Retained Placenta Percreta with Acquired Uterine Arteriovenous Malformation—Case Report and Short Review of the Literature
Abstract
1. Introduction
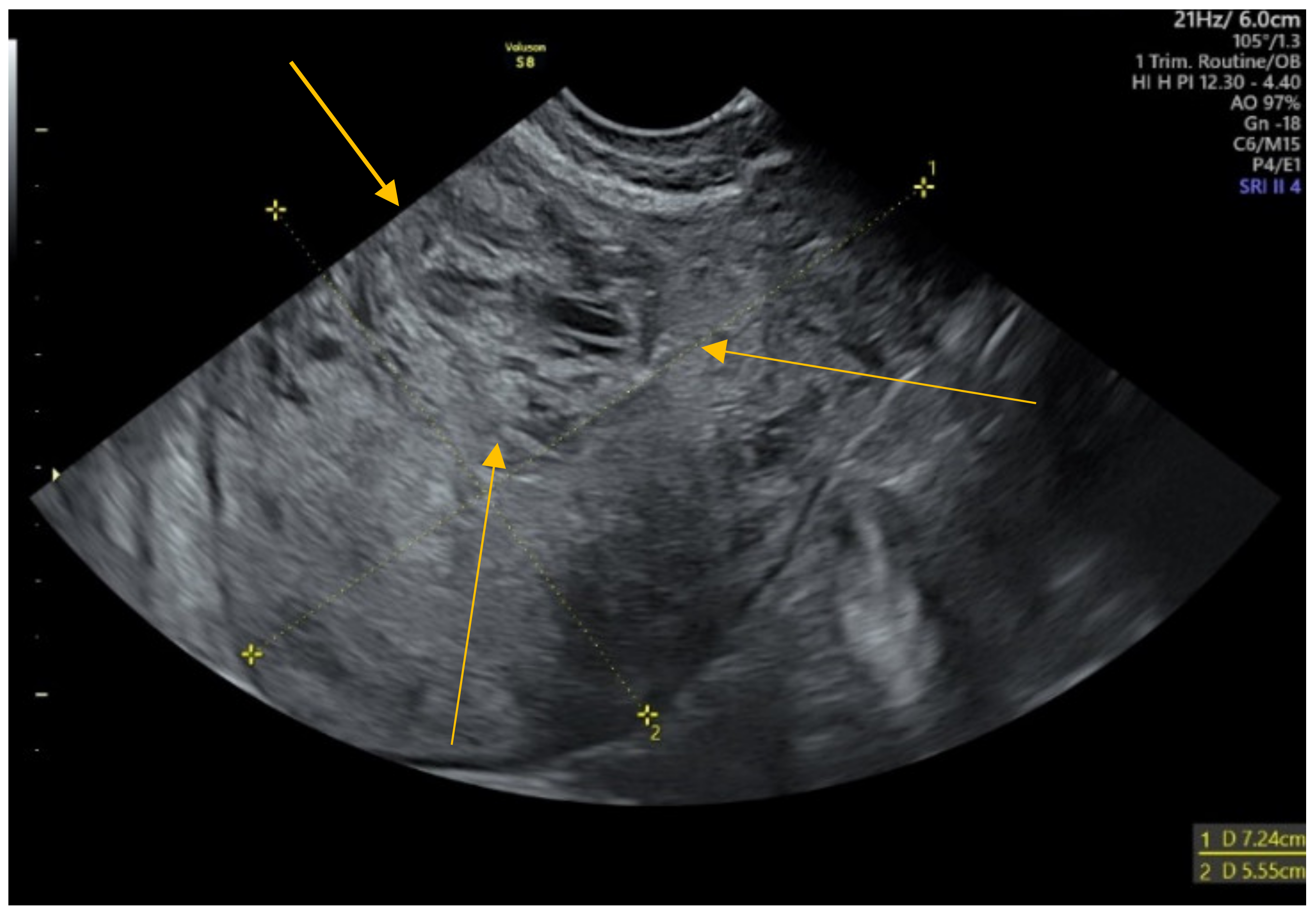
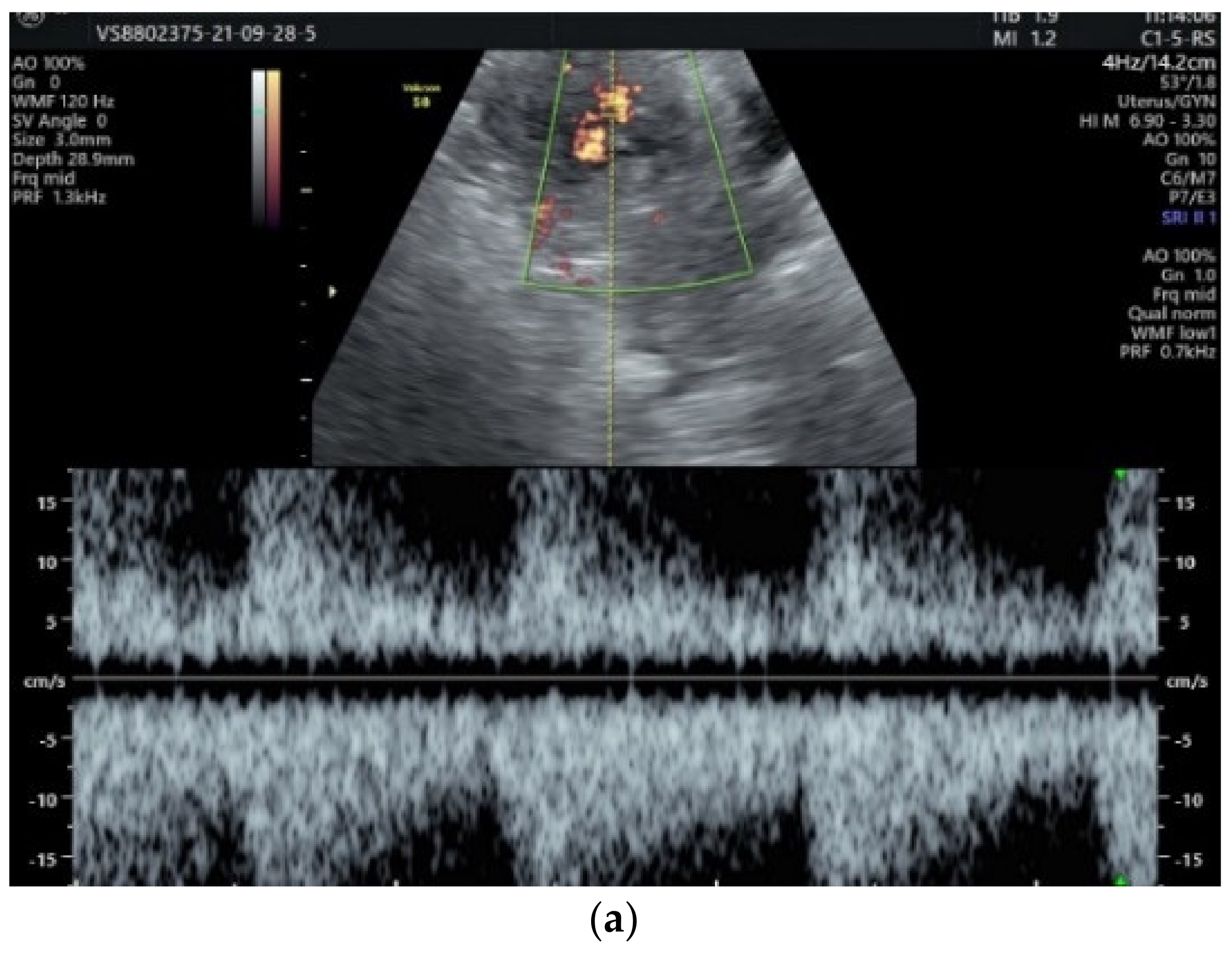
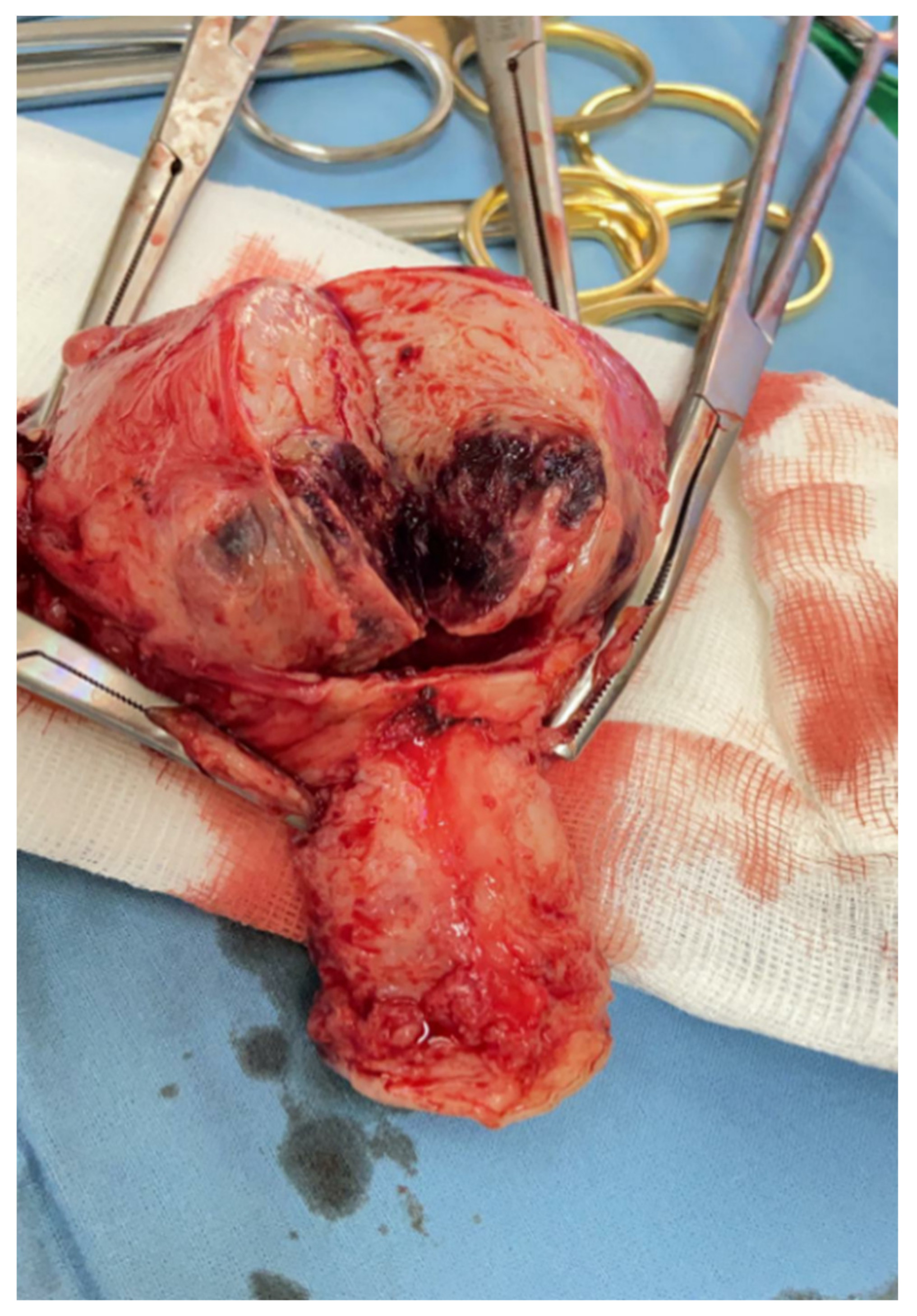
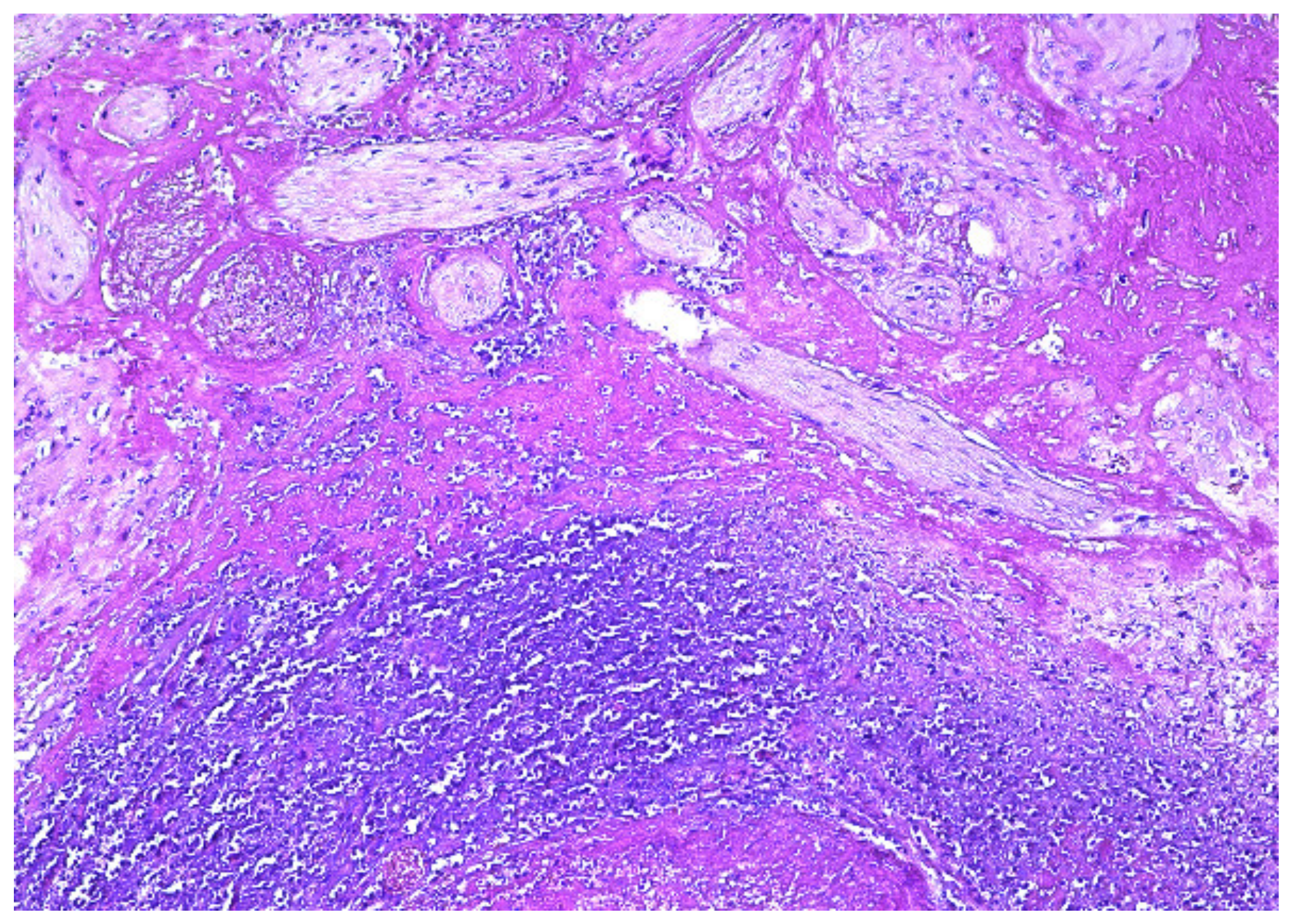
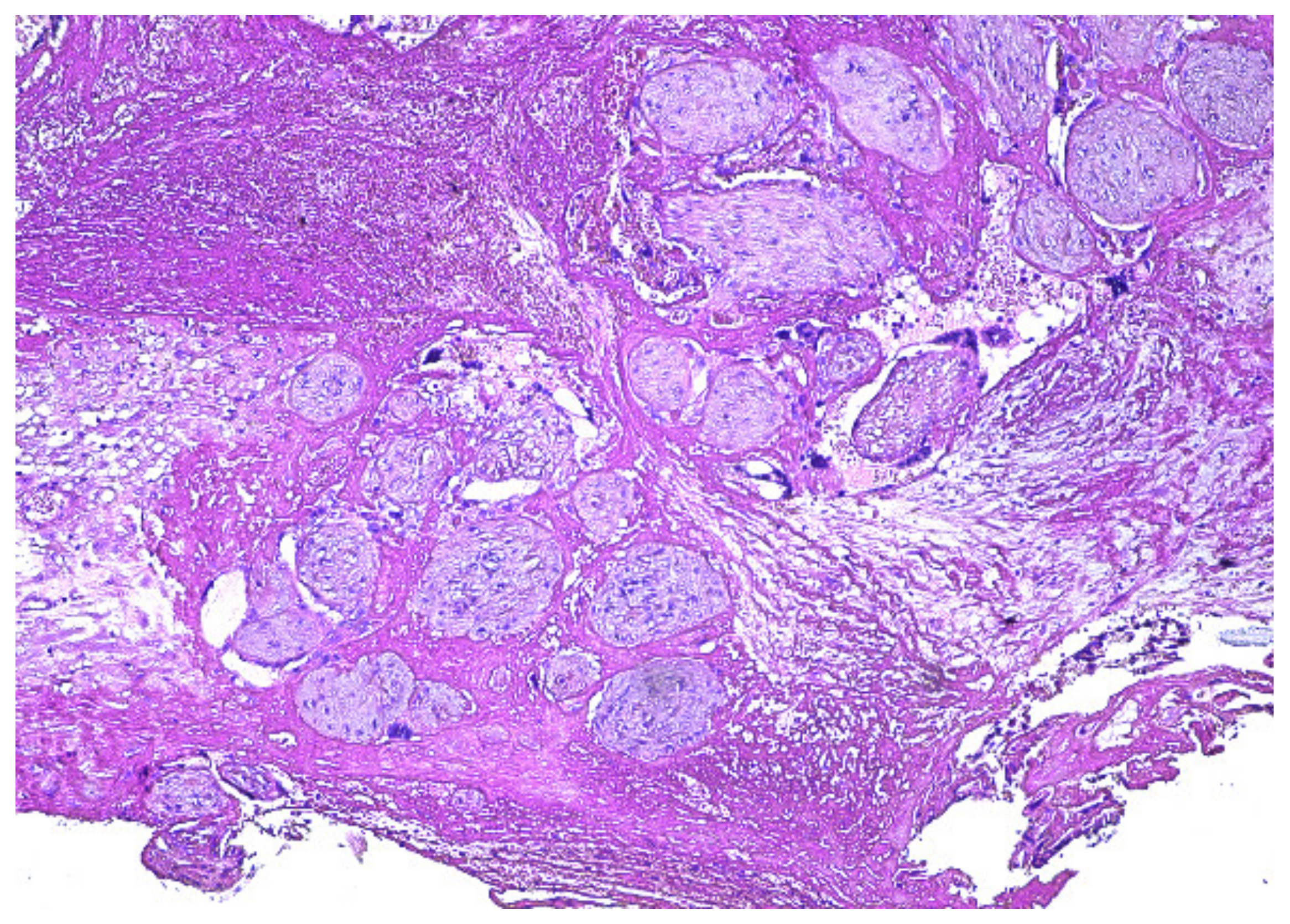
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohi, M.P.; Rizzuto, G.A.; Fidelman, N.; Lucero, J.; Thiet, M.P. Retained Placenta Accreta Mimicking Choriocarcinoma. Case Rep. Pathol. 2015, 2015, 167986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oyelese, Y.; Smulian, J.C. Placenta previa, placenta accreta, and vasa previa. Obstet. Gynecol. 2006, 107, 927–941. [Google Scholar] [CrossRef]
- Morlando, M.; Collins, S. Placenta Accreta Spectrum Disorders: Challenges, Risks, and Management Strategies. Int. J. Womens Health 2020, 12, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, D.; Zhong, M.; He, Y.; Su, C. Management of patients with placenta accreta in association with fever following vaginal delivery. Medicine 2017, 96, e6279. [Google Scholar] [CrossRef]
- Wortman, A.C.; Alexander, J.M. Placenta accreta, increta, and percreta. Obstet. Gynecol. Clin. N. Am. 2013, 40, 137–154. [Google Scholar] [CrossRef]
- Oppenheimer, L. Diagnosis and management of placenta previa. J. Obstet. Gynaecol. Can. 2007, 29, 261–266. [Google Scholar] [CrossRef]
- Eller, A.G.; Bennett, M.A.; Sharshiner, M.; Masheter, C.; Soisson, A.P.; Dodson, M.; Silver, R.M. Maternal Morbidity in Cases of Placenta Accreta Managed by a Multidisciplinary Care Team Compared With Standard Obstetric Care. Obstet. Gynecol. 2011, 117, 331–337. [Google Scholar] [CrossRef]
- Abramowicz, J.S.; Sheiner, E. In utero imaging of the placenta: Importance for diseases of pregnancy. Placenta 2007, 28, S14–S22. [Google Scholar] [CrossRef]
- Sellmyer, M.A.; Desser, T.S.; Maturen, K.E.; Jeffrey, R.B., Jr.; Kamaya, A. Physiologic, histologic, and imaging features of retained products of conception. Radiographics 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Mekaru, K.; Oishi, S.; Akamine, K.; Heshiki, C.; Aoki, Y. Spontaneous Regression of Uterine Arteriovenous Malformations with Conservative Management. Case Rep. Obstet. Gynecol. 2017, 2017, 6437670. [Google Scholar] [CrossRef]
- Kim, S.M.; Ahn, H.Y.; Choi, M.J.; Kang, Y.D.; Park, J.W.; Park, C.H.; Kim, J.S. Uterine arteriovenous malformation with positive serum beta-human chorionic gonadotropin: Embolization of both uterine arteries and extra-uterine feeding arteries. Obstet. Gynecol. Sci. 2016, 59, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kim, G.S. Obstetric iatrogenic arterial injuries of the uterus: Diagnosis with US and treatment with transcatheter arterial embolization. Radiographics 2002, 22, 35–46. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.; Neyastani, A.; Buckley, A.R.; Chang, S.D.; Legiehn, G.M. Uterine arteriovenous malformations: From diagnosis to treatment. J. Ultrasound Med. 2006, 25, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Park, J.K.; Park, S.H.; Kim, H.B.; Park, S.T. Uterine arteriovenous malformation caused by intrauterine instrumentation for laparoscopic surgery due to left tubal pregnancy. Obstet. Gynecol. Sci. 2014, 57, 419–423. [Google Scholar] [CrossRef]
- Mishina, M.; Hasegawa, J.; Nakamura, M.; Ichizuka, K.; Sekizawa, A.; Okai, T. Sonohysterography is a useful diagnostic approach for uterine arteriovenous malformation. J. Obstet. Gynaecol. Res. 2014, 40, 1811–1813. [Google Scholar] [CrossRef]
- Lee, T.Y.; Kim, S.H.; Lee, H.J.; Kim, M.J.; Lee, S.K.; Kim, Y.H.; Cho, S.H. Ultrasonographic indications for conservative treatment in pregnancy-related uterine arteriovenous malformations. Acta Radiol. 2014, 55, 1145–1152. [Google Scholar] [CrossRef]
- Masood, L.; Rana, A.I.; Khan, Z.A.; Nosheen, S.; Ali, H.; Anwar, J. Imaging spectrum of acquired uterine vascular abnormalities with angiographic correlates, a pictorial review. Egypt. J. Radiol. Nucl. Med. 2022, 53, 6. [Google Scholar] [CrossRef]
- Touhami, O.; Gregoire, J.; Noel, P.; Trinh, X.B.; Plante, M. Uterine arteriovenous malformations following gestational trophoblastic neoplasia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 54–59. [Google Scholar] [CrossRef]
- Eller, A.; Porter, T.; Soisson, P.; Silver, R. Optimal management strategies for placenta accreta. Int. J. Obstet. Gynaecol. 2009, 116, 648–654. [Google Scholar] [CrossRef]
- Schoolmeester, J.K.; Bakkum-Gamez, J.N. Retained Products of Conception After Cesarean Section and Occult Placenta Accreta. Mayo Clin. Proc. 2020, 95, 2462–2463. [Google Scholar] [CrossRef]
- Takeda, A.; Koike, W. Conservative endovascular management of retained placenta accreta with marked vascularity after abortion or delivery. Arch. Gynecol. Obstet. 2017, 296, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Soeda, S.; Kyozuka, H.; Suzuki, S.; Yasuda, S.; Nomura, Y.; Fujimori, K. Uterine artery embolization for uterine arteriovenous malformation is associated with placental abnormalities in the subsequent pregnancy: Two cases report. Fukushima J. Med. Sci. 2014, 60, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.K.; Thomassee, M.S. Acquired Uterine Arteriovenous Malformation and Retained Placenta Increta. Obstet. Gynecol. 2015, 126, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.T.; Tressler, T.B.; Willis, G.S.; Martinez, F.J.; Peisner, D.B.; Goodman, J.D.; Taboada, C.D. Arteriovenous malformation identification after conservative management of placenta percreta with uterine artery embolization and adjunctive therapy. Am. J. Obstet. Gynecol. 2011, 204, e4–e8. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.P.; Chandraharan, E. Placenta accreta spectrum: Risk factors, diagnosis and management with special reference to the Triple P procedure. Women’s Health 2019, 15, 1745506519878081. [Google Scholar] [CrossRef]
- Silver, R.M.; Barbour, K.D. Placenta accreta spectrum: Accreta, increta, and percreta. Obstet. Gynecol. Clin. N. Am. 2015, 42, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Gersell, D.; Kraus, F. Diseases of the placenta. In Blaustein’s Pathology of the Female Genital Tract, 7th ed.; Kurman, R.J., Ellenson, L.H., Ronnett, B.M., Eds.; Springer International Publishing AG: New York, NY, USA, 2019; pp. 999–1073. [Google Scholar] [CrossRef]
- Okunowo, A.A.; Ohazurike, E.O.; Habeebu-Adeyemi, F.M. Undiagnosed placenta praevia percreta: A rare case report and review of management. Niger. Postgrad. Med. J. 2019, 26, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Collins, S.L.; Jurkovic, D.; Burton, G.J. Accreta placentation: A systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am. J. Obstet. Gynecol. 2016, 215, 712–721. [Google Scholar] [CrossRef]
- Jauniaux, E.; Bhide, A.; Kennedy, A.; Woodward, P.; Hubinont, C.; Collins, S.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int. J. Gynecol. Obstet. 2018, 140, 274–280. [Google Scholar] [CrossRef]
- Jauniaux, E.; Hussein, A.M.; Fox, K.A.; Collins, S.L. New evidence-based diagnostic and management strategies for placenta accreta spectrum disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 61, 75–88. [Google Scholar] [CrossRef]
- Pather, S.; Strockyj, S.; Richards, A.; Campbell, N.; de Vries, B.; Ogle, R. Maternal outcome after conservative management of placenta percreta at caesarean section: A report of three cases and a review of the literature. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Perez-Delboy, A.; Wright, J.D. Surgical management of placenta accreta: To leave or remove the placenta? Int. J. Obstet. Gynaecol. 2014, 121, 163–169, discussion 169-70. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.; Battarbee, A.N.; Ernst, L.M.; Peaceman, A.M. Occult placenta accreta: Risk factors, adverse obstetrical outcomes, and recurrence in subsequent pregnancies. Am. J. Perinatol. 2019, 36, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.J.; Jones, M.; Taani, J.A.; Buhimschi, C.; Dowell, J.D. A Systematic Review of Acquired Uterine Arteriovenous Malformations: Pathophysiology, Diagnosis, and Transcatheter Treatment. AJP Rep. 2016, 6, e6–e14. [Google Scholar] [CrossRef]
- Mungen, E.; Yergok, Y.Z.; Ertekin, A.A.; Ergur, A.R.; Uçmakli, E.; Aytaçlar, S. Color Doppler sonographic features of uterine arteriovenous malformations: Report of two cases. Ultrasound Obstet. Gynecol. 1997, 10, 215–219. [Google Scholar] [CrossRef]
- Moro, F.; Mavrelos, D.; Pateman, K.; Holland, T.; Hoo, W.L.; Jurkovic, D. Prevalence of pelvic adhesions on ultrasound examination in women with a history of Cesarean section. Ultrasound Obstet. Gynecol. 2014, 45, 223–228. [Google Scholar] [CrossRef]
- Butureanu, S.A.; Butureanu, T.A.S. Pathophysiology of adhesions. Chirurgia 2014, 109, 293–298. [Google Scholar]
- Zhu, Y.-P.; Sun, Z.-J.; Lang, J.-H.; Pan, J. Clinical Characteristic and Management of Acquired Uterine Arteriovenous Malformation. Chin. Med. J. 2018, 131, 2489–2491. [Google Scholar] [CrossRef]
- Nakashololo, T.; Khan, N.; Dunn, Z.; Snyman, L.; Ismail, S.M. Uterine arteriovenous malformations, clinical and radiological considerations: A report of two cases. Radiol. Case Rep. 2021, 16, 1924–1929. [Google Scholar] [CrossRef]
- Lollie, T.K.; Raman, S.S.; Qorbani, A.; Farzaneh, T.; Moatamed, N.A. Rare occurrence of uterine arteriovenous malformation clinically mimicking a malignant growth: A critical reminder for pathologists. Autops. Case Rep. 2020, 10, e2020144. [Google Scholar] [CrossRef]
- Timmerman, D.; Wauters, J.; Van Calenbergh, S.; Van Schoubroeck, D.; Maleux, G.; Van Den Bosch, T.; Spitz, B. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound Obstet. Gynecol. 2003, 21, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.S.; Rebarber, A.; Eckstein, D.A.; Lookstein, R.A.; Saltzman, D.H. Successful Bilateral Uterine Artery Embolization During an Ongoing Pregnancy. Obstet. Gynecol. 2009, 113, 554–556. [Google Scholar] [CrossRef]
- Kido, A.; Togashi, K.; Koyama, T.; Ito, H.; Tatsumi, K.; Fujii, S.; Konishi, J. Retained Products of Conception Masquerading as Acquired Arteriovenous Malformation. J. Comput. Assist. Tomogr. 2003, 27, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Soper, J.T.; Mutch, D.G.; Schink, J.C. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol. Oncol. 2004, 93, 575–585. [Google Scholar] [CrossRef]
- Ju, D.H.; Yi, S.W.; Sohn, W.S.; Lee, S.S. Acquired uterine vascular abnormalities associated with persistent human chorionic gonadotropin: Experience at a Korean teaching hospital. Taiwan J. Obstet. Gynecol. 2015, 54, 654–659. [Google Scholar] [CrossRef][Green Version]
- Zeng, X.; Liu, X.; Tian, Q.; Xue, Y.; An, R. Placental site trophoblastic tumor: A case report and literature review. Intractable Rare Dis. Res. 2015, 4, 147–151. [Google Scholar] [CrossRef]
- Chen, J.; Samson, S.-L.; Bentley, J.; Chen, Y. Persistent mild increase of human chorionic gonadotropin levels in a 31-year-old woman after spontaneous abortion. Can. Med. Assoc. J. 2016, 188, 17–18. [Google Scholar] [CrossRef][Green Version]
- Fleming, H.; Ostör, A.G.; Pickel, H.; Fortune, D.W. Arteriovenous malformations of the uterus. Obstet. Gynecol. 1989, 73, 209–214. [Google Scholar]
- Peitsidis, P.; Manolakos, E.; Tsekoura, V.; Kreienberg, R.; Schwentner, L. Uterine arteriovenous malformations induced after diagnostic curettage: A systematic review. Arch. Gynecol. Obstet. 2011, 284, 1137–1151. [Google Scholar] [CrossRef]
- Clark, M.; Tchrakian, N.; Clarke, B.; Metser, U.; Bouchard-Fortier, G. Placenta increta mimicking placental site trophoblastic tumor. Int. J. Gynecol. Cancer 2021, 31, 1481–1485. [Google Scholar] [CrossRef]
- Calzolari, S.; Cozzolino, M.; Castellacci, E.; Dubini, V.; Farruggia, A.; Sisti, G. Hysteroscopic Management of Uterine Arteriovenous Malformation. J. Soc. Laparoendosc. Surg. 2017, 21, e2016.00109. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Carinelli, S.; Guerrieri, M.E.; Aletti, G.D. Placental site trophoblastic tumor and epithelioid trophoblastic tumor: Clinical and pathological features, prognostic variables and treatment strategy. Gynecol. Oncol. 2019, 153, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Li, Y.; He, J.; Liu, J. Placental site trophoblastic tumor: Lymphatic spread and possible target markers. Gynecol. Oncol. 2010, 116, 430–437. [Google Scholar] [CrossRef]
- Sentilhes, L.; Ambroselli, C.; Kayem, G.; Provansal, M.; Fernandez, H.; Perrotin, F.; Winer, N.; Pierre, F.; Benachi, A.; Dreyfus, M.; et al. Maternal Outcome After Conservative Treatment of Placenta Accreta. Obstet. Gynecol. 2010, 115, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Esposito, R.; Saccone, G.; Della Corte, L.; Sarno, L.; Morlando, M.; Maruotti, G.M.; Migliorini, S.; D’Alessandro, P.; Arduino, B.; et al. Use of routine ureteral stents in cesarean hysterectomy for placenta accreta. J. Matern. Neonatal Med. 2021, 34, 386–389. [Google Scholar] [CrossRef] [PubMed]





| Differential Diagnosis | Characteristic Ultrasound Features |
|---|---|
| Uterine fibroids | Capsule |
| Adenomyosis | Alteration of endo-myometrial line |
| Intrauterine characteristic ectopic foci | |
| Location near endometrium | |
| Isthmocele | Triangular appearance of cesarean scar hernia |
| Differential Diagnosis | Characteristic Ultrasound Features | β-HGC Values |
|---|---|---|
| Invasive mole Choriocarcinoma | Arteriovenous abnormalities | Higher Over 50,000 mUI/mL With rapid increase |
| PSTT | Arteriovenous shunt | Lower Over 100 mUI/mL With variable increase |
| RPC | Increased systolic arterial type vascularization | Moderately increased Over 100 mUI/mL Slight decrease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butureanu, T.; Balan, R.A.; Socolov, R.; Ioanid, N.; Socolov, D.; Gafitanu, D. Retained Placenta Percreta with Acquired Uterine Arteriovenous Malformation—Case Report and Short Review of the Literature. Diagnostics 2022, 12, 904. https://doi.org/10.3390/diagnostics12040904
Butureanu T, Balan RA, Socolov R, Ioanid N, Socolov D, Gafitanu D. Retained Placenta Percreta with Acquired Uterine Arteriovenous Malformation—Case Report and Short Review of the Literature. Diagnostics. 2022; 12(4):904. https://doi.org/10.3390/diagnostics12040904
Chicago/Turabian StyleButureanu, Tudor, Raluca Anca Balan, Razvan Socolov, Nicolae Ioanid, Demetra Socolov, and Dumitru Gafitanu. 2022. "Retained Placenta Percreta with Acquired Uterine Arteriovenous Malformation—Case Report and Short Review of the Literature" Diagnostics 12, no. 4: 904. https://doi.org/10.3390/diagnostics12040904
APA StyleButureanu, T., Balan, R. A., Socolov, R., Ioanid, N., Socolov, D., & Gafitanu, D. (2022). Retained Placenta Percreta with Acquired Uterine Arteriovenous Malformation—Case Report and Short Review of the Literature. Diagnostics, 12(4), 904. https://doi.org/10.3390/diagnostics12040904








