Abstract
Pericardial effusions can be caused by diverse etiologies, including heart-related conditions, kidney failure, trauma, infections, autoimmune diseases, and cancer. This systematic review aimed to assess the role of cytology in identifying the most prevalent cancers related to malignant pericardial effusions (MPEs), the ability of cytology, compared to histology, to detect cancer while evaluating pericardial effusions, and the prognostic impact of MPEs. Four electronic databases were investigated using a predefined algorithm, and specific inclusion and exclusion criteria. We found that the most prevalent primaries associated with MPEs were lung (especially NSCLCs), breast, hematolymphoid, and gastrointestinal cancers. MPEs tended to be hemorrhagic rather than serous or serosanguinous and to occupy larger volumes compared to non-neoplastic effusions. In addition, cytology was shown to exhibit an enhanced ability to detect cancer compared to biopsy in most of the included studies. Lastly, the presence of an MPE was associated with poor prognosis, while survival depended on the specific cancer type detected. Particularly, prognosis was found to be worse when MPEs were caused by lung or gastric cancer, rather than breast or hematolymphoid malignancies. In conclusion, evidence suggests that cytologic evaluation has a significant diagnostic and prognostic impact in patients with MPEs.
1. Introduction
A pericardial effusion, which is formed by the accumulation of excessive fluid within the pericardial cavity, is a prevalent manifestation clinicians face [1]. It can be caused by diverse etiologies, such as heart-related conditions (e.g., myocardial infarction or cardiac surgery), kidney failure, trauma, infections, autoimmune diseases, and cancer [1,2,3]. Cytology is one of the diagnostic modalities used to identify the cause of a pericardial effusion, particularly to detect whether cancer cells are present within the fluid [3,4]. Cancer-associated pericardial effusions might be either free from malignancy—for instance, when induced by chemotherapy or radiotherapy—or show evidence of cancer cells (malignant pericardial effusions; MPEs); the latter are most often derived from a metastasis rather than a primary lesion. Furthermore, the presence of a MPE has been associated with poor prognosis, while its management necessitates a multidisciplinary approach [3,5,6,7]. Recently, the International System for Reporting Serous Fluid Cytology (ISRSFC) has been reported with the goal to improve diagnosis, standardize reporting, and facilitate communication among physicians [8].
To our knowledge, while the cytology of MPEs has previously been described in multiple studies, no systematic review has been performed up to date. Our study aimed to highlight the diagnostic and prognostic impact of cytology while evaluating pericardial effusions.
2. Materials and Methods
2.1. Search Strategy
We performed this systematic review following the Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) guidelines [9]. We comprehensively investigated four electronic databases (PubMed, Embase, Scopus, and Web of Science) for articles published until May 2021 reporting on the cytopathology of MPEs. We used the following search algorithm: “pericardi* AND (cytolog* OR cytopatholog*) AND (effusion* OR fluid) AND (cancer* OR carcinoma* OR metasta*)”, while no specific search filters were applied (e.g., publication date). Duplicates were removed with the Paperpile reference manager. Then, the remaining records were inserted into the Rayyan App for title–abstract selection [10].
2.2. Study Selection
Two authors (Ranim Shartouni, and Ilias P. Nikas) selected independently the eligible studies using predefined inclusion and exclusion criteria (Table 1). This was done in two steps: first, an initial title-abstract screening was performed in the Rayyan App, and its results underwent a full-text evaluation to select the final eligible study list. Any disagreements were resolved by a consensus between the two authors.

Table 1.
Inclusion and exclusion criteria of this systematic review.
2.3. Data Extraction
The following data were extracted from each eligible study onto an Excel® spreadsheet: first author and year, country, study design, study period, follow-up duration, mean and/or median age of the enrolled patients, total number of patients and samples included, number of samples with malignant cytology and histology, malignancy rate between females and males, number of serous, hemorrhagic, serosanguinous, or purulent effusions, mean or median effusion volume of the benign, malignant or all effusions evaluated, main symptoms reported, cancer types described, and selected prognostic data (e.g., OR, overall survival; median survival; HR, hazard ratio).
2.4. Study Outcomes
The outcomes of this systematic review were used to assess the following:
- The role of cytology in identifying specific cancer primaries associated with MPEs;
- The ability of cytology, compared to histology, to detect cancer while evaluating pericardial effusions;
- The prognostic impact of MPEs.
3. Results
3.1. Literature Search
The flowchart of our study is displayed in Figure 1. The search yielded initially a total number of 2148 articles (PubMed, 397; Scopus, 633; Embase, 594; Web of Science, 524); of them, 1228 were removed as duplicates. Then, 920 reports were screened in a title–abstract fashion. The whole process resulted in 42 studies included in our review for subsequent data extraction and analysis.
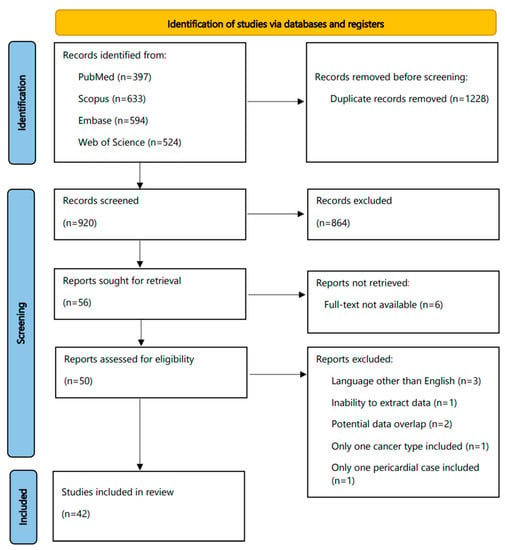
Figure 1.
Flowchart of this systematic review.
3.2. Study and Patient Characteristics
Table S1 displays the characteristics of the 42 eligible studies, conducted from 1972 to 2020 across various countries, most often (n = 21) in the U.S.A. The majority of them had a retrospective design. Table 2 shows the percentage of pericardial malignancy in females vs. males, also highlights the percentage of samples where cancer was detected. A total number of 9570 patients was included (4490 females and 5080 males), while a total number of 9156 samples was also recorded, of which 2807 were positive for malignancy. In seven studies [3,4,11,12,13,14,15], females had a higher malignancy prevalence, ranging from 57% to 82%; in contrast, MPEs were more prevalent in males in three studies [16,17,18], ranging from 70.7% to 72.3%. MPEs tended to be hemorrhagic [12,16,19,20,21,22], rather than serous or serosanguinous, and also to occupy larger volumes than benign effusions [13,14,15,19,23] (Table S2).

Table 2.
Presence of pericardial malignancy in the included studies.
3.3. Cancer Types Associated with MPEs
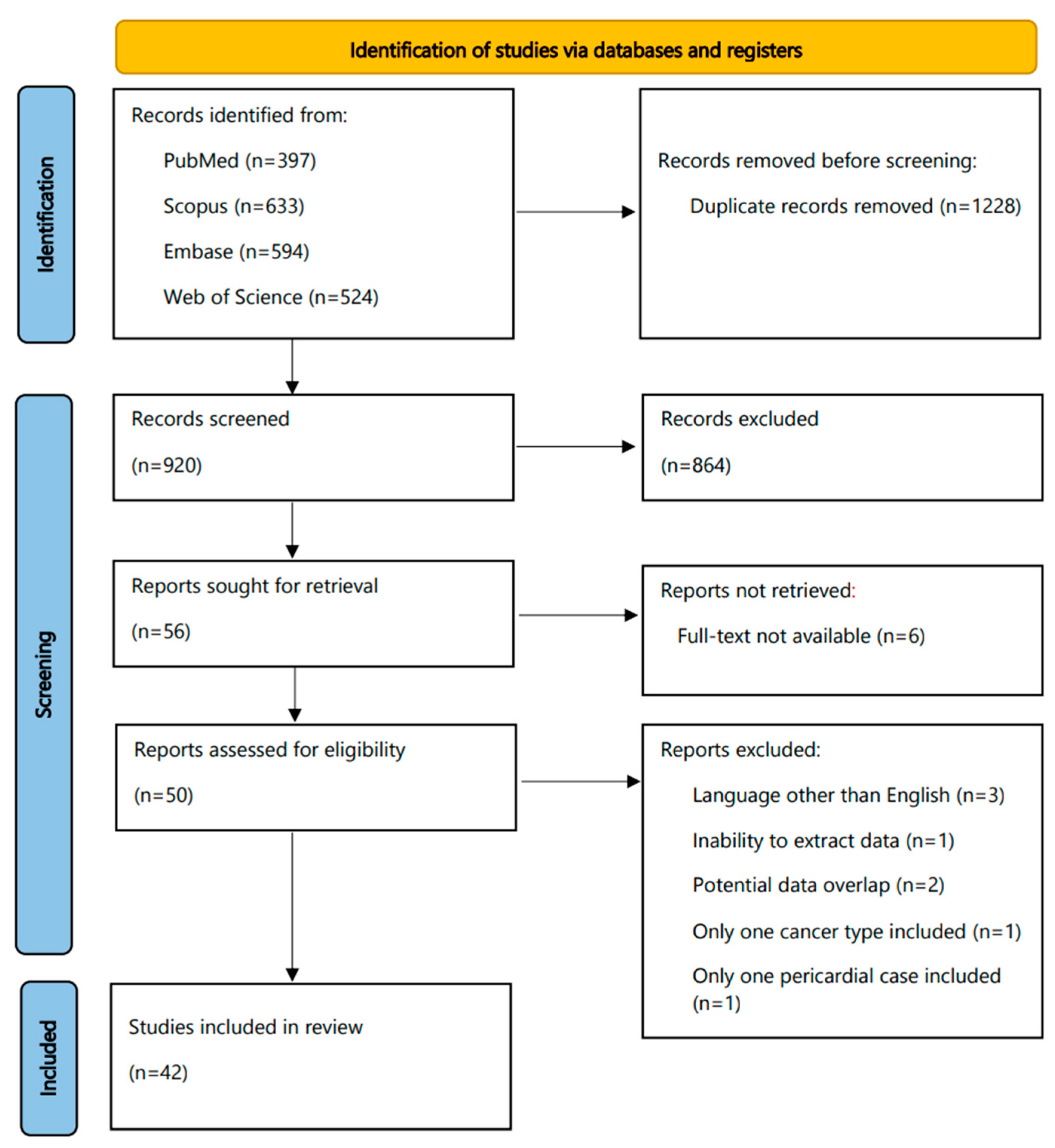
Table S3 shows the most prevalent cancer primaries in the patients with cancer-associated pericardial effusions (effusions in patients with clinical history of cancer, associated with presence or absence of malignant cells) included in each eligible report. Lung cancer was the most common primary in most studies, followed by breast cancer, hematolymphoid, and gastrointestinal malignancies. Other primaries comprised gynecological cancers, thyroid and urinary malignancies, melanomas, sarcomas, germ cell tumors, and thymomas. Concerning mesotheliomas, these occupied only a minority of the cases extracted. We found 18 cases in total, of which 6 were reported as pleural mesotheliomas. In the two studies focusing on pediatric MPEs, hematolymphoid neoplasms and sarcomas were the ones most often reported [37,39].
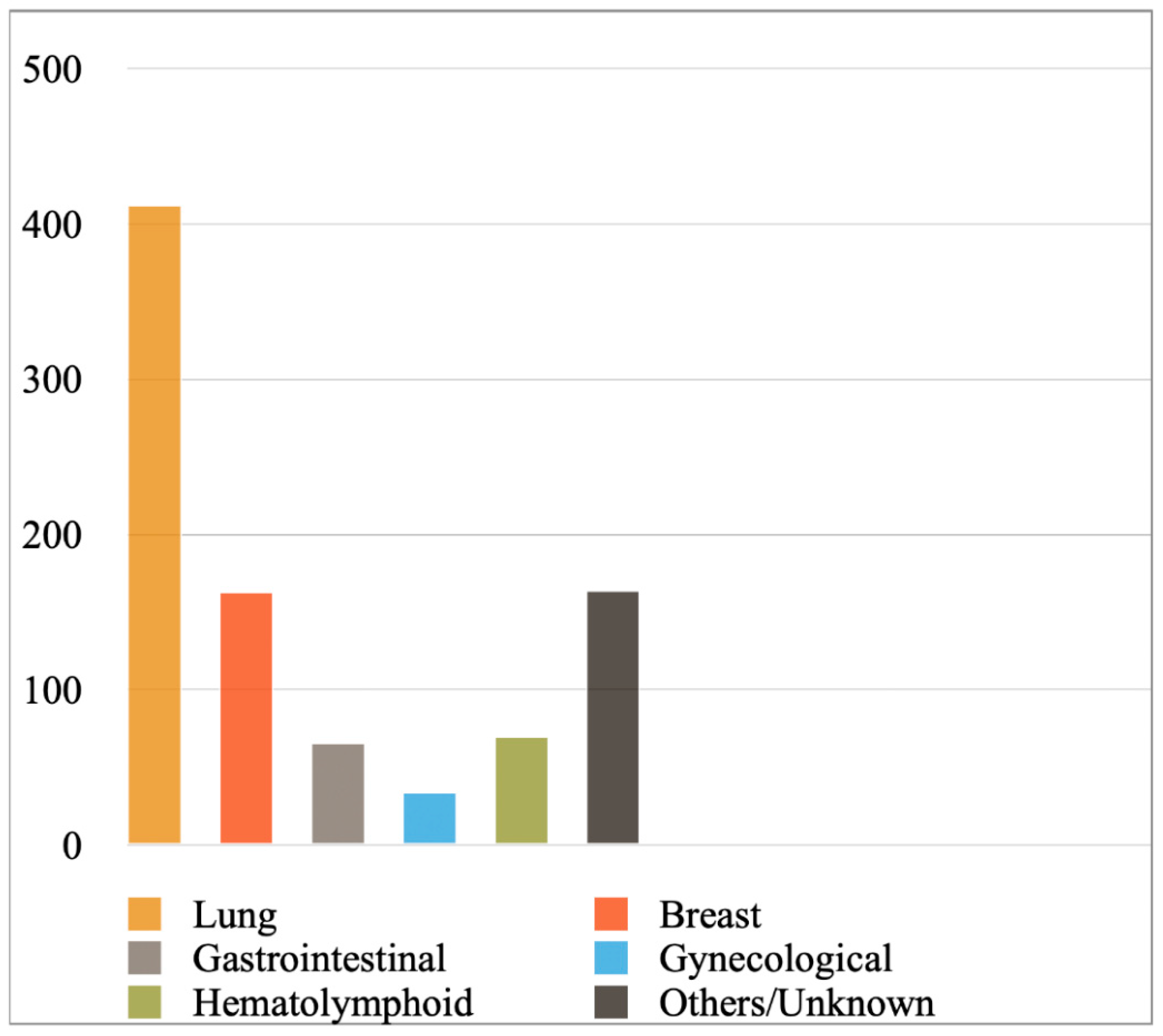
Figure 2 shows the most prevalent cancer primaries in MPEs diagnosed with cytology or a combination of cytology and biopsy in the included studies, while Figure 3 shows their prevalence when results were reported exclusively with cytology. Lung cancer (comprising mainly adenocarcinomas) was reported to be the most common primary, followed by breast cancer, hematolymphoid and gastrointestinal malignancies. Briefly, from the studies reporting findings derived either from cytology or a combination of cytology and biopsy (Figure 2), we calculated the following frequencies: lung cancer 45%, breast cancer 18%, hematolymphoid neoplasms 8%, gastrointestinal cancers 7%, gynecological cancers 4%, and others/unknown 18%. In this analysis, the study by Wilkes et al. [46] was excluded, as it did not report separately the numbers of gastrointestinal and gynecological cancer diagnoses. In addition, from the studies reporting only cytologic findings (Figure 3), we calculated the following frequencies: lung cancer 44%, breast cancer 18%, hematolymphoid neoplasms 7%, gastrointestinal cancers 7%, gynecological cancers 4%, and others/unknown 19%. Analytical results of the reported cancer types in each eligible study are shown in Tables S4 and S5, respectively. Concerning lung cancer, non-small cell lung carcinoma (NSCLC) was much more common than small cell lung carcinoma (SCLC); in the MPEs diagnosed with cytology only, just 13 cases were confirmed as SCLC (out of 330 lung cancer cases in total; Table S5).
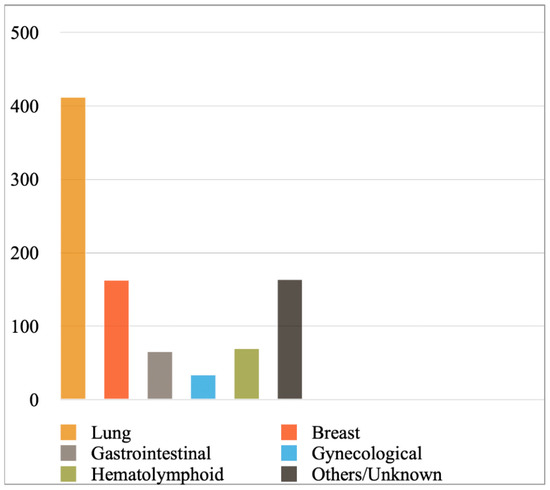
Figure 2.
Most prevalent cancer primaries in malignant pericardial effusions. In the included studies, results were reported with cytology or a combination of cytology and biopsy.
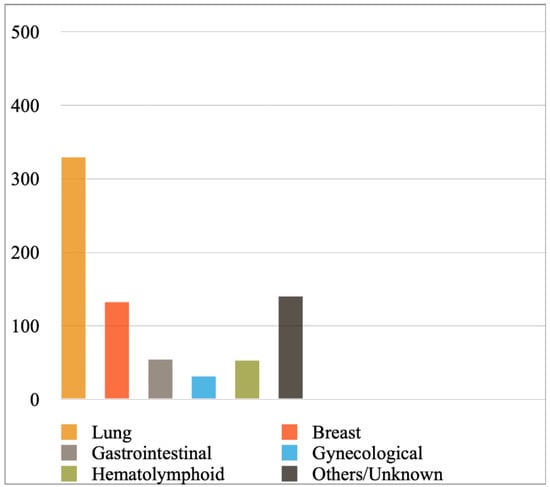
Figure 3.
Most prevalent cancer primaries in malignant pericardial effusions diagnosed exclusively with cytology.
3.4. Cytology vs. Histology for Cancer Detection While Evaluating Pericardial Effusions
Table 3 compares the performance of cytology and histology for diagnosing MPEs in the studies that applied both modalities. For this analysis, and wherever present, cytologically suspicious interpretations were regarded as positive, and atypical or indeterminate interpretations as negative. Cytology tended to be positive for malignancy in cases where histology was negative [3,11,12,13,16,23,38,40,43,44,46] more often than the opposite scenario [15,34,48,49]. Although in most of these studies the difference was not statistically significant (Table 3), the following three studies exhibited a significantly higher ability of cytology to detect cancer in the pericardium, compared to biopsy. First, in the report by Maisch et al. [16], 32 cases were found to be positive for malignancy only with cytology, as their paired biopsies yielded negative results; meanwhile, histology samples detected just five cancers missed with cytology. Thus, cytology detected more cancers involving the pericardium than histology (p < 0.001). Likewise, statistically significant results were reported by Cullinane et al. [24] and Lopez et al. [12] (p = 0.02 and p = 0.04, respectively), both highlighting the value of cytology detecting MPEs. In contrast, in none of the studies where histology detected more cancers than cytology [15,34,48,49] was the result statistically significant.

Table 3.
Comparison of cytology and histology for detecting cancer in pericardial effusions. The studies showing differences in their cytology vs. histology findings are compared, and the resulting p-values are shown in the Table. Results exhibiting differences between cytology and histology, also p-values < 0.05, are highlighted with Bold.
Notably, the combination of both cytology and biopsy could increase the overall diagnostic performance. For instance, Wilkes et al. reported sensitivities of 90% and 56% with cytology and histology, respectively, while their combination increased the overall sensitivity to 94% [46].
Three studies reported their pericardial effusion findings using the newly developed ISRSFC [11,14,30], stratifying their interpretations into each of the five reporting categories linked with a different risk of malignancy (ROM). Lobo et al. reclassified 64 samples using the criteria of ISRSFC and reported a 100% risk of malignancy (ROM) for the malignant category; in contrast, the negative and atypical categories exhibited a 0% ROM.
3.5. Prognostic Impact of MPEs
The presence of MPEs, diagnosed either with cytology or biopsy, was correlated with dismal prognosis [6,13,20,22,24,26,31,33,38]. Notably, the specific cancer primary seems to be important. Cullinane et al. reported that lung cancer was associated with shorter survival than breast or hematolymphoid malignancies [24], while Gornik et al. reported that patients with breast and hematolymphoid cancers exhibited longer survival compared to lung and other solid malignancies [26]. Kil et al. also displayed that patients with breast cancer or lymphoma showed longer survival than stomach cancer or unknown metastases [33], while He et al. reported that cases with lung cancer exhibited worse prognosis than non-cancer cases [22]. Lekhakul et al. reported that a diagnosis of chronic myelogenous leukemia or lymphoma was linked with a better prognosis compared to carcinoma or sarcoma [35]. Lastly, Wilkes et al. studied a cohort largely composed of malignant effusions from hematopoietic neoplasms and chemo/radiosensitive breast cancers; of interest, survival did not statistically differ from non-malignant effusions [46]. A summary of the relevant prognostic findings in the included studies is shown in Table 4.

Table 4.
Studies highlighting the prognostic impact of cancer-associated and malignant pericardial effusions.
4. Discussion
To our knowledge, this is the first systematic review examining the role of cytology in the evaluation of MPEs. We found that the most prevalent malignancies detected in MPEs, either with cytology or biopsy, were derived from lung, breast, hematopoietic, or gastrointestinal primaries. In addition, most studies showed that cytology exhibited an enhanced ability to detect cancer compared to biopsy. Lastly, the presence of a MPE was associated with dismal prognosis, while survival depended on the specific cancer type detected.
Similar to our study that focused on pericardial effusions, the prevalence of various cancers has also been shown in pleural and peritoneal effusions. Dermawan et al. reported that the most common malignancies associated with malignant pleural effusions in males were derived from lung (especially adenocarcinoma), hematolymphoid, genitourinary, and gastrointestinal primaries, whereas they were derived from breast, Mullerian, lung, and hematolymphoid primaries in females. They also found that hematolymphoid, gastrointestinal, and genitourinary malignancies were the most common ones associated with malignant peritoneal effusions in males; in contrast, primaries of Mullerian origin, the breast, and the gastrointestinal system were most often found in females [4]. Of interest, Flanagan et al. investigated the Irish National Cancer Registry for extra-abdominal cancers metastasizing to the peritoneum, reporting that the most common primaries were the breast cancer, lung cancer, and melanoma. In addition, peritoneal metastasis was associated with poor prognosis compared to stage IV metastatic cancers devoid of peritoneal cancer spread [50].
Our study additionally reported that MPEs were more likely to be hemorrhagic and occupy larger volumes than non-malignant effusions. Likewise, the presence of hemorrhage has also been associated with malignant pleural effusions [51]. A potential cause could be the high levels of VEGF secreted by the cancer cells [52]. Notably, a recent study compared malignant pleural effusions with and without hemorrhage and found that the former was associated with more severe dyspnea and larger effusion volumes, in addition to worse prognosis and response to therapy [53].
According to our results, cytology tended to be positive for malignancy in cases where histology was negative [3,11,12,13,16,23,38,40,43,44,46] more often than the opposite scenario [15,34,48,49], potentially exhibiting an enhanced cancer detection ability while evaluating the pericardium. The lower performance of histology could be because of sampling error, as the pericardium is more difficult to biopsy than the pleura or peritoneum [18]. Other reasons could be the fact that cancer cells first spread to the visceral, before reaching the parietal, pericardium, in addition to the reported dispersed involvement of the pericardium in metastatic disease [46]. Notably, results from a recent study suggest another potential explanation for the enhanced ability of cytology to detect MPEs. Karpathiou et al. showed that pericardial metastases often exhibit a pattern where cells float inside the cavity. In contrast, pleural metastases tend to present with an invasive pattern [54]. Consequently, cytologic sampling seems a suitable modality to detect cancers involving the pericardium, whereas tissue biopsy could be more likely to be successful when evaluating pleural metastases. However, the combination of both cytology and tissue biopsy may increase the overall diagnostic accuracy while evaluating MPEs [46]. Of interest, the application of the recent ISRSFC could further enhance cytology’s diagnostic performance, facilitate communication, and minimize interobserver variability [11,14,30,55,56]; however, more studies are needed to unravel its potential.
Our review highlighted that MPEs were linked with higher mortality rates, while positive pericardial cytology was a significantly dismal prognostic factor [6,13,20,22,24,26,31,33,38]. Notably, the specific cancer primary seemed to be important, as lung cancer and gastric cancer were associated with worse prognosis than breast or hematolymphoid malignancies [24,26,33]. Of interest, a recent study showed that survival was shorter for patients with MPEs associated with gastric cancer, compared to lung, breast, or other malignancies [56]. Prognosis has been reported to be similarly poor in patients with malignant pleural or peritoneal effusions [57,58]. In a study enrolling patients with malignant pleural effusions, lung and gastric cancers were found to be worse prognostic factors compared to breast, ovarian, or renal carcinomas [57].
This systematic review has some important limitations. It was composed mainly of retrospective studies, while most were of a small size. In addition, many of the included studies exhibited possible selection bias, while their results were largely reported in a heterogenous way. Furthermore, a cytohistological correlation could not be performed in all studies, let alone in all samples in each study individually. In five of the studies included in the cytology/histology correlation analysis (Table 3), cytology interpretations originally reported as suspicious or positive were both considered as being cytologically positive for this analysis; this could have potentially overestimated the ability of cytology to detect cancer in these particular studies. Lastly, the heterogeneity in the reporting of prognostic findings (various outcomes) prohibited their pooled analysis.
5. Conclusions
This study showed that the most prevalent primaries associated with MPEs are lung (especially NSCLCs), breast, hematolymphoid, and gastrointestinal cancers. MPEs tend to be hemorrhagic rather than serous or serosanguinous and occupy larger volumes than non-neoplastic effusions. Notably, cytology may exhibit enhanced cancer detection ability while assessing pericardial effusions, compared to histology. Lastly, MPEs show poor prognosis, while evidence suggests that the latter is worse when MPEs are caused by lung or gastric cancer, rather than breast or hematolymphoid malignancies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12020367/s1, Table S1: Study Characteristics; Table S2: Gross findings of pericardial effusions and their association with malignancy; Table S3: Most prevalent primary cancer sites/neoplasms in patients presenting with cancer-associated pericardial effusions; Table S4: Most prevalent primary cancer sites/neoplasms in patients presenting with malignant pericardial effusions diagnosed with cytology and/or biopsy; Table S5: Most prevalent primary cancer sites/neoplasms in patients presenting with malignant pericardial effusions diagnosed exclusively with cytology.
Author Contributions
Conceptualization: I.P.N.; methodology: R.S. (Ranim Shartouni), I.P.N.; software: R.S. (Ranim Shartouni), I.P.N.; validation: R.S. (Ranim Shartouni), R.S. (Roy Shartouni), M.M., I.P.N.; formal analysis: R.S. (Ranim Shartouni), R.S. (Roy Shartouni), M.M., I.P.N.; investigation: R.S. (Ranim Shartouni), R.S. (Roy Shartouni), M.M., I.P.N.; resources: R.S. (Ranim Shartouni), R.S. (Roy Shartouni), M.M., I.P.N.; data curation: R.S. (Ranim Shartouni), R.S. (Roy Shartouni), M.M., I.P.N.; writing—original draft preparation: R.S. (Ranim Shartouni), I.P.N.; writing—review and editing: R.S. (Ranim Shartouni), R.S. (Roy Shartouni), M.M., I.P.N.; visualization: R.S. (Ranim Shartouni), I.P.N.; supervision: I.P.N.; project administration: I.P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sagristà-Sauleda, J.; Mercé, A.S.; Soler-Soler, J. Diagnosis and Management of Pericardial Effusion. World J. Cardiol. 2011, 3, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.-Y.; Corey, R.; Berger, P.; Habib, G.; Bonnet, J.-L.; Levy, S.; Messana, T.; Djiane, P.; Frances, Y.; Botta, C.; et al. Etiologic Diagnosis of 204 Pericardial Effusions. Medicine 2003, 82, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Dragoescu, E.A.; Liu, L. Pericardial Fluid Cytology: An Analysis of 128 Specimens over a 6-Year Period. Cancer Cytopathol. 2013, 121, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.T.; Policarpio-Nicolas, M.L. Malignancies in Pleural, Peritoneal, and Pericardial Effusions. Arch. Pathol. Lab. Med. 2020, 144, 1086–1091. [Google Scholar] [CrossRef]
- Burazor, I.; Imazio, M.; Markel, G.; Adler, Y. Malignant Pericardial Effusion. Cardiology 2013, 124, 224–232. [Google Scholar] [CrossRef]
- Neragi-Miandoab, S.; Linden, P.A.; Ducko, C.T.; Bueno, R.; Richards, W.G.; Sugarbaker, D.J.; Jaklitsch, M.T. VATS Pericardiotomy for Patients with Known Malignancy and Pericardial Effusion: Survival and Prognosis of Positive Cytology and Metastatic Involvement of the Pericardium: A Case Control Study. Int. J. Surg. 2008, 6, 110–114. [Google Scholar] [CrossRef]
- De Filippo, O.; Gatti, P.; Rettegno, S.; Iannaccone, M.; D’Ascenzo, F.; Lazaros, G.; Brucato, A.; Tousoulis, D.; Adler, Y.; Imazio, M. Is Pericardial Effusion a Negative Prognostic Marker? Meta-Analysis of Outcomes of Pericardial Effusion. J. Cardiovasc. Med. 2019, 20, 39–45. [Google Scholar] [CrossRef]
- Pinto, D.; Chandra, A.; Crothers, B.A.; Kurtycz, D.F.I.; Schmitt, F. The International System for Reporting Serous Fluid Cytopathology-Diagnostic Categories and Clinical Management. J. Am. Soc. Cytopathol. 2020, 9, 469–477. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Lobo, C.; Costa, J.; Petronilho, S.; Monteiro, P.; Leça, L.; Schmitt, F. Cytohistological Correlation in Serous Effusions Using the Newly Proposed International System for Reporting Serous Fluid Cytopathology: Experience of an Oncological Center. Diagn. Cytopathol. 2021, 49, 596–605. [Google Scholar] [CrossRef] [PubMed]
- López, J.M.; Delgado, J.L.; Tovar, E.; González, A.G. Massive Pericardial Effusion Produced by Extracardiac Malignant Neoplasms. Arch. Intern. Med. 1983, 143, 1815–1816. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Van Trigt, P.; Wall, T.C.; Kenney, R.T.; O’Connor, C.M.; Sheikh, K.H.; Kisslo, J.A.; Baker, M.E.; Corey, G.R. Subxiphoid Pericardiotomy in the Diagnosis and Management of Large Pericardial Effusions Associated with Malignancy. Chest 1992, 101, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.F.; Jones, R.; Gabrielson, M.; Santos, D.; Pastorello, R.G.; Maleki, Z. Application of the International System for Reporting Serous Fluid Cytopathology (ISRSFC) on Reporting Pericardial Effusion Cytology. Acta Cytol. 2020, 64, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Robles, R.; Piñero, A.; Luján, J.A.; Fernández, J.A.; Torralba, J.A.; Acosta, F.; Villegas, M.; Parrilla, P. Thoracoscopic Partial Pericardiectomy in the Diagnosis and Management of Pericardial Effusion. Surg. Endosc. 1997, 11, 253–256. [Google Scholar] [CrossRef]
- Maisch, B.; Ristic, A.; Pankuweit, S. Evaluation and Management of Pericardial Effusion in Patients with Neoplastic Disease. Prog. Cardiovasc. Dis. 2010, 53, 157–163. [Google Scholar] [CrossRef]
- García-Riego, A.; Cuiñas, C.; Vilanova, J.J. Malignant Pericardial Effusion. Acta Cytol. 2001, 45, 561–566. [Google Scholar] [CrossRef]
- Bardales, R.H.; Stanley, M.W.; Schaefer, R.F.; Liblit, R.L.; Owens, R.B.; Surhland, M.J. Secondary Pericardial Malignancies: A Critical Appraisal of the Role of Cytology, Pericardial Biopsy, and DNA Ploidy Analysis. Am. J. Clin. Pathol. 1996, 106, 29–34. [Google Scholar] [CrossRef][Green Version]
- Edoute, Y.; Malberger, E.; Kuten, A.; Ben-Haim, S.A.; Moscovitz, M. Cytologic Analysis of Pericardial Effusion Complicating Extracardiac Malignancy. Am. J. Cardiol. 1992, 69, 568–571. [Google Scholar] [CrossRef]
- Strobbe, A.; Adriaenssens, T.; Bennett, J.; Dubois, C.; Desmet, W.; McCutcheon, K.; Van Cleemput, J.; Sinnaeve, P.R. Etiology and Long-Term Outcome of Patients Undergoing Pericardiocentesis. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Gecmen, C.; Gecmen, G.G.; Ece, D.; Kahyaoğlu, M.; Kalayci, A.; Karabay, C.Y.; Candan, O.; Isik, M.E.; Yilmaz, F.; Akgun, O.; et al. Cytopathology of Pericardial Effusions: Experience from a Tertiary Center of Cardiology. Herz 2017, 43, 543–547. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yang, Z.; Zhao, P.; Li, Y.-J.; Wang, J.-G. Cytopathologic Analysis of Pericardial Effusions in 116 Cases: Implications for Poor Prognosis in Lung Cancer Patients with Positive Interpretations. Diagn. Cytopathol. 2017, 45, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sarigül, A.; Farsak, B.; Ateş, M.Ş.; Demircin, M.; Paşaoğlu, İ. Subxiphoid Approach for Treatment of Pericardial Effusion. Asian Cardiovasc. Thorac. Ann. 1999, 7, 297–300. [Google Scholar] [CrossRef]
- Cullinane, C.A.; Paz, I.B.; Smith, D.; Carter, N.; Grannis, F.W., Jr. Prognostic Factors in the Surgical Management of Pericardial Effusion in the Patient with Concurrent Malignancy. Chest 2004, 125, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Di Liso, E.; Menichetti, A.; Dieci, M.V.; Ghiotto, C.; Banzato, A.; Bianchi, A.; Pintacuda, G.; Padovan, M.; Nappo, F.; Cumerlato, E.; et al. Neoplastic Pericardial Effusion: A Monocentric Retrospective Study. J. Palliat. Med. 2019, 22, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Gerhard-Herman, M.; Beckman, J.A. Abnormal Cytology Predicts Poor Prognosis in Cancer Patients with Pericardial Effusion. J. Clin. Oncol. 2005, 23, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Kenwright, D.N.; Fauck, R.; Lallu, S.; Naran, S. The Usefulness of a Panel of Immunostains in the Diagnosis and Differentiation of Metastatic Malignancies in Pericardial Effusions. Cytopathology 2000, 11, 312–321. [Google Scholar] [CrossRef]
- Gupta, S.; Sodhani, P.; Jain, S. Cytomorphological Profile of Neoplastic Effusions: An Audit of 10 Years with Emphasis on Uncommonly Encountered Malignancies. J. Cancer Res. Ther. 2012, 8, 602–609. [Google Scholar] [CrossRef]
- Haskell, R.J.; French, W.J. Cardiac Tamponade as the Initial Presentation of Malignancy. Chest 1985, 88, 70–73. [Google Scholar] [CrossRef]
- Hou, T.; Landon, G.; Stewart, J.; Roy-Chowdhuri, S. The Value of a Tiered Cytology Diagnostic Reporting System in Assessing the Risk of Malignancy in Indeterminate Serous Effusions. Cancer Cytopathol. 2020. [Google Scholar] [CrossRef]
- Jeon, H.W.; Cho, D.G.; Park, J.K.; Hyun, K.Y.; Choi, S.Y.; Suh, J.H.; Kim, Y.-D. Prognostic Factors Affecting Survival of Patients with Cancer-Related Pericardial Effusion Managed by Surgery. World J. Surg. Oncol. 2014, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Kabukcu, M.; Demircioglu, F.; Yanik, E.; Basarici, I.; Ersel, F. Pericardial Tamponade and Large Pericardial Effusions: Causal Factors and Efficacy of Percutaneous Catheter Drainage in 50 Patients. Tex. Heart Inst. J. 2004, 31, 398–403. [Google Scholar] [PubMed]
- Kil, U.H.; Jung, H.O.; Koh, Y.S.; Park, H.J.; Park, C.S.; Kim, P.J.; Baek, S.-H.; Seung, K.-B.; Choi, K.-B. Prognosis of Large, Symptomatic Pericardial Effusion Treated by Echo-Guided Percutaneous Pericardiocentesis. Clin. Cardiol. 2008, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, J.G.; Hancock, E.W. Pericardiocentesis. Am. J. Med. 1978, 65, 808–814. [Google Scholar] [CrossRef]
- Lekhakul, A.; Assawakawintip, C.; Fenstad, E.R.; Pislaru, S.V.; Thaden, J.J.; Sinak, L.J.; Kane, G.C. Safety and Outcome of Percutaneous Drainage of Pericardial Effusions in Patients with Cancer. Am. J. Cardiol. 2018, 122, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Malamou-Mitsi, V.D.; Zioga, A.P.; Agnantis, N.J. Diagnostic Accuracy of Pericardial Fluid Cytology: An Analysis of 53 Specimens from 44 Consecutive Patients. Diagn. Cytopathol. 1996, 15, 197–204. [Google Scholar] [CrossRef]
- Medary, I.; Steinherz, L.J.; Aronson, D.C.; La Quaglia, M.P. Cardiac Tamponade in the Pediatric Oncology Population: Treatment by Percutaneous Catheter Drainage. J. Pediatr. Surg. 1996, 31, 197–199, discussion 199–200. [Google Scholar] [CrossRef]
- Mirhosseini, S.M.; Fakhri, M.; Mozaffary, A.; Lotfaliany, M.; Behzadnia, N.; Ansari Aval, Z.; Ghiasi, S.M.S.; Boloursaz, M.R.; Masjedi, M.R. Risk Factors Affecting the Survival Rate in Patients with Symptomatic Pericardial Effusion Undergoing Surgical Intervention. Interact. Cardiovasc. Thorac. Surg. 2012, 16, 495–500. [Google Scholar] [CrossRef]
- Parsons, L.N.; Jarzembowski, J.A. Clinicopathologic Analysis of Malignant Effusions in Pediatric Patients. J. Am. Soc. Cytopathol. 2016, 6, 41–47. [Google Scholar] [CrossRef]
- Patel, N.; Rafique, A.M.; Eshaghian, S.; Mendoza, F.; Biner, S.; Cercek, B.; Siegel, R.J. Retrospective Comparison of Outcomes, Diagnostic Value, and Complications of Percutaneous Prolonged Drainage versus Surgical Pericardiotomy of Pericardial Effusion Associated with Malignancy. Am. J. Cardiol. 2013, 112, 1235–1239. [Google Scholar] [CrossRef]
- Razek, A.A.K.A.; Samir, S. Differentiation Malignant from Benign Pericardial Effusion with Diffusion-Weighted MRI. Clin. Radiol. 2019, 74, 325.e19–325.e24. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Bizzarro, T.; Schmitt, F.; Longatto-Filho, A. The Role of Liquid-Based Cytology and Ancillary Techniques in Pleural and Pericardic Effusions: An Institutional Experience. Cancer Cytopathol. 2015, 123, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Saab, J.; Hoda, R.S.; Narula, N.; Hoda, S.A.; Geraghty, B.E.; Nasar, A.; Alperstein, S.A.; Port, J.L.; Giorgadze, T. Diagnostic Yield of Cytopathology in Evaluating Pericardial Effusions: Clinicopathologic Analysis of 419 Specimens. Cancer Cytopathol. 2016, 125, 128–137. [Google Scholar] [CrossRef]
- Volk, L.; Lee, L.Y.; Lemaire, A. Surgical Pericardial Drainage Procedures Have a Limited Diagnostic Sensitivity. J. Card. Surg. 2019, 34, 1573–1576. [Google Scholar] [CrossRef]
- Wagner, P.L.; McAleer, E.; Stillwell, E.; Bott, M.; Rusch, V.W.; Schaffer, W.; Huang, J. Pericardial Effusions in the Cancer Population: Prognostic Factors after Pericardial Window and the Impact of Paradoxical Hemodynamic Instability. J. Thorac. Cardiovasc. Surg. 2010, 141, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.D.; Fidias, P.; Vaickus, L.; Perez, R.P. Malignancy-Related Pericardial Effusion: 127 Cases from the Roswell Park Cancer Institute. Cancer 1995, 76, 1377–1387. [Google Scholar] [CrossRef]
- Yonemori, K.; Kunitoh, H.; Tsuta, K.; Tamura, T.; Arai, Y.; Shimada, Y.; Fujiwara, Y.; Sasajima, Y.; Asamura, H.; Tamura, T. Prognostic Factors for Malignant Pericardial Effusion Treated by Pericardial Drainage in Solid-Malignancy Patients. Med. Oncol. 2007, 24, 425–430. [Google Scholar] [CrossRef]
- Zhu, H.; Booth, C.N.; Reynolds, J.P. Clinical Presentation and Cytopathologic Features of Malignant Pericardial Cytology: A Single Institution Analysis Spanning a 29-Year Period. J. Am. Soc. Cytopathol. 2015, 4, 203–209. [Google Scholar] [CrossRef]
- Zipf, R.E., Jr.; Johnston, W.W. The Role of Cytology in the Evaluation of Pericardial Effusions. Chest 1972, 62, 593–596. [Google Scholar] [CrossRef]
- Flanagan, M.; Solon, J.; Chang, K.H.; Deady, S.; Moran, B.; Cahill, R.; Shields, C.; Mulsow, J. Peritoneal Metastases from Extra-Abdominal Cancer—A Population-Based Study. Eur. J. Surg. Oncol. 2018, 44, 1811–1817. [Google Scholar] [CrossRef]
- Ferrer, J.; Roldán, J.; Teixidor, J.; Pallisa, E.; Gich, I.; Morell, F. Predictors of Pleural Malignancy in Patients with Pleural Effusion Undergoing Thoracoscopy. Chest 2005, 127, 1017–1022. [Google Scholar] [CrossRef]
- Ishimoto, O.; Saijo, Y.; Narumi, K.; Kimura, Y.; Ebina, M.; Matsubara, N.; Asou, N.; Nakai, Y.; Nukiwa, T. High Level of Vascular Endothelial Growth Factor in Hemorrhagic Pleural Effusion of Cancer. Oncology 2002, 63, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lending, G.; El Ghani, Y.A.; Kaykov, E.; Svirsky, B.; Cohen, H.I.; Altman, E. Hemorrhagic Malignant Pleural Effusion: Diagnosis, Survival Rate, and Response to Talc Pleurodesis. Indian J. Surg. Oncol. 2021, 12, 54–60. [Google Scholar] [CrossRef]
- Karpathiou, G.; Mobarki, M.; Stachowicz, M.L.; Hathroubi, S.; Patoir, A.; Tiffet, O.; Froudarakis, M.; Peoc’h, M. Pericardial and Pleural Metastases: Clinical, Histologic, and Molecular Differences. Ann. Thorac. Surg. 2018, 106, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.J.; Baloch, Z. Are We Ready to Develop a Tiered Scheme for the Effusion Cytology? A Comprehensive Review and Analysis of the Literature. Diagn. Cytopathol. 2019, 47, 1145–1159. [Google Scholar] [CrossRef]
- Song, M.J.; Jo, U.; Jeong, J.-S.; Cho, K.-J.; Gong, G.; Cho, Y.M.; Song, J.S. Clinico-Cytopathologic Analysis of 574 Pericardial Effusion Specimens: Application of the International System for Reporting Serous Fluid Cytopathology (ISRSFC) and Long-Term Clinical Follow-Up. Cancer Med. 2021, 10, 8899–8908. [Google Scholar] [CrossRef] [PubMed]
- Ozyurtkan, M.O.; Balci, A.E.; Cakmak, M. Predictors of Mortality within Three Months in the Patients with Malignant Pleural Effusion. Eur. J. Intern. Med. 2010, 21, 30–34. [Google Scholar] [CrossRef]
- Ayantunde, A.A.; Parsons, S.L. Pattern and Prognostic Factors in Patients with Malignant Ascites: A Retrospective Study. Ann. Oncol. 2007, 18, 945–949. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).