Multidrug-Resistant Tuberculosis—Diagnostic Procedures and Treatment of Two Beijing-like TB Cases
Abstract
1. Introduction
2. Case Study
2.1. 51-Year-Old Male Patient from Ukraine. MDR-TB, Beijing 541 Genotype, Successful Treatment
2.2. 58-Year-Old Male Patient. Pre-XDR-TB, Beijing 265 Genotype, Unsuccessful Treatment
| Patient 1 | Patient 2 | |
|---|---|---|
| Sex | Male | Male |
| Age | 51 | 58 |
| Origin | Ukraine | Poland |
| Comorbidities | HIV Nicotine dependence Alcohol dependence | Nicotine dependence Alcohol dependence |
| Symptoms on admission to hospital | Stable; productive cough An abscess with a large cutaneous fistula in the right supraclavicular area | Productive cough, cachexia, lack of appetite, 16 kg weight loss |
| TB | ||
| Medical history | 2015—Pulmonary tuberculosis | 2019—Pulmonary tuberculosis, drug-susceptible, treated irregularly, no follow-up visits, treatment discontinued |
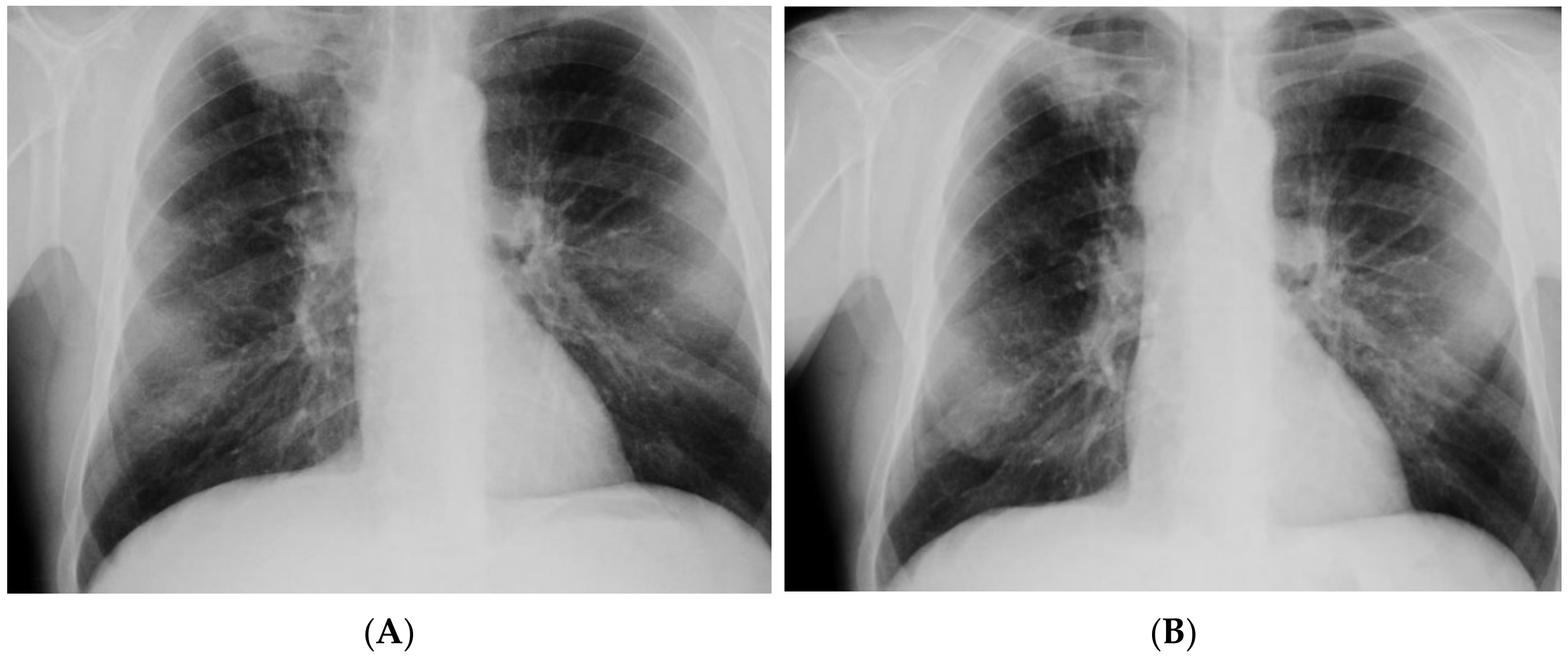
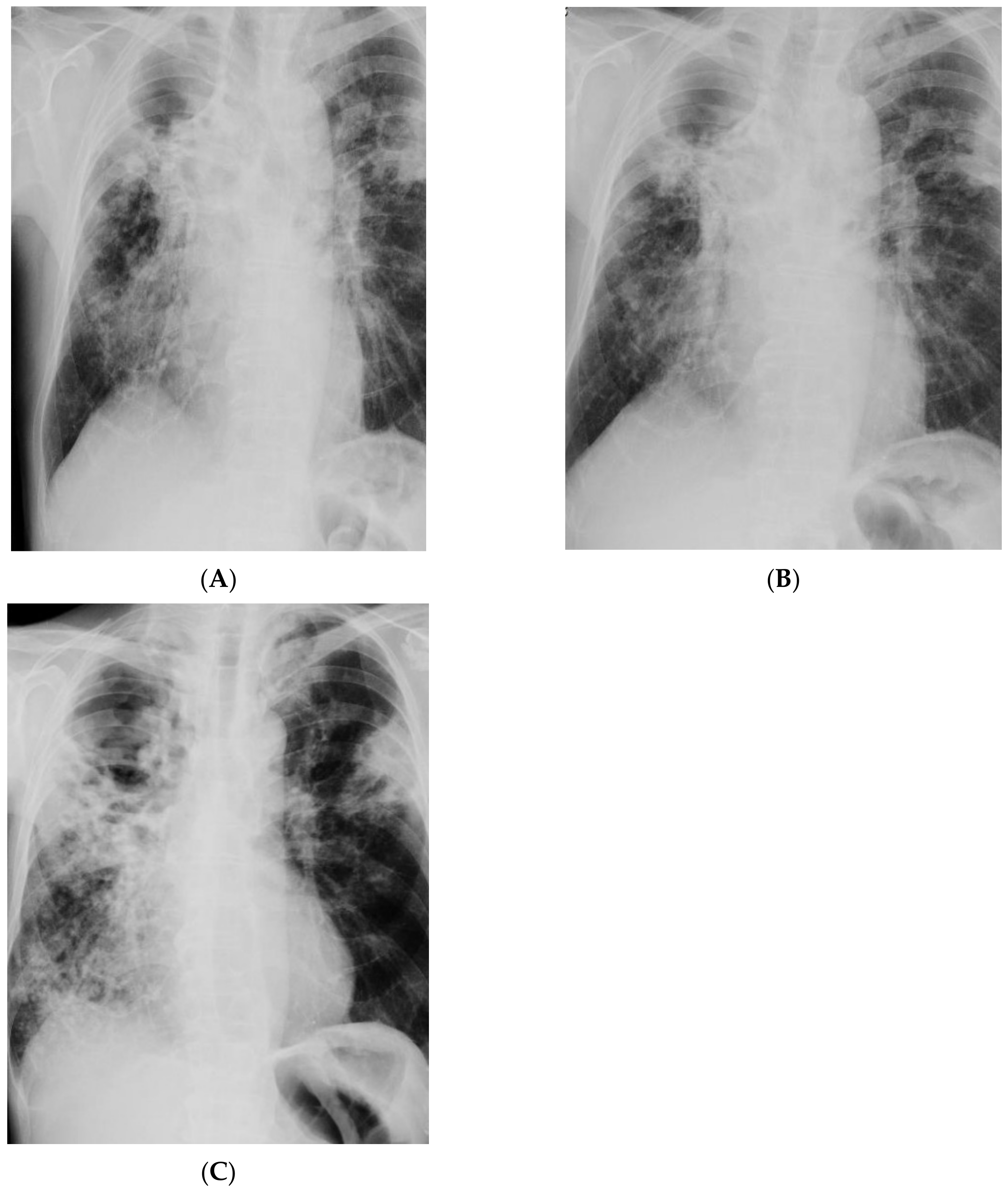
| Imaging studies | Chest CT: Lesions in the lungs (cavities and nodules), enlarged mediastinal lymph nodes, 2 abscesses in the subcutaneous tissue in the right supraclavicular area | Chest X-ray: Extensive bilateral infiltrative lesions, large cavity in the upper area of the right lung (Figure 5A) |
| Bacteriological assay | Sputum AFB (+++)—MTBC cultured; Abscess swab—AFB (++)—MTBC cultured; | Sputum AFB (+++)—MTBC cultured |
| Genetic assay | GeneXpert Sputum (+), mutation in the rpoB gene; Abscess swab (+), mutation in the rpoB gene | GeneXpert Sputum (+), mutation in the rpoB gene |
| Drug resistance profile | MDR Phenotypic resistance to SM, INH, RMP, EMB, RIF | Pre-XDR Molecular resistance to INH, RMP, FLQ, KM; Phenotypic resistance to INH, RMP, OFX, PZA, RIF, SM, KM |
| Spoligotyping | Beijing 541 □□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□■■■■■□□■■ | Beijing 265 □□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□□■■□■■■■■■ |
| TB treatment | PZA, LZD, CS, LFX, ETO, CFZ | EMB, LZD, CS, LFX, ETO, CFZ, AMK, TMC-207 |
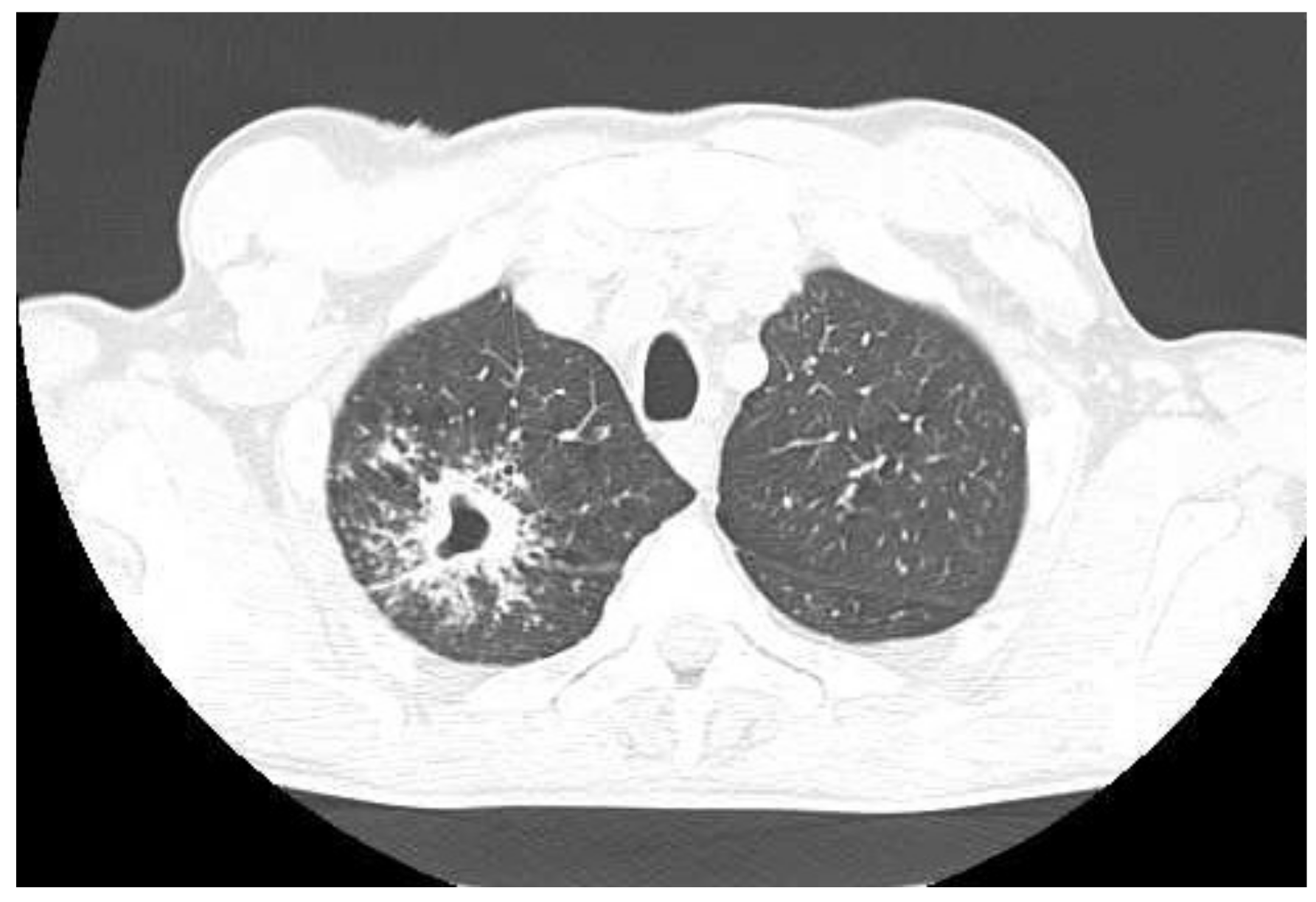
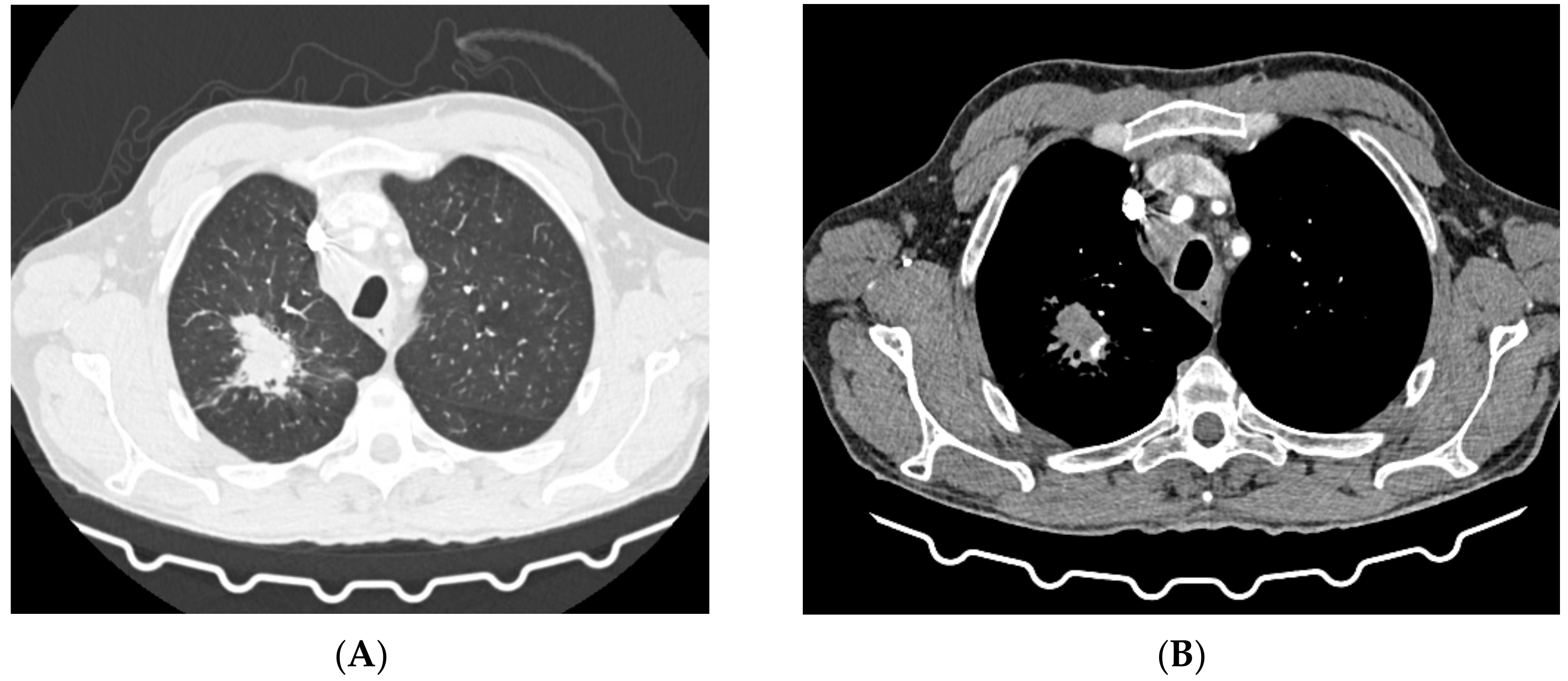
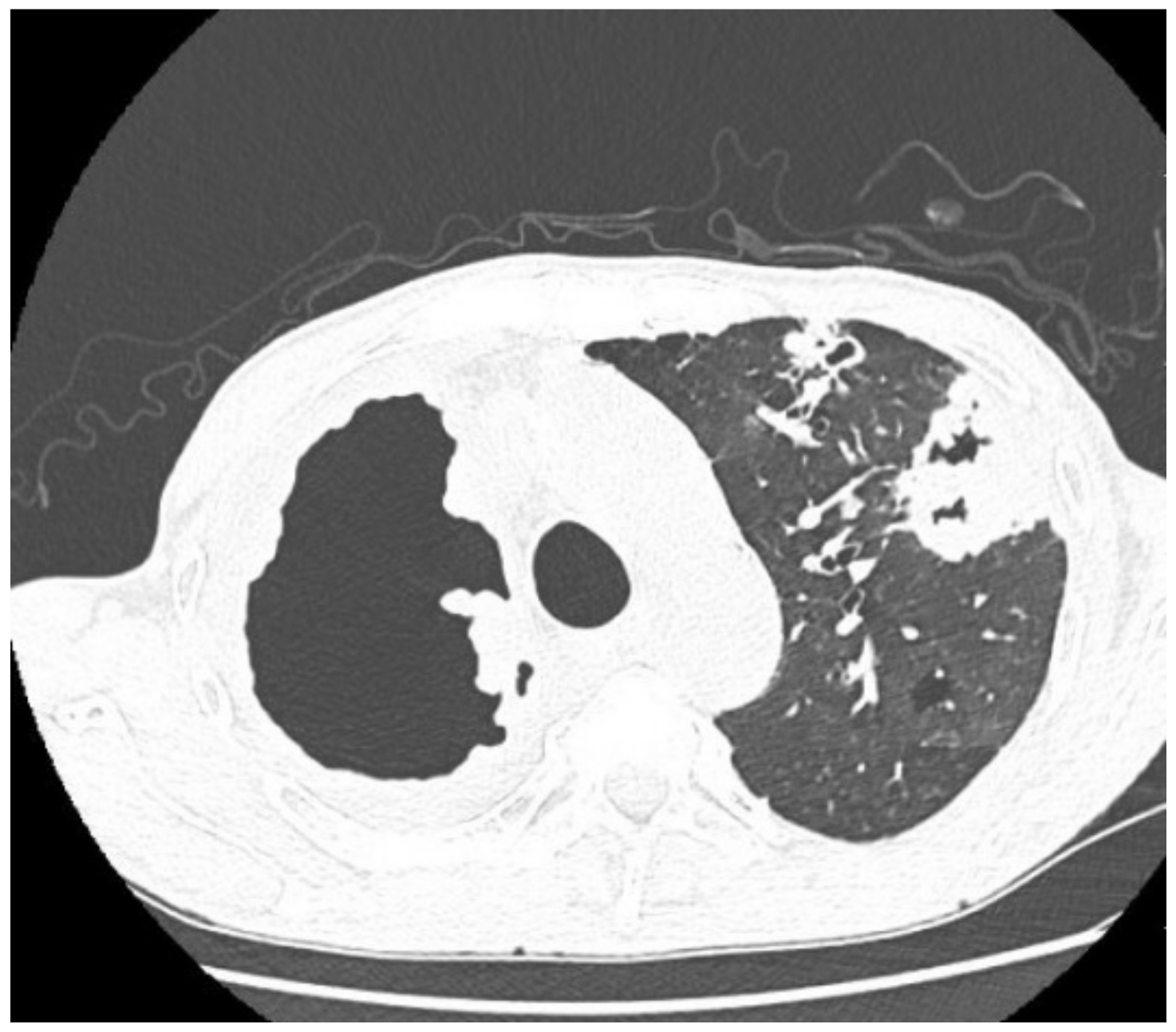
| Imaging studies during antimycobacterial treatment | Chest X-ray: After 2 mo of treatment—an irregular infiltration in the upper area of the right lung (Figure 2A). After 5 mo of treatment—reduced infiltration (Figure 2B) Chest CT: After 5 mo of treatment an oval nodular lesion with calcification, enlarged right and left mediastinal nodes, nodular lesions in the lungs (Figure 3A,B) | Chest X-ray: No improvement by radiographic criteria after 17 mo of treatment (Figure 5B). Still no improvement by radiographic criteria after further 2 years of treatment (Figure 5C) Chest CT: An extensive cavity in the upper lobe of the right lung, infiltrative lesions in the middle lobe, cavernous lesions in the left lung (Figure 4) |
| Additional tests performed during hospitalization | anti-HIV antibodies (+), HIV-RNA (+), p24 antigen (−), CD4+ lymphocytes—32 cells/mm3Bronchofiberoscopy: Histopathological analysis—squamous cell lung carcinoma (p40+, TTF−, CK7−) | No data |
| Course and outcome of treatment | Improved general health, 4 kg weight gain, reduction of cough, abscess resolution and reduction in fistula size; Recovery from TB, oncological treatment postponed | Therapy periodically interrupted; Still no recovery from TB after 2 years of treatment |
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kumar, B. The End TB Strategy: A global rally. Lancet Respir. Med. 2014, 2, 943. [Google Scholar] [CrossRef]
- Dooley, K.E.; Chaisson, R.E. Tuberculosis and diabetes mellitus: Convergence of two epidemics. Lancet Infect. Dis. 2009, 9, 737–746. [Google Scholar] [CrossRef]
- Gandhi, N.R.; Nunn, P.P.; Dheda, K.; Schaaf, H.S.; Zignol, M.; van Soolingen, D.; Jensen, P.; Bayona, J. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet 2010, 375, 1830–1843. [Google Scholar] [CrossRef]
- Kozińska, M.; Podlasin, R.; Ropelewska-Łącka, K.; Wojtycha-Kwaśnica, B.; Bajera-Mitschein, I.; Augustynowicz-Kopeć, E. TB and COVID-19 coinfection. Int. J. Tuberc. Lung Dis. 2021, 25, 776–777. [Google Scholar] [CrossRef]
- Parwati, I.; van Crevel, R.; van Soolingen, D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 2010, 10, 103–111. [Google Scholar] [CrossRef]
- Steenwinkel, J.E.; ten Kate, M.T.; de Knegt, G.J.; Kremer, K.; Aarnoutse, R.E.; Boeree, M.J.; Verbrugh, H.A.; van Soolingen, D.; Bakker-Woudenberg, I.A. Drug susceptibility of Mycobacterium tuberculosis Beijing genotype and association with MDR TB. Emerg. Infect. Dis. 2012, 18, 660–663. [Google Scholar] [CrossRef]
- Shabbeer, A.; Cowan, L.S.; Ozcaglar, C.; Rastogi, N.; Vandenberg, S.L.; Yener, B.; Bennett, K.P. TB-Lineage: An online tool for classification and analysis of strains of Mycobacterium tuberculosis complex. Infect. Genet. Evol. 2012, 12, 789–797. [Google Scholar] [CrossRef]
- Van Soolingen, D.; Qian, L.; de Haas, P.E. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 1995, 33, 3234–3238. [Google Scholar] [CrossRef]
- Luo, T.; Comas, I.; Luo, D.; Lu, B.; Wu, J.; Wei, L.; Yang, C.; Liu, Q.; Gan, M.; Sun, G.; et al. Southern East Asian origin and coexpansion of Mycobacterium tuberculosis Beijing family with Han Chinese. Proc. Natl. Acad. Sci. USA 2015, 112, 8136–8141. [Google Scholar] [CrossRef]
- Mokrousov, I.; Narvskaya, O.; Otten, T.; Vyazovaya, A.; Limeschenko, E.; Steklova, L.; Vyshnevskyi, B. Phylogenetic reconstruction within Mycobacterium tuberculosis Beijing genotype in northwestern Russia. Res. Microbiol. 2002, 153, 629–637. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Gomes, L.L.; Amaral, E.P.; Andrade, M.R.; Almeida, F.M.; Rezende, A.L.; Lanes, V.R.; Carvalho, E.C.; Suffys, P.N.; Mokrousov, I.; et al. Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. J. Clin. Microbiol. 2014, 52, 2615–2624. [Google Scholar] [CrossRef]
- Anh, D.D.; Borgdorff, M.W.; Van, L.N.; Lan, N.T.; van Gorkom, T.; Kremer, K.; van Soolingen, D. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 2000, 6, 302–305. [Google Scholar] [CrossRef]
- Drobniewski, F.; Balabanova, Y.; Nikolayevsky, V.; Ruddy, M.; Kuznetzov, S.; Zakharova, S.; Melentyev, A.; Fedorin, I. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA 2005, 293, 2726–2731. [Google Scholar] [CrossRef]
- Glynn, J.R.; Kremer, K.; Borgdorff, M.W.; Mar, P.R.; van Soolingen, D. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. European concerted action on new generation genetic markers and techniques for the epidemiology and control of tuberculosis. Emerg. Infect. Dis. 2006, 12, 736–743. [Google Scholar] [CrossRef]
- Mokrousov, I.; Jiao, W.W.; Sun, G.Z.; Liu, J.W.; Valcheva, V.; Li, M.; Narvskaya, O.; Shen, A.D. Evolution of drug resistance in different sublineages of Mycobacterium tuberculosis Beijing genotype. Antimicrob. Agents Chemother. 2006, 50, 2820–2823. [Google Scholar] [CrossRef][Green Version]
- Abebe, F.; Bjune, G. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille Calmette–Guerin (BCG) vaccines: Is there a link? Clin. Exp. Immunol. 2006, 145, 389–397. [Google Scholar] [CrossRef]
- Kremer, K.; van-der-Werf, M.J.; Au, B.K.; Anh, D.D.; Kam, K.M.; van-Doorn, H.R.; Borgdorff, M.W.; van-Soolingen, D. Vaccine-induced immunity circumvented by typical Mycobacterium tuberculosis Beijing strains. Emerg. Infect. Dis. 2009, 15, 335–339. [Google Scholar] [CrossRef]
- Toungoussova, O.S.; Caugant, D.A.; Sandven, P.; Mariandyshev, A.O.; Bjune, G. Impact of drug resistance on fitness of Mycobacterium tuberculosis strains of the W-Beijing genotype. FEMS Immunol. Med. Microbiol. 2004, 42, 281–290. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Li, W.; Zhang, X.; Wang, W.; Li, C. The study on the association between Beijing genotype family and drug susceptibility phenotypes of Mycobacterium tuberculosis in Beijing. Sci. Rep. 2017, 7, 15076. [Google Scholar] [CrossRef]
- Kozińska, M.; Augustynowicz-Kopeć, E. Drug Resistance and Population Structure of Mycobacterium tuberculosis Beijing Strains Isolated in Poland. Pol. J. Microbiol. 2015, 64, 399–401. [Google Scholar] [CrossRef][Green Version]
- Cerezo-Cortés, M.I.; Rodríguez-Castillo, J.G.; Hernández-Pando, R.; Murcia, M.I. Circulation of M. tuberculosis Beijing genotype in Latin America and the Caribbean. Pathog. Glob. Health 2019, 113, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Hanekom, M.; van der Spuy, G.D.; Streicher, E.; Ndabambi, S.L.; McEvoy, C.R.; Kidd, M.; Beyers, N.; Victor, T.C.; van Helden, P.D.; Warren, R.M. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability tospread and cause disease. J. Clin. Microbiol. 2007, 45, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, D.; Hanekom, M.; Mata, D.; Gey van Pittius, N.C.; van Helden, P.D.; Warren, R.M.; Hernandez-Pando, R. Mycobacterium tuberculosis strains with the Beijing genotype demonstrate variability in virulence associated with transmission. Tuberculosis 2010, 90, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Augustynowicz-Kopeć, E.; Zozio, T.; Rastogi, N.; Zwolska, Z. Spoligotype-based comparative population structure analysis of multidrug-resistant and isoniazid-monoresistant Mycobacterium tuberculosis complex clinical isolates in Poland. J. Clin. Microbiol. 2010, 48, 3899–3909. [Google Scholar] [CrossRef]
- Demay, C.; Liens, B.; Burguière, T.; Hill, V.; Couvin, D.; Millet, J.; Mokrousov, I.; Sola, C.; Zozio, T.; Rastogi, N. SITVITWEB--a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect. Genet. Evol. 2012, 12, 755–766. [Google Scholar] [CrossRef]
- WHO. Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27–29 October 2020; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Palanisamy, G.S.; Smith, E.E.; Shanley, C.A.; Ordway, D.J.; Orme, I.M.; Basaraba, R.J. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis 2008, 88, 295–306. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chang, J.R.; Huang, W.F.; Hsu, S.C.; Kuo, S.C.; Sun, J.R.; Dou, H.Y. The pattern of cytokine production in vitro induced by ancient and modern Beijing Mycobacterium tuberculosis strains. PLoS ONE 2014, 9, e94296. [Google Scholar] [CrossRef]
- Wang, C.; Peyron, P.; Mestre, O.; Kaplan, G.; van Soolingen, D.; Gao, Q.; Gicquel, B.; Neyrolles, O. Innate immune response to Mycobacterium tuberculosis Beijing and other genotypes. PLoS ONE 2010, 5, e13594. [Google Scholar] [CrossRef]
- Zhang, J.; Mi, L.; Wang, Y.; Liu, P.; Liang, H.; Huang, Y.; Lv, B.; Yuan, L. Genotypes and drug susceptibility of Mycobacterium tuberculosis Isolates in Shihezi, Xinjiang Province, China. BMC Res. Notes 2012, 5, 309. [Google Scholar] [CrossRef]
- Augustynowicz-Kopeć, E.; Jagielski, T.; Kozińska, M.; Kremer, K.; van Soolingen, D.; Bielecki, J.; Zwolska, Z. Transmission of tuberculosis within family-households. J. Infect. 2012, 64, 596–608. [Google Scholar] [CrossRef]
- Brudey, K.; Driscoll, J.R.; Rigouts, L.; Prodinger, W.M.; Gori, A.; Al-Hajoj, S.A.; Allix, C.; Aristimuño, L.; Arora, J.; Baumanis, V.; et al. Mycobacterium tuberculosis complex genetic diversity: Mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006, 6, 2384–2387. [Google Scholar] [CrossRef]
- Iwamoto, T. Population structure analysis of Mycobacterium tuberculosis Beijing family in Japan. Kekkaku 2009, 84, 755–759. [Google Scholar]
- Schurch, A.C.; Kremer, K.; Warren, R.M.; Hung, N.V.; Zhao, Y.; Wan, K.; Boeree, M.J.; Siezen, R.J.; Smith, N.H.; van Soolingen, D. Mutations in the regulatory network underlie the recent clonal expansionof a dominant subclone of the Mycobacterium tuberculosis Beijing genotype. Infect. Genet. Evol. 2011, 11, 587–597. [Google Scholar] [CrossRef]
- Lopez, B.; Aguilar, D.; Orozco, H.; Burger, M.; Espitia, C.; Ritacco, V.; Barrera, L.; Kremer, K.; Hernandez-Pando, R.; Huygen, K.; et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 2003, 133, 30–37. [Google Scholar] [CrossRef]
- Van Laarhoven, A.; Mandemakers, J.J.; Kleinnijenhuis, J.; Enaimi, M.; Lachmandas, E.; Joosten, L.A.; Ottenhoff, T.H.; Netea, M.G.; van Soolingen, D.; van Crevel, R. Low induction of proinflammatory cytokines parallels evolutionary success of modern strains within the Mycobacterium tuberculosis Beijing genotype. Infect. Immun. 2013, 81, 3750–3756. [Google Scholar] [CrossRef]
- Nieto Ramirez, L.M.; Ferro, B.E.; Diaz, G.; Anthony, R.M.; de Beer, J.; van Soolingen, D. Genetic profiling of Mycobacterium tuberculosis revealed “modern” Beijing strains linked to MDR-TB from Southwestern Colombia. PLoS ONE 2020, 15, e0224908. [Google Scholar] [CrossRef]
- María Irene, C.C.; Juan Germán, R.C.; Gamaliel, L.L.; Dulce Adriana, M.E.; Estela Isabel, B.; Brenda Nohemí, M.C.; Payan Jorge, B.; Zyanya Lucía, Z.B.; Myriam, B.D.V.; Fernanda, C.G.; et al. Profiling the immune response to Mycobacterium tuberculosis Beijing family infection: A perspective from the transcriptome. Virulence 2021, 12, 1689–1704. [Google Scholar] [CrossRef]
- Murcia, M.I.; Manotas, M.; Jiménez, Y.J.; Hernández, J.; Cortès, M.I.; López, L.E.; Zozio, T.; Rastogi, N. First case of multidrug-resistant tuberculosis caused by a rare “Beijing-like” genotype of Mycobacterium tuberculosis in Bogotá, Colombia. Infect. Genet. Evol. 2010, 10, 678–681. [Google Scholar] [CrossRef]
- Buu, T.N.; Huyen, M.N.; Lan, N.T.; Quy, H.T.; Hen, N.V.; Zignol, M.; Borgdorff, M.W.; Cobelens, F.G.; van Soolingen, D. The Beijing genotype is associated with young age and multidrug-resistant tuberculosis in rural Vietnam. Int. J. Tuberc. Lung Dis. 2009, 13, 900–906. [Google Scholar] [PubMed]
- Lan, N.T.; Lien, H.T.; Tung, L.B.; Borgdorff, M.W.; Kremer, K.; van Soolingen, D. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg. Infect. Dis. 2003, 9, 1633–1635. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozińska, M.; Skowroński, M.; Gruszczyński, P.; Augustynowicz-Kopeć, E. Multidrug-Resistant Tuberculosis—Diagnostic Procedures and Treatment of Two Beijing-like TB Cases. Diagnostics 2022, 12, 1699. https://doi.org/10.3390/diagnostics12071699
Kozińska M, Skowroński M, Gruszczyński P, Augustynowicz-Kopeć E. Multidrug-Resistant Tuberculosis—Diagnostic Procedures and Treatment of Two Beijing-like TB Cases. Diagnostics. 2022; 12(7):1699. https://doi.org/10.3390/diagnostics12071699
Chicago/Turabian StyleKozińska, Monika, Marcin Skowroński, Paweł Gruszczyński, and Ewa Augustynowicz-Kopeć. 2022. "Multidrug-Resistant Tuberculosis—Diagnostic Procedures and Treatment of Two Beijing-like TB Cases" Diagnostics 12, no. 7: 1699. https://doi.org/10.3390/diagnostics12071699
APA StyleKozińska, M., Skowroński, M., Gruszczyński, P., & Augustynowicz-Kopeć, E. (2022). Multidrug-Resistant Tuberculosis—Diagnostic Procedures and Treatment of Two Beijing-like TB Cases. Diagnostics, 12(7), 1699. https://doi.org/10.3390/diagnostics12071699








