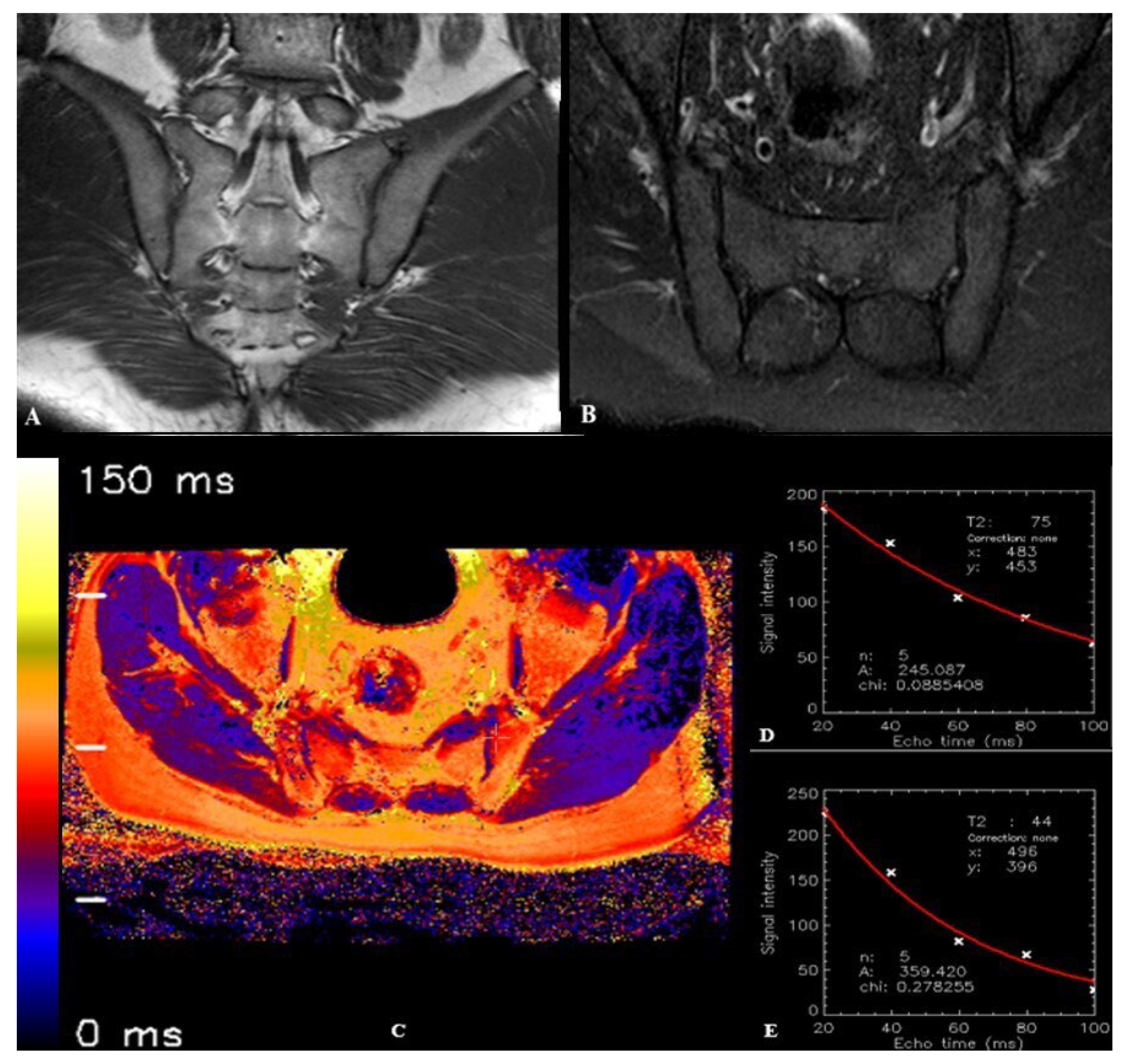
2.3. Image Processing and T2 Relaxation Time Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.P. Sacroiliac joint pain: A comprehensive review of anatomy, diagnosis, and treatment. Anesth. Analg. 2005, 101, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, C.; Griffith, J.F.; Lee, R.K.L.; Wong, P.C.H.; Tam, L.S. Imaging of sacroiliitis: Current status, limitations and pitfalls. Quant. Imaging Med. Surg. 2019, 9, 318–335. [Google Scholar] [CrossRef] [PubMed]
- Cihan, O.F.; Karabulut, M.; Kilincoglu, V.; Yavuz, N. The variations and degenerative changes of sacroiliac joints in asymptomatic adults. Folia Morphol. 2021, 80, 87–96. [Google Scholar] [CrossRef]
- Demir, M.; Mavi, A.; Gümüsburun, E.; Bayram, M.; Gürsoy, S.; Nishio, H. Anatomical variations with joint space measurements on CT. Kobe J. Med. Sci. 2007, 53, 209–217. [Google Scholar] [PubMed]
- Sudol-Szopinska, I.; Urbanik, A. Diagnostic imaging of sacroiliac joints and the spine in the course of spondyloarthropathies. Pol. J. Radiol. 2013, 78, 43–49. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Khan, M.A.; Sieper, J. The challenge of diagnosis and classification in early ankylosing spondylitis: Do we need new criteria? Arthritis Rheum. 2005, 52, 1000–1008. [Google Scholar] [CrossRef]
- Sieper, J.; Rudwaleit, M.; Baraliakos, X.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Dougados, M.; Hermann, K.G.; Landewé, R.; Maksymowych, W.; et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: A guide to assess spondyloarthritis. Ann. Rheum. Dis. 2009, 68 (Suppl. S2), ii1–ii44. [Google Scholar] [CrossRef]
- Navallas, M.; Ares, J.; Beltrán, B.; Lisbona, M.P.; Maymó, J.; Solano, A. Sacroiliitis associated with axial spondyloarthropathy: New concepts and latest trends. Radiographics 2013, 33, 933–956. [Google Scholar] [CrossRef]
- Ozgen, A. The Value of the T2-Weighted Multipoint Dixon Sequence in MRI of Sacroiliac Joints for the Diagnosis of Active and Chronic Sacroiliitis. AJR Am. J. Roentgenol. 2017, 208, 603–608. [Google Scholar] [CrossRef]
- Banegas Illescas, M.E.; López Menéndez, C.; Rozas Rodríguez, M.L.; Fernández Quintero, R.M. New ASAS criteria for the diagnosis of spondyloarthritis: Diagnosing sacroiliitis by magnetic resonance imaging. Radiologia 2014, 56, 7–15. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Zhu, Y.; Li, W.; Li, X.; Zheng, J.; Hong, G. Synthetic MRI in the detection and quantitative evaluation of sacroiliac joint lesions in axial spondyloarthritis. Front. Immunol. 2022, 13, 1000314. [Google Scholar] [CrossRef] [PubMed]
- Martín-Noguerol, T.; Casado-Verdugo, O.L.; Beltrán, L.S.; Aguilar, G.; Luna, A. Role of advanced MRI techniques for sacroiliitis assessment and quantification. Eur. J. Radiol. 2023, 163, 110793. [Google Scholar] [CrossRef] [PubMed]
- Bollow, M.; Biedermann, T.; Kannenberg, J.; Paris, S.; Schauer-Petrowski, C.; Minden, K.; Schöntube, M.; Hamm, B.; Sieper, J.; Braun, J. Use of dynamic magnetic resonance imaging to detect sacroiliitis in HLA-B27 positive and negative children with juvenile arthritides. J. Rheumatol. 1998, 25, 556–564. [Google Scholar] [PubMed]
- Ai, F.; Ai, T.; Li, X.; Hu, D.; Zhang, W.; Morelli, J.N. Value of diffusion-weighted magnetic resonance imaging in early diagnosis of ankylosing spondylitis. Rheumatol. Int. 2012, 32, 4005–4013. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Liang, Z.; Liu, Y. Diagnostic efficacy of dual-energy CT virtual non-calcium technique in the diagnosis of bone marrow edema of sacroiliac joints in ankylosing spondylitis. J. Orthop. Surg. Res. 2025, 20, 28. [Google Scholar] [CrossRef]
- Quirbach, S.; Trattnig, S.; Marlovits, S.; Zimmermann, V.; Domayer, S.; Dorotka, R.; Mamisch, T.C.; Bohndorf, K.; Welsch, G.H. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009, 38, 751–760. [Google Scholar] [CrossRef]
- Lefebvre, G.; Bergere, A.; Rafei, M.E.; Duhamel, A.; Teixeira, P.; Cotten, A. T2 Mapping of the Sacroiliac Joints With 3-T MRI: A Preliminary Study. AJR Am. J. Roentgenol. 2017, 209, 389–394. [Google Scholar] [CrossRef]
- Albano, D.; Chianca, V.; Cuocolo, R.; Bignone, R.; Ciccia, F.; Sconfienza, L.M.; Midiri, M.; Brunetti, A.; Lagalla, R.; Galia, M. T2-mapping of the sacroiliac joints at 1.5 Tesla: A feasibility and reproducibility study. Skeletal Radiol. 2018, 47, 1691–1696. [Google Scholar] [CrossRef]
- Huang, T.Y.; Liu, Y.J.; Stemmer, A.; Poncelet, B.P. T2 measurement of the human myocardium using a T2-prepared transient-state TrueFISP sequence. Magn. Reson. Med. 2007, 57, 960–966. [Google Scholar] [CrossRef]
- Baessler, B.; Schaarschmidt, F.; Stehning, C.; Schnackenburg, B.; Maintz, D.; Bunck, A.C. Cardiac T2-mapping using a fast gradient echo spin echo sequence—First in vitro and in vivo experience. J. Cardiovasc. Magn. Reson. 2015, 17, 67. [Google Scholar] [CrossRef]
- Sabouri, S.; Chang, S.D.; Savdie, R.; Zhang, J.; Jones, E.C.; Goldenberg, S.L.; Black, P.C.; Kozlowski, P. Luminal Water Imaging: A New MR Imaging T2 Mapping Technique for Prostate Cancer Diagnosis. Radiology 2017, 284, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.; Bronskill, M.J.; Henkelman, R.M. Magnetization transfer and T2 relaxation components in tissue. Magn. Reson. Med. 1995, 33, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, R.M.; Stanisz, G.J.; Kim, J.K.; Bronskill, M.J. Anisotropy of NMR properties of tissues. Magn. Reson. Med. 1994, 32, 592–601. [Google Scholar] [CrossRef]
- Packer, K.J. The dynamics of water in heterogeneous systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977, 278, 59–87. [Google Scholar] [CrossRef]
- Zhou, N.; Chu, C.; Dou, X.; Chen, W.; He, J.; Yan, J.; Zhou, Z.; Yang, X. Early evaluation of radiation-induced parotid damage in patients with nasopharyngeal carcinoma by T2 mapping and mDIXON Quant imaging: Initial findings. Radiat. Oncol. 2018, 13, 22. [Google Scholar] [CrossRef]
- Ece, B.; Yigit, H.; Ergun, E.; Koseoglu, E.N.; Karavas, E.; Aydin, S.; Kosar, P.N. Quantitative Analysis of Supraspinatus Tendon Pathologies via T2/T2* Mapping Techniques with 1.5 T MRI. Diagnostics 2023, 13, 2534. [Google Scholar] [CrossRef]
- Baessler, B.; Luecke, C.; Lurz, J.; Klingel, K.; von Roeder, M.; de Waha, S.; Besler, C.; Maintz, D.; Gutberlet, M.; Thiele, H.; et al. Cardiac MRI Texture Analysis of T1 and T2 Maps in Patients with Infarctlike Acute Myocarditis. Radiology 2018, 289, 357–365. [Google Scholar] [CrossRef]
- Hueper, K.; Lang, H.; Hartleben, B.; Gutberlet, M.; Derlin, T.; Getzin, T.; Chen, R.; Abou-Rebyeh, H.; Lehner, F.; Meier, M.; et al. Assessment of liver ischemia reperfusion injury in mice using hepatic T(2) mapping: Comparison with histopathology. J. Magn. Reson. Imaging 2018, 48, 1586–1594. [Google Scholar] [CrossRef]
- Dunn, T.C.; Lu, Y.; Jin, H.; Ries, M.D.; Majumdar, S. T2 relaxation time of cartilage at MR imaging: Comparison with severity of knee osteoarthritis. Radiology 2004, 232, 592–598. [Google Scholar] [CrossRef]
- Lazovic-Stojkovic, J.; Mosher, T.J.; Smith, H.E.; Yang, Q.X.; Dardzinski, B.J.; Smith, M.B. Interphalangeal joint cartilage: High-spatial-resolution in vivo MR T2 mapping--a feasibility study. Radiology 2004, 233, 292–296. [Google Scholar] [CrossRef]
- Maizlin, Z.V.; Clement, J.J.; Patola, W.B.; Fenton, D.M.; Gillies, J.H.; Vos, P.M.; Jacobson, J.A. T2 mapping of articular cartilage of glenohumeral joint with routine MRI correlation--initial experience. HSS J. 2009, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 relaxation time mapping: Overview and applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Matzat, S.J.; McWalter, E.J.; Kogan, F.; Chen, W.; Gold, G.E. T2 Relaxation time quantitation differs between pulse sequences in articular cartilage. J. Magn. Reson. Imaging 2015, 42, 105–113. [Google Scholar] [CrossRef]
- Keenan, K.E.; Besier, T.F.; Pauly, J.M.; Han, E.; Rosenberg, J.; Smith, R.L.; Delp, S.L.; Beaupre, G.S.; Gold, G.E. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthr. Cartil. 2011, 19, 171–179. [Google Scholar] [CrossRef]
- White, L.M.; Sussman, M.S.; Hurtig, M.; Probyn, L.; Tomlinson, G.; Kandel, R. Cartilage T2 assessment: Differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 2006, 241, 407–414. [Google Scholar] [CrossRef]
- Wang, D.; Yin, H.; Liu, W.; Li, Z.; Ren, J.; Wang, K.; Han, D. Comparative analysis of the diagnostic values of T2 mapping and diffusion-weighted imaging for sacroiliitis in ankylosing spondylitis. Skeletal Radiol. 2020, 49, 1597–1606. [Google Scholar] [CrossRef]
- Kasar, S.; Ozturk, M.; Polat, A.V. Quantitative T2 mapping of the sacroiliac joint cartilage at 3T in patients with axial spondyloarthropathies. Eur. Radiol. 2022, 32, 1395–1403. [Google Scholar] [CrossRef]
- Francavilla, M.L.; Serai, S.D.; Brandon, T.G.; Biko, D.M.; Khrichenko, D.; Nguyen, J.C.; Xiao, R.; Chauvin, N.A.; Gendler, L.; Weiss, P.F. Feasibility of T2 Mapping of the Sacroiliac Joints in Healthy Control Subjects and Children and Young Adults with Sacroiliitis. ACR Open Rheumatol. 2022, 4, 74–82. [Google Scholar] [CrossRef]
- Koyun, M. Sakroiliak Eklem Patolojilerinde T2 Mappin’in Tanısal Değeri. Tıpta Uzmanlık Tezi (Medical Specialty Thesis), Sağlık Bilimleri Üniversitesi, Ankara, Türkiye, 2021. [Google Scholar]
- Messroghli, D.R.; Rudolph, A.; Abdel-Aty, H.; Wassmuth, R.; Kuhne, T.; Dietz, R.; Schulz-Menger, J. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med. Imaging 2010, 10, 16. [Google Scholar] [CrossRef]
- SourceForge. MRmap v1.4. Available online: https://sourceforge.net/p/mrmap/mailman/message/30100362/ (accessed on 12 January 2021).
- Albano, D.; Bignone, R.; Chianca, V.; Cuocolo, R.; Messina, C.; Sconfienza, L.M.; Ciccia, F.; Brunetti, A.; Midiri, M.; Galia, M. T2 mapping of the sacroiliac joints in patients with axial spondyloarthritis. Eur. J. Radiol. 2020, 131, 109246. [Google Scholar] [CrossRef]
- Lambert, R.G.; Bakker, P.A.; van der Heijde, D.; Weber, U.; Rudwaleit, M.; Hermann, K.G.; Sieper, J.; Baraliakos, X.; Bennett, A.; Braun, J.; et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: Update by the ASAS MRI working group. Ann. Rheum. Dis. 2016, 75, 1958–1963. [Google Scholar] [CrossRef]
- Hermann, K.G.; Bollow, M. Magnetic resonance imaging of sacroiliitis in patients with spondyloarthritis: Correlation with anatomy and histology. Rofo 2014, 186, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tuite, M.J. Sacroiliac joint imaging. Semin. Musculoskelet. Radiol. 2008, 12, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Dautry, R.; Bousson, V.; Manelfe, J.; Perozziello, A.; Boyer, P.; Loriaut, P.; Koch, P.; Silvestre, A.; Schouman-Claeys, E.; Laredo, J.D.; et al. Correlation of MRI T2 mapping sequence with knee pain location in young patients with normal standard MRI. J. Belg. Soc. Radiol. 2014, 97, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Chen, W.; Xu, X.Q.; Wu, F.Y. T2 mapping of the extraocular muscles in healthy volunteers: Preliminary research on scan-rescan and observer-observer reproducibility. Acta Radiol. 2020, 61, 804–812. [Google Scholar] [CrossRef]
- Lüsse, S.; Claassen, H.; Gehrke, T.; Hassenpflug, J.; Schünke, M.; Heller, M.; Glüer, C.C. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn. Reson. Imaging 2000, 18, 423–430. [Google Scholar] [CrossRef]
- Wiesmueller, M.; Wuest, W.; Heiss, R.; Treutlein, C.; Uder, M.; May, M.S. Cardiac T2 mapping: Robustness and homogeneity of standardized in-line analysis. J. Cardiovasc. Magn. Reson. 2020, 22, 39. [Google Scholar] [CrossRef]


| Parameters | T1-Weighted TSE | SPAIR TSE |
|---|---|---|
| Plane | Coronal oblique | Axial/Coronal oblique |
| TE (ms) | 8 | 80 |
| TR (ms) | 593 | 3431 |
| FOV (mm) | 200 | 200 |
| Flip angle (°) | 90 | 90 |
| No. of signal averages | 2 | 2 |
| Slice thickness (mm) | 4 | 4 |
| Echo train length | 6 | 19 |
| Parameters | Multi-Echo TSE |
|---|---|
| Plane | Axial oblique |
| TE (ms) | 20, 40, 60, 80, 100 |
| TR (ms) | 3825 |
| No. of echoes | 5 |
| Echo spacing (ms) | 20 |
| FOV (mm) | 200 |
| Flip angle (°) | 90 |
| No. of signal averages | 1 |
| Slice thickness (mm) | 4 |
| Bandwidth (pixels) | 219 |
| Acquisition matrix | 268 × 265 |
| Acquisition time | 6.53 min |
| Acquired pixel resolution (mm) | 0.75 × 0.75 |
| Gender |
SpA Group
(n = 31) | Control Group (n = 25) |
Total
(n = 56) | p Value |
|---|---|---|---|---|
| Female, n (%) | 15 (48.3) | 11 (44) | 26 (100) | >0.05 |
| Male, n (%) | 16 (51.7) | 14 (56) | 30 (100) | >0.05 |
| Age, Mean ± SD | 35.4 ± 13.0 | 37.2 ± 9.6 | 36.2 ± 12.0 | >0.05 |
| ASAS Criteria | n (%) | |
|---|---|---|
| Inflammatory Back Pain | 19 (61.2) | |
| Arthritis | 12 (38.7) | |
| Enthesitis | 4 (12.9) | |
| Uveitis | 2 (6.4) | |
| Dactylitis | 3 (9.6) | |
| Psoriasis | 2 (6.4) | |
| Crohn’s/Ulcerative Colitis | 0 (0) | |
| Family history for SpA | 6 (19.3) | |
| HLA-B27 | Negative | 16 (51.6) |
| Positive | 7 (22.6) | |
| Unknown | 8 (25.8) | |
| Good Response to NSAIDs | No | 5 (16.1) |
| Yes | 12 (38.7) | |
| Unknown | 14 (45.2) | |
| CRP Level | <5 mg/L | 7 (22.6) |
| >5 mg/L | 24 (77.4) | |
| Active Sacroiliitis on MRI | 31 (100) | |
| SpA Group (n = 31) | Control Group (n = 25) | p Value | |
|---|---|---|---|
| T2 relaxation time—bone (ms) *, (Mean ± SD) | 100.23 ± 7.41 | 69.44 ± 4.37 | <0.001 |
| T2 relaxation time—cartilage (ms) **, (Mean ± SD) | 44.0 ± 3.19 | 43.2 ± 3.41 | 0.249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyun, M.; Arda, K.N. Diagnostic Value of T2 Mapping in Sacroiliitis Associated with Spondyloarthropathy. Diagnostics 2025, 15, 1634. https://doi.org/10.3390/diagnostics15131634
Koyun M, Arda KN. Diagnostic Value of T2 Mapping in Sacroiliitis Associated with Spondyloarthropathy. Diagnostics. 2025; 15(13):1634. https://doi.org/10.3390/diagnostics15131634
Chicago/Turabian StyleKoyun, Mustafa, and Kemal Niyazi Arda. 2025. "Diagnostic Value of T2 Mapping in Sacroiliitis Associated with Spondyloarthropathy" Diagnostics 15, no. 13: 1634. https://doi.org/10.3390/diagnostics15131634
APA StyleKoyun, M., & Arda, K. N. (2025). Diagnostic Value of T2 Mapping in Sacroiliitis Associated with Spondyloarthropathy. Diagnostics, 15(13), 1634. https://doi.org/10.3390/diagnostics15131634






