Electrochemical Polymerisation of Glutamic Acid on the Surface of Graphene Paste Electrode for the Detection and Quantification of Rutin in Food and Medicinal Samples
Abstract
1. Introduction
2. Experimental Procedure
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Fabrication of Working Electrodes
2.4. Preparation of RU Containing Real Sample Solutions
3. Results and Discussions
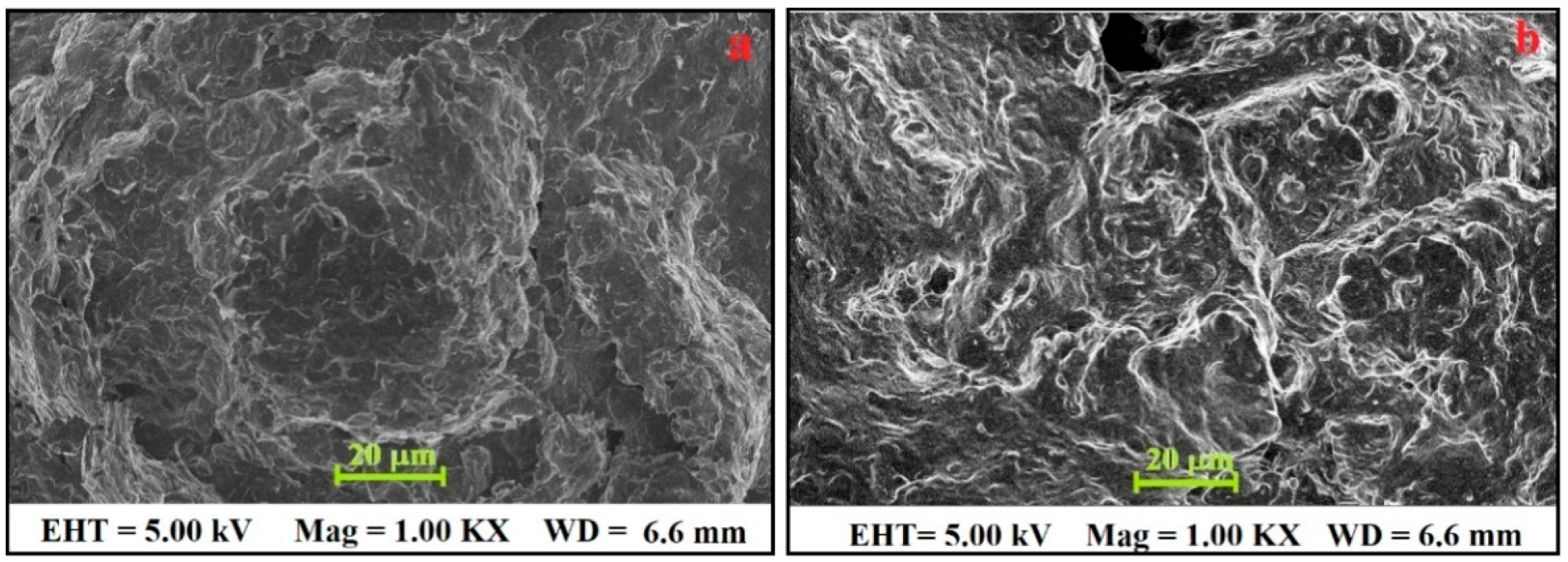
3.1. FE-SEM Characterisation of BGPE and PGAMGPE
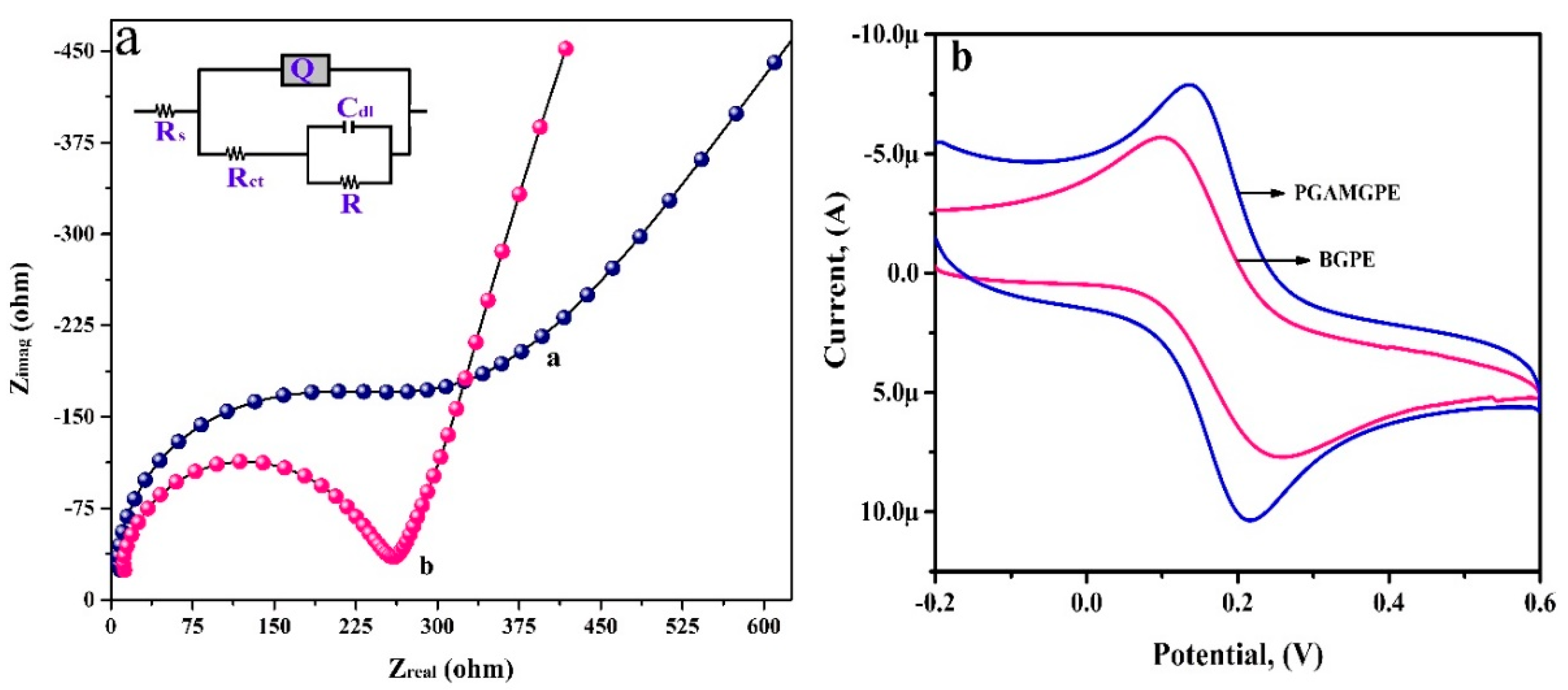
3.2. Electrochemical Impedance Studies and Electroactive Surface Area Calculation
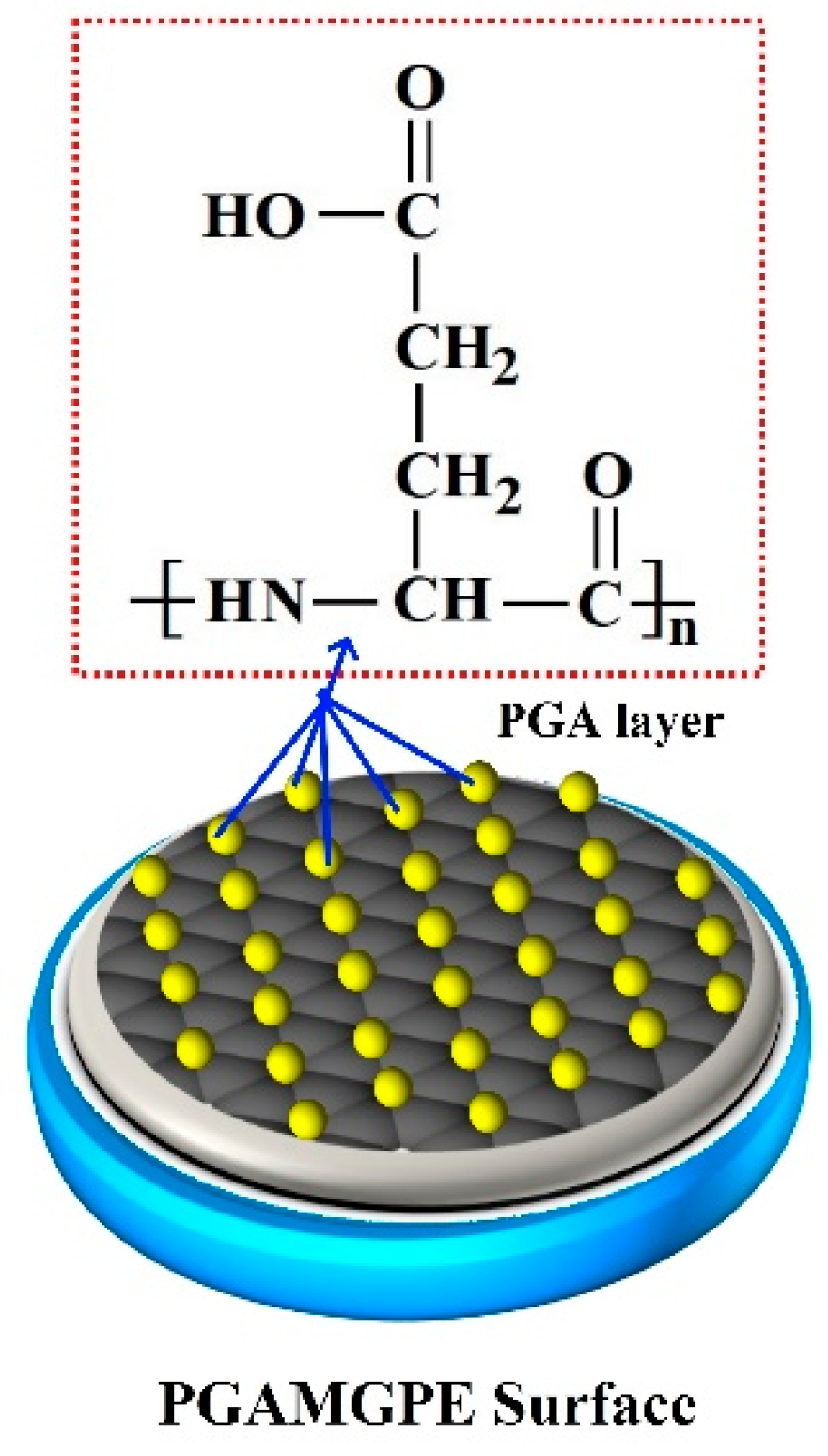
3.3. Electrochemical Deposition of PGA Film on the Surface of the BGPE
3.4. Influence of Supporting Electrolyte
3.5. Optimisation of Accumulation Potential and Time
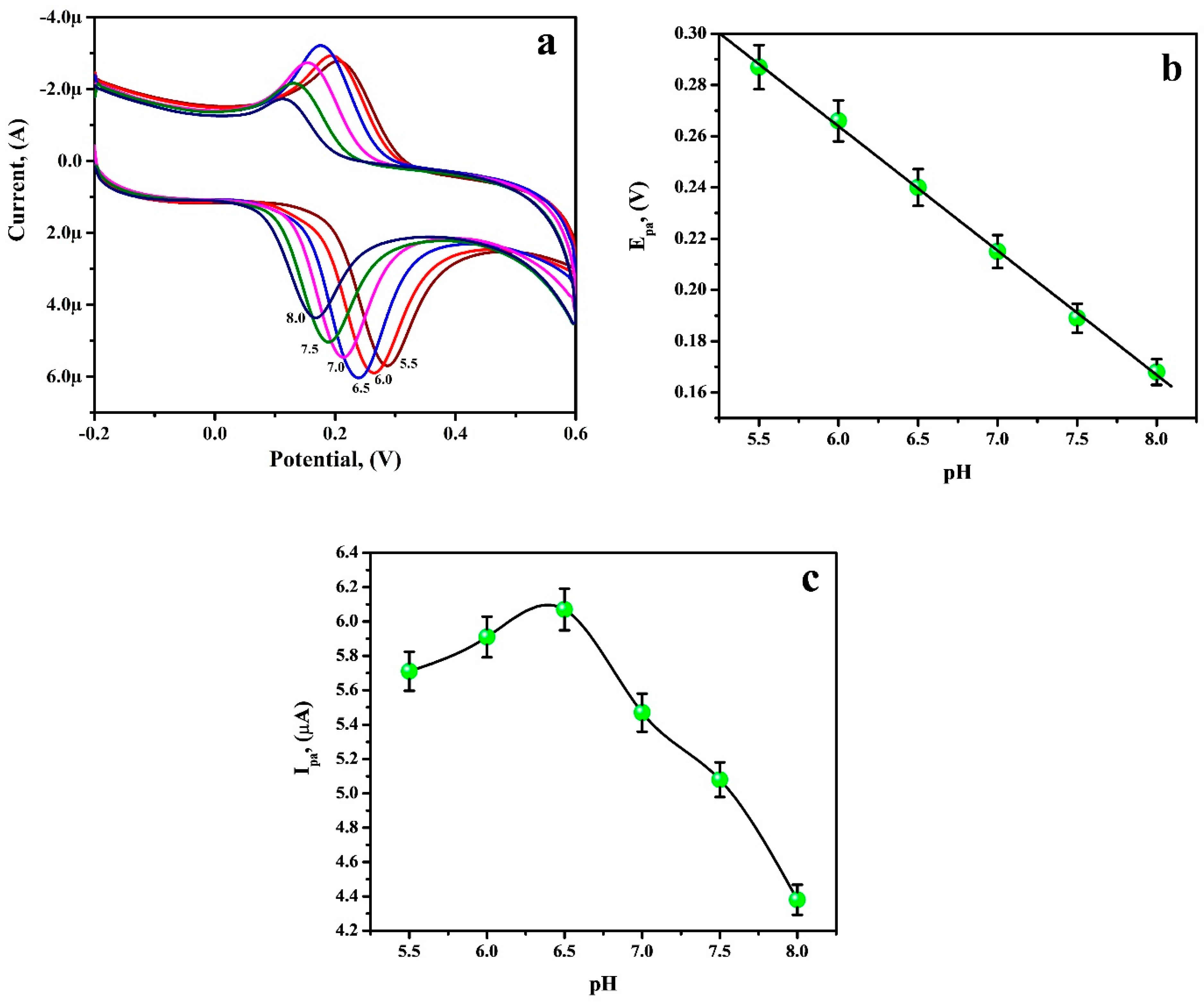
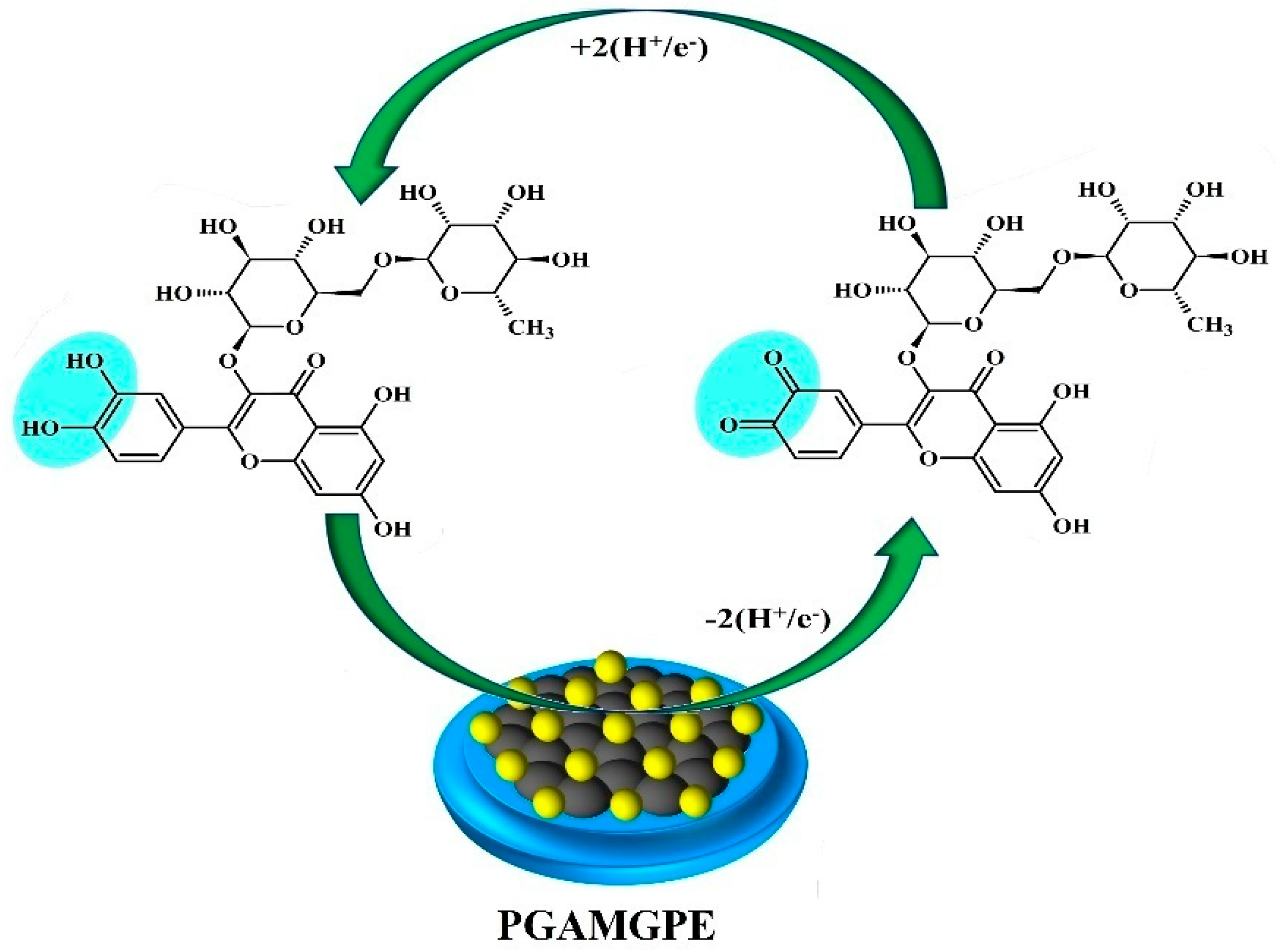
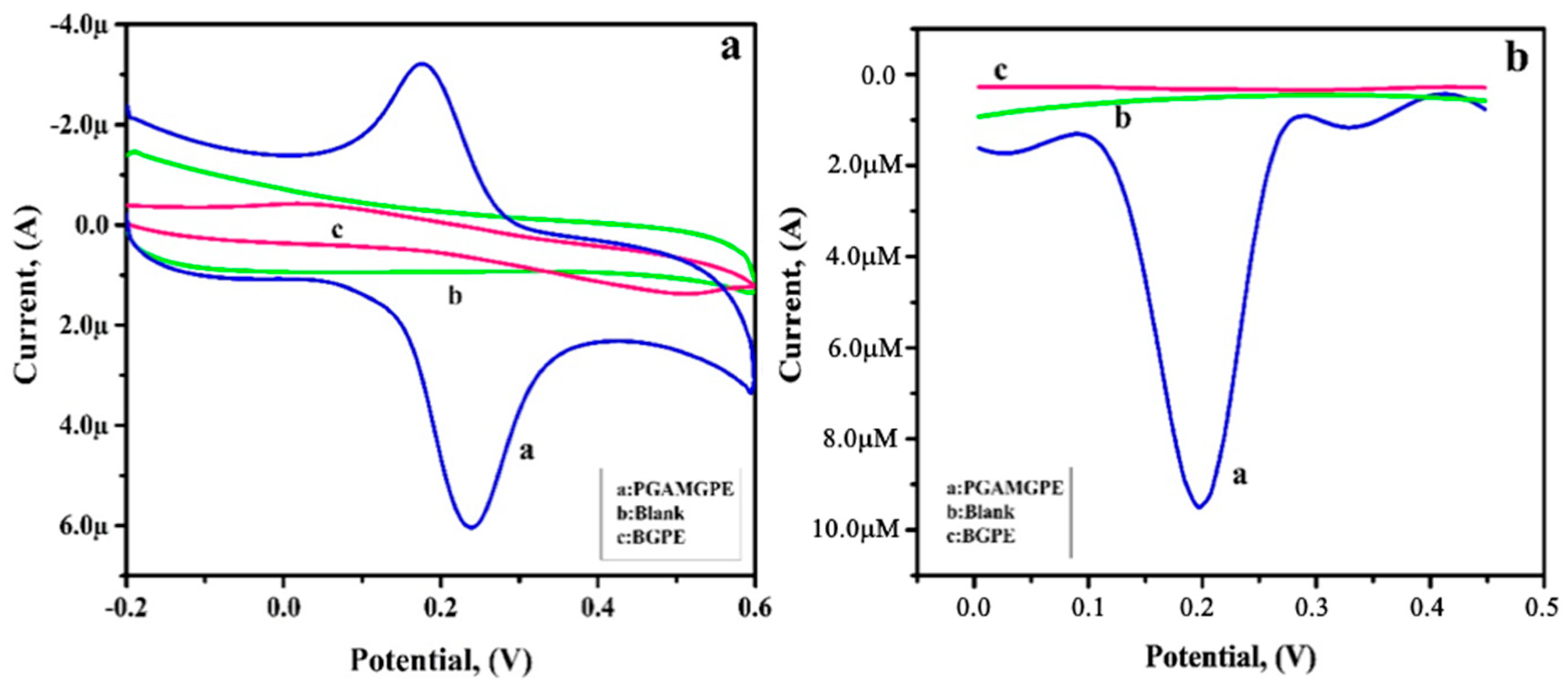
3.6. Electrochemical Sensing of RU at the PGAMGPE
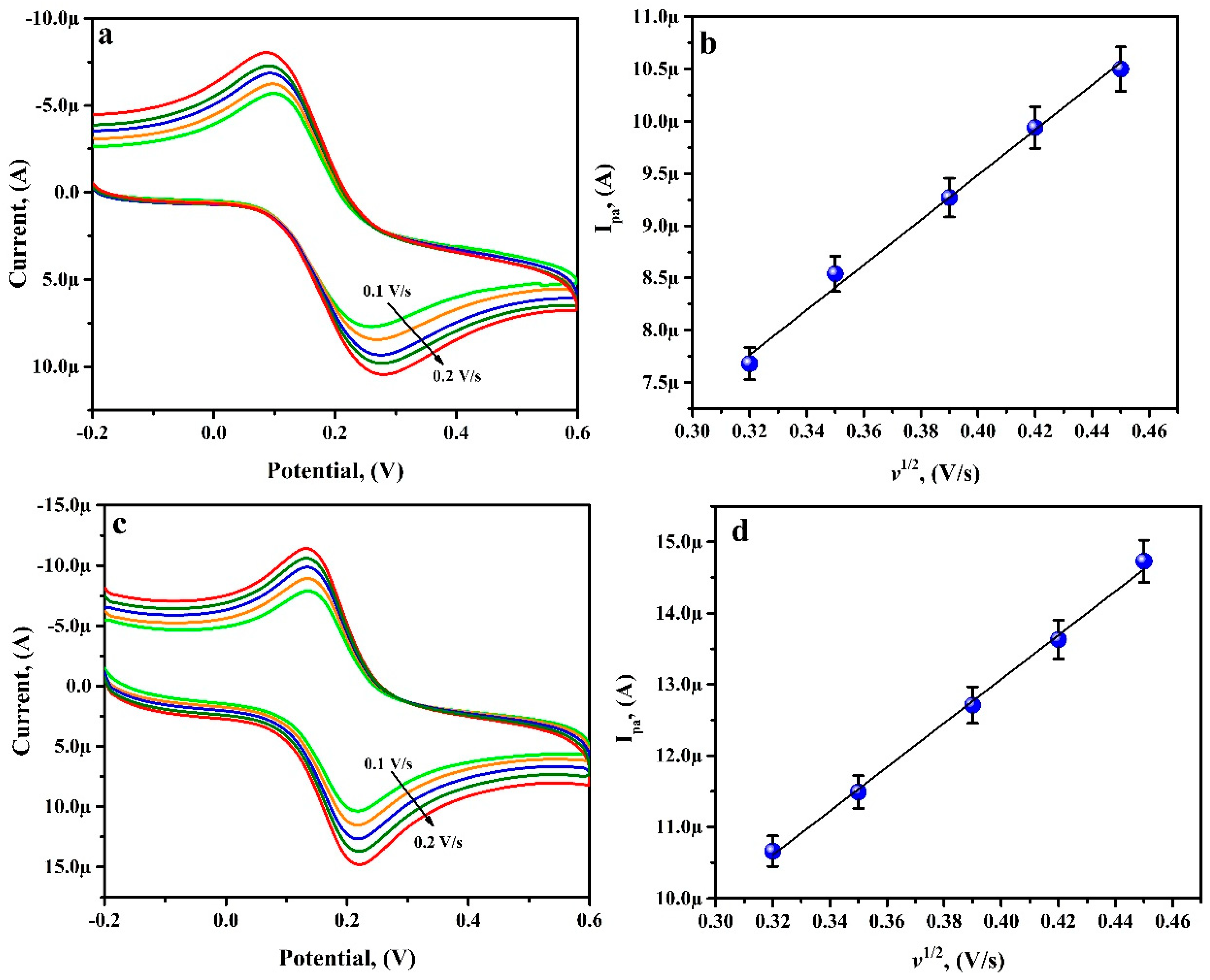
3.7. Potential Scan Rate Effect
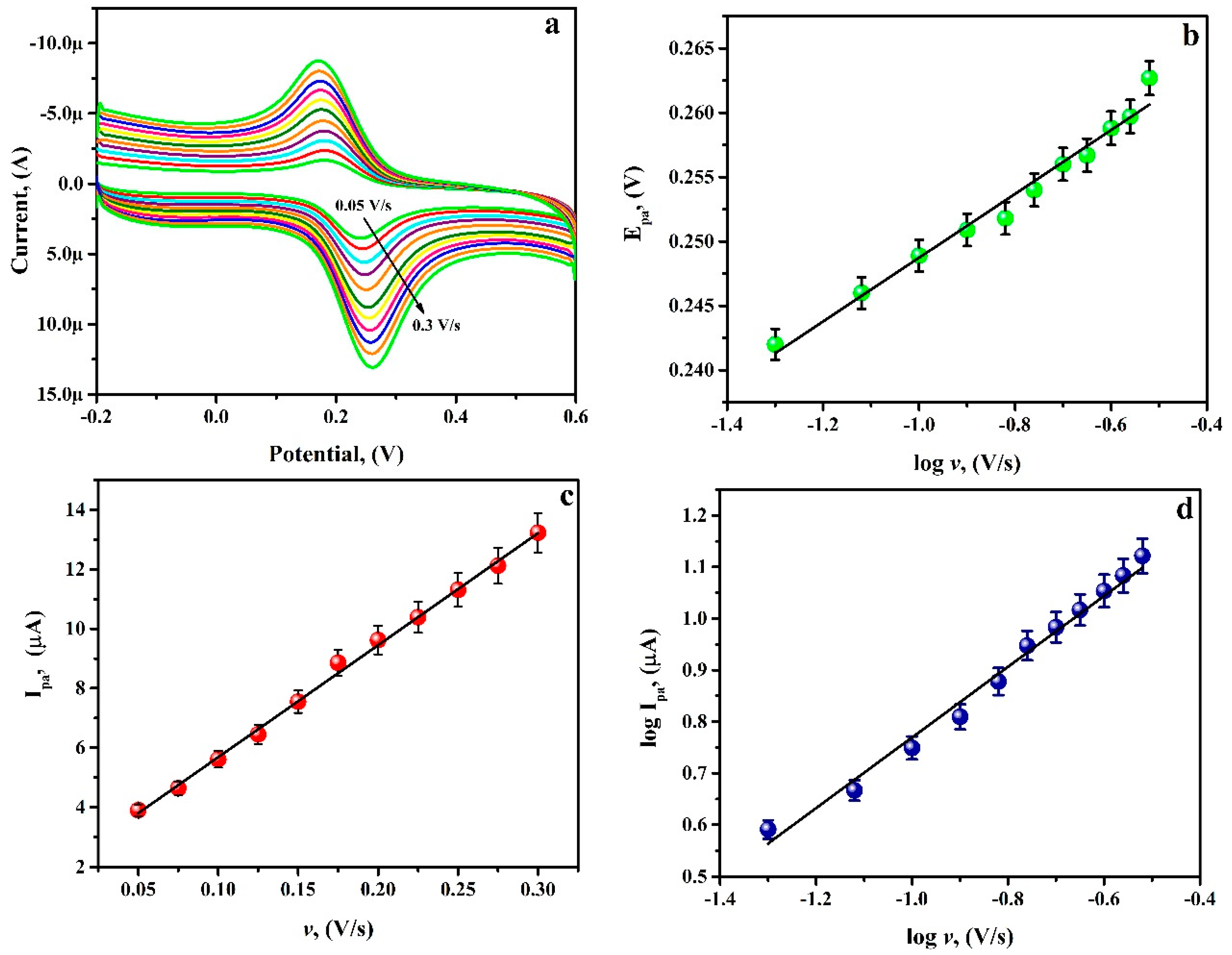
3.8. Analysing Capability of PGAMGPE towards RU
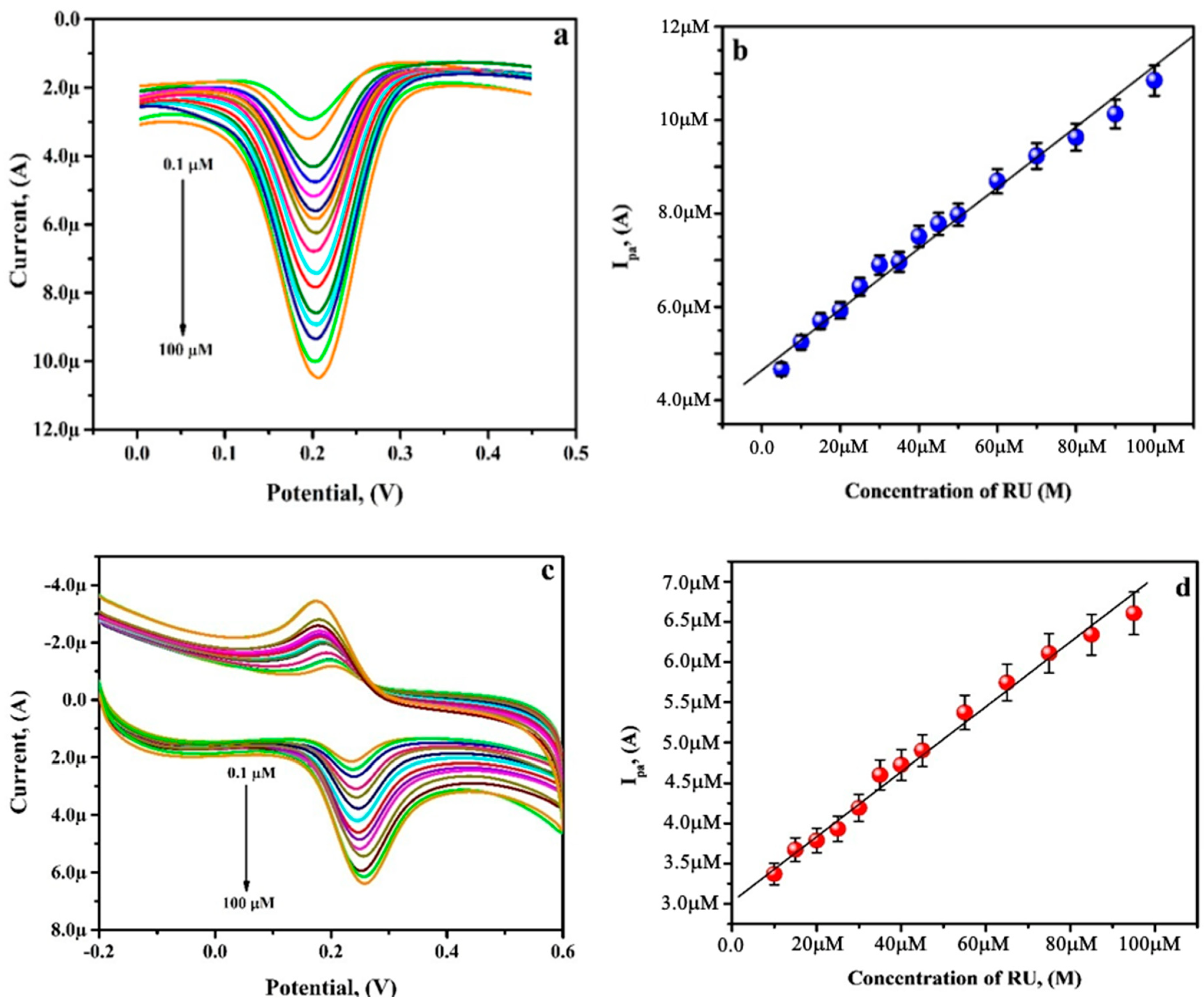
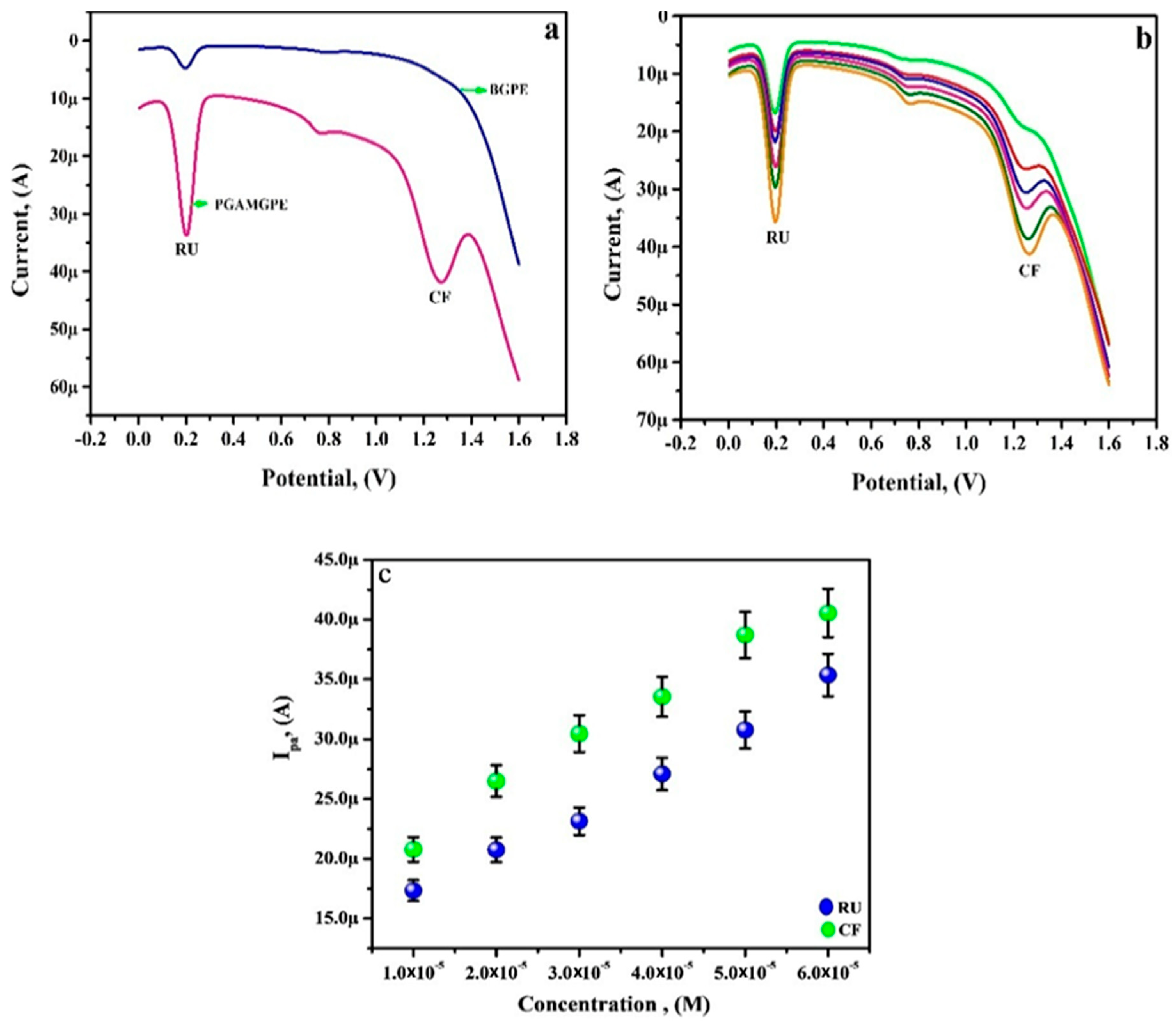
3.9. Simultaneous Analysis of RU and CF
3.10. Interference Analysis
3.11. Assessment of Stability, Reproducibility, and Repeatability
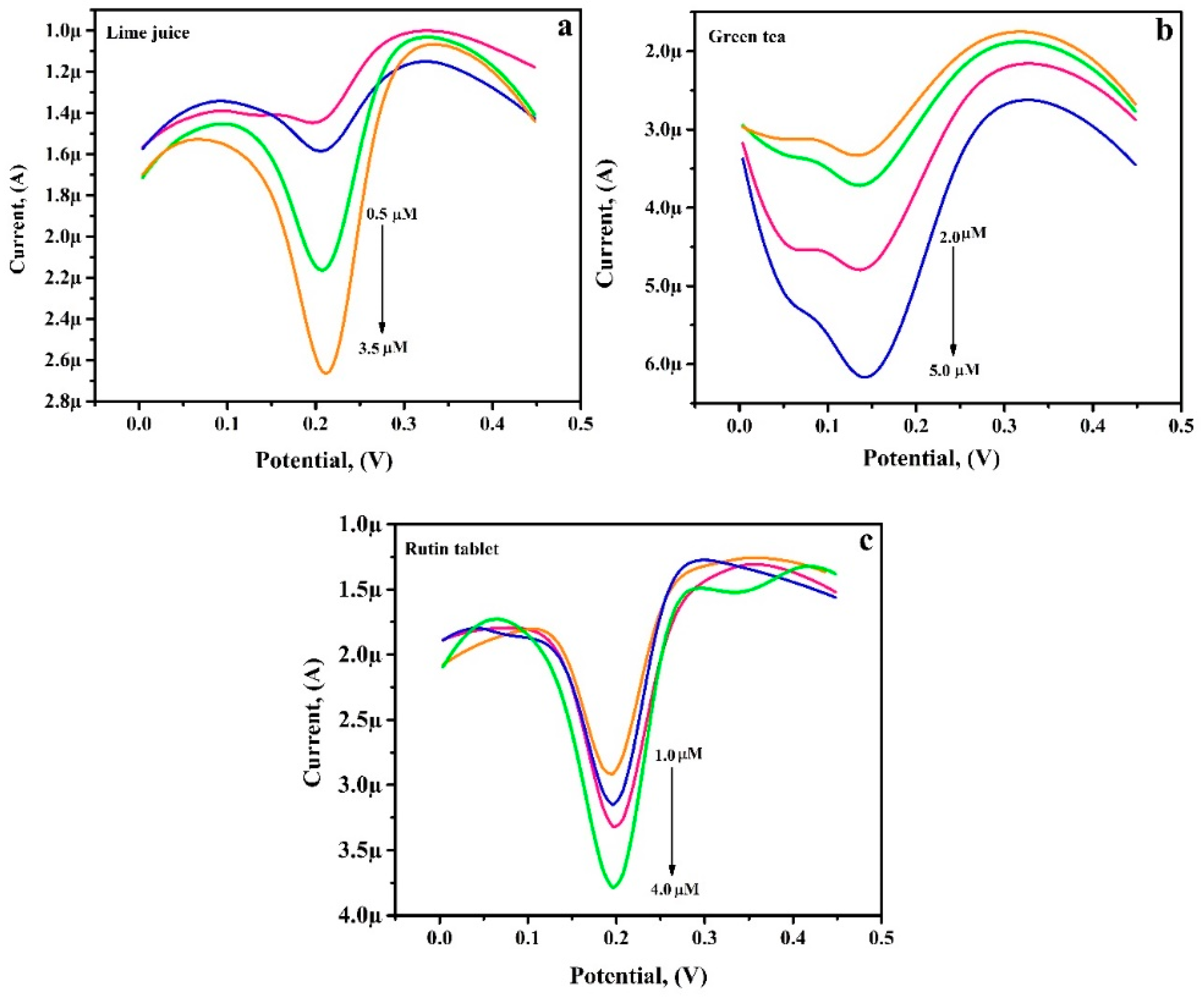
3.12. Analytical Application of PGAMGPE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Asgar, A.; Javed, A.; Jasjeet, K.S.; Sanjula, B. Rutin: Therapeutic Potential and Recent Advances in Drug Delivery. Expert. Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Dizaj, S.M.; Saadat, Y.R.; Khezri, K.; Jafari, S.; Ahmadian, E.; Jahandizi, N.G.; Raeesi, S. Therapeutic Benefits of Rutin and its Nanoformulations. Phytother. Res. 2020, 35, 1719–1738. [Google Scholar] [CrossRef]
- Rodriguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Vazquez-Sanchez, M.; Alvarez-Bernal, D.; Oregel-Zamudio, E.; Ceja-Torres, L.F.; Medina-Medrano, J.R. Quantitative Analysis of Rutin by HPTLC and In Vitro Antioxidant and Antibacterial Activities of Phenolic-Rich Extracts from Verbesina Sphaerocephala. Plants 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qi, X.; Zou, M.; Li, J. Analysis of Rutin from Lespedeza Virgata (Thunb) DC. by Microwave-Assisted Extraction and Capillary Electrophoresis. J. Chem. 2013, 2013, 324294. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Hong-Wu, T.; Chun-Mei, L.; Qiong-Shui, W. Determination of Rutin with UV-Vis Spectrophotometric and Laser-Induced Fluorimetric Detections Using a Non-Scanning Spectrometer. Anal. Lett. 2010, 43, 893–904. [Google Scholar] [CrossRef]
- Liu, D.; Mei, Q.; Wan, X.; Que, H.; Li, L.; Wan, D. Determination of Rutin and Isoquercetin Contents in Hibiscimutabilis Folium in Different Collection Periods by HPLC. J. Chromatogr. Sci. 2015, 53, 1680–1684. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, Y.; Chen, P.; Jing, P.; Yue, J.; Yu, L.L. Chromatographic Fingerprint Analysis and Rutin and Quercetin Compositions in the Leaf and Whole-Plant Samples of Di- and Tetraploid Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 3042–3049. [Google Scholar] [CrossRef]
- Song, Z.; Hou, S. Sensitive Determination of Sub-Nanogram Amounts of Rutin by its Inhibition on Chemiluminescence with Immobilized Reagents. Talanta 2002, 57, 59–67. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, F.; Li, H.; He, P.; Fang, Y. Determination of Hydrochlorothiazide and Rutin in Chinese Herb Medicine and Human Urine by Capillary Zone Electrophoresis with Amperometricdetection. J. Pharm. Biomed. Anal. 2003, 30, 1507–1514. [Google Scholar] [CrossRef]
- Bojarowicz, H.; Marszall, M.P.; Wnuk, M.; Gorynski, K.; Bucinski, A. Determination of Rutin in Plant Extracts and Emulsions by HPLC-MS. Anal. Lett. 2011, 44, 1728–1737. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Khoobi, A.; Behpour, M.; Masoum, S. Application of Multivariate Curve Resolution Alternating Least Squares to Biomedical Analysis Using Electrochemical Techniques at a Nanostructure-Based Modified Sensor. Electrochim. Acta 2014, 130, 271–278. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Khoobi, A.; Masoum, S. Application of Experimental Design for Quantification and Voltammetric Studies of Sulfapyridine Based on a Nanostructure Electrochemical Sensor. Arab. J. Chem. 2017, 10, S3156–S3166. [Google Scholar] [CrossRef]
- Amrutha, B.M.; Manjunatha, J.G.; Aarti, S.B.; Hareesha, N. Sensitive and Selective Electrochemical Detection of Vanillin at Graphene Based Poly (Methyl Orange) Modified Electrode. J. Sci.-Adv. Mater. Dev. 2021, 6, 415–424. [Google Scholar] [CrossRef]
- Berkes, B.B.; Bandarenka, A.S.; Inzelt, G. Electropolymerization: Further Insight into the Formation of Conducting Polyindole Thin Films. J. Phys. Chem. C 2015, 119, 1996–2003. [Google Scholar] [CrossRef]
- Amrutha, B.M.; Manjunatha, J.G.; Aarti, S.B.; Pushpanjali, P.A. Fabrication of a Sensitive and Selective Electrochemical Sensing Platform Based on Poly-l-Leucine Modifed Sensor for Enhanced Voltammetric Determination of Ribofavin. J. Food Meas. Charact. 2020, 14, 3633–3643. [Google Scholar] [CrossRef]
- Khoobi, A.; Shahdost-fard, F.; Arbabi, M.; Akbari, M.; Mirzaei, H.; Nejati, M.; Banafshe, H.R. Sonochemical Synthesis of ErVO4/MnWO4 Heterostructures: Application as a Novel Nanostructured Surface for Electrochemical Determination of Tyrosine in Biological Samples. Polyhedron 2019, 177, 114302. [Google Scholar] [CrossRef]
- Feminus, J.J.; Manikandan, R.; Narayanan, S.S.; Deepa, P.N. Determination of Gallic Acid Using Poly (Glutamic Acid): Graphene Modified Electrode. J. Chem. Sci. 2019, 131, 11. [Google Scholar] [CrossRef]
- Xiao, L.; Liqiang, L.; Yaping, D.; Daixin, Y. Poly-Glutamic Acid Modified Carbon Nanotube-Doped Carbon Paste Electrode for Sensitive Detection of L-Tryptophan. Bioelectrochemistry 2011, 82, 38–45. [Google Scholar] [CrossRef]
- Wei, Y.; Zihua, H.; Junjie, F.; Xiaohua, H. Sensitive Electrochemical Sensor Based on Poly (L=Glutamic Acid)/Graphene Oxide Composite Material for Simultaneous Detection of Heavy Metal Ions. RSC Adv. 2019, 9, 17325–17334. [Google Scholar] [CrossRef]
- Nazari, F.; Ghoreishi, S.M.; Khoobi, A. Bio-based Fe3O4/Chitosan Nanocomposite Sensor for Response Surface Methodology and Sensitive Determination of Gallic acid. Int. J. Biol. Macromol. 2020, 160, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Pushpanjali, P.A.; Manjunatha, J.G.; Hareesha, N.; Amrutha, B.M.; Raril, C.; Zeid, A.A.; Amer, M.A.; Anup, P. Fabrication of Poly (ʟ-Aspartic Acid) Layer on Graphene Nanoplatelets Paste Electrode for Riboflavin Sensing. Mater. Chem. Phys. 2022, 276, 125392. [Google Scholar] [CrossRef]
- Matthew, R.; Andrew, G.W.; Steven, H.; Paolo, B. Voltammetry at Hexamethyl-P-Terphenyl Poly(Benzimidazolium) (HMT-PMBI)-Coated Glassy Carbon Electrodes: Charge Transport Properties and Detection of Uric and Ascorbic Acid. Sensors 2020, 20, 443. [Google Scholar] [CrossRef]
- Chen, C.; Xuchu, L.; Lei, W.; Wu, Y.; Feng, S.; Ding, Y.; Jingjing, L.; Hao, Q.; Shen-Ming, C. Amoxicillin on Polyglutamic Acid Composite Three-Dimensional Graphene Modified Electrode: Reaction Mechanism of Amoxicillin Insights by Computational Simulations. Anal. Chim. Acta 2019, 1073, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hareesha, N.; Manjunatha, J.G.; Amrutha, B.M.; Sreeharsha, N.; Asdaq, S.M.B.; Anwer, M.K. A Fast and Selective Electrochemical Detection of Vanillin in Food Samples on the Surface of Poly (Glutamic Acid) Functionalized Multiwalled Carbon Nanotubes and Graphite Composite Paste Sensor. Colloids Surf. A Physicochem. Eng. 2021, 626, 127042. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P.; Raviraj, M.K. Construction of Nanoparticles Composite Sensor for Atorvastatin and its Determination in Pharmaceutical and Urine Samples. Sens. Actuators B Chem. 2018, 255, 1462–1470. [Google Scholar] [CrossRef]
- Amrutha, B.M.; Manjunatha, J.G.; Aarti, S.B. Design of a Sensitive and Selective Voltammetric Sensor Based on a Cationic Surfactant-Modified Carbon Paste Electrode for the Determination of Alloxan. ACS Omega 2020, 36, 23481–23490. [Google Scholar] [CrossRef]
- Zeng, B.; Wei, S.; Xiao, F.; Zhao, F. Voltammetric Behavior and Determination of Rutin at a Single-Walled Carbon Nanotubes Modified Gold Electrode. Sens. Actuators B Chem. 2006, 115, 240–246. [Google Scholar] [CrossRef]
- Chen, L.; Chaisiwamongkhol, K.; Chen, Y.; Compton, R.G. Rapid electrochemical detection of vanillin in natural vanilla. Electroanalysis 2019, 31, 1067–1074. [Google Scholar] [CrossRef]
- Lei, Y.; Du, D.; Tang, L.; Tan, C.; Chen, K.; Guo-Jun, Z. Determination of Rutin by a Graphene-Modified Glassy Carbon Electrode. Anal. Lett. 2015, 48, 894–906. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G. A Simple Approach for the Electrochemical Determination of Vanillin at Ionic Surfactant Modified Graphene Paste Electrode. Microchem. J. 2020, 154, 104575. [Google Scholar] [CrossRef]
- Hanustiak, P.; Mikelova, R.; Potesil, D.; Hodek, P.; Stiborova, M.; Kizek, R. Electrochemical Behaviour of Flavonoids on a Surface of a Carbon Paste Electrode. Biomed. Pap. 2005, 149, 44–47. [Google Scholar]
- Joon-Hyung, J.; Eunae, C.; Chanho, K.; Seunho, J. Selective Monitoring of Rutin and Quercetin Based on a Novel Multi-Wall Carbon Nanotube-Coated Glassy Carbon Electrode Modifed with Microbial Carbohydrates α-Cyclosophorohexadecaose and Succinoglycan Monomer M3. Bull. Korean Chem. Soc. 2010, 31, 1897–1901. [Google Scholar] [CrossRef][Green Version]
- Yalikun, N.; Mamat, X.; Li, Y.; Hu, X.; Wang, P.; Hu, G. Taraxacum-like Mg-AlSi@Porous Carbon Nanoclusters for Electrochemical Rutin Detection. Microchim. Acta 2019, 186, 379. [Google Scholar] [CrossRef]
- Liu, M.; Deng, J.; Chen, Q.; Huang, Y.; Wang, L.; Zhao, Y.; Zhang, Y.; Li, H.; Yao, S. Sensitive Detection of Rutin with Novel Ferrocene Benzyne Derivative Modified Electrodes. Biosens. Bioelectron. 2013, 41, 275–281. [Google Scholar] [CrossRef]
- Senocak, A.; Khataee, A.; Demirbas, E.; Doustkhah, E. Ultrasensitive Detection of Rutin Antioxidant Through a Magnetic Micro-Mesoporous Graphitized Carbon Wrapped Co Nanoarchitecture. Sens. Actuators B Chem. 2020, 312, 127939. [Google Scholar] [CrossRef]
- Kupendrian, S.; Sakthivel, R.; Chen, S.; Qin-Jin, Y.; Bhuvanenthiran, M.; Thirumalraj, B. “Design of Novel WO3/CB Nanohybrids” An Affordable and Efficient Electrochemical Sensor for the Detection of Multifunctional Flavonoid Rutin. Inorg. Chem. Front. 2018, 5, 1085–1093. [Google Scholar] [CrossRef]
- Oliveira-Roberth, A.D.; Santos, D.I.V.; Cordeiro, D.D.; Lino, F.M.D.A.; Bara, M.T.F.; Gil, E.D.S. Voltammetric determination of Rutin at Screen-Printed carbon disposable electrodes. Cent. Eur. J. Chem. 2012, 10, 1609–1616. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Alothman, Z.A.; Sillanpa, M. Simple and Affordable Graphene Nano-Platelets and Carbon Nanocomposite Surface Decorated with Cetrimonium Bromide as a Highly Responsive Electrochemical Sensor for Rutin Detection. J. Electroanal. Chem. 2022, 917, 116388. [Google Scholar] [CrossRef]
- Attia, T.Z. Simultaneous Determination of Rutin and Ascorbic Acid Mixture in their Pure Forms and Combined Dosage Form. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 169, 82–86. [Google Scholar] [CrossRef]
- Lourenc, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Mazoa, L.H.; Fatibello-Filhoa, O. Simultaneous Voltammetric Determination of Paracetamol and Caffeine in Pharmaceutical Formulations Using a Boron-Doped Diamond Electrode. Talanta 2009, 78, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.; Bairagi, S.M. The In-Vivo Effects of Caffeine and Rutin Combination on Caffeine Intoxication in Rodent Model. Acta Pharm. Sci. 2017, 1, 20–24. [Google Scholar]


| Technique | Electrode | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|---|
| SWV a | CPE b | 1–100 | 0.850 | [32] |
| SWV | α-C16-GCE-MWCNT c | 2–8 | 0.604 | [33] |
| DPV | Mg-Al-Si-PC-GCE d | 1–10 | 0.01 | [34] |
| DPV | MWCNTs-IL-gel-GCE e | 0.04–100 | 0.01 | [35] |
| DPV | Co-ZIF-C-GCE f | 0.1–30 | 0.022 | [36] |
| DPV | CB-WO3-SPCE g | 0.01–75.46 | 0.002 | [37] |
| CV | SPE h | 2.0–15 | 0.1 | [38] |
| CV | CMB-GNPs-CBCPE i | 0.1–8 | 0.023 | [39] |
| HPLC-UV | / | 16.3–98.27 | 3.0 | [40] |
| CV | PGAMGPE | 0.1–100 | 0.06 | Present |
| DPV | PGAMGPE | 0.1–100 | 0.04 | work |
| Sample | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| Lime Juice | 0.5 | 0.48 | 96.83 |
| 1.0 | 0.97 | 97.67 | |
| 2.0 | 1.90 | 95.00 | |
| 3.0 | 2.83 | 97.88 | |
| Green Tea | 2.0 | 1.89 | 94.64 |
| 3.0 | 2.91 | 97.13 | |
| 4.0 | 4.10 | 102.68 | |
| 5.0 | 4.95 | 99.04 | |
| RU Tablet | 1.0 | 0.99 | 99.89 |
| 2.0 | 2.06 | 100.31 | |
| 3.0 | 2.95 | 98.40 | |
| 4.0 | 3.98 | 99.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrutha, B.M.; Manjunatha, J.G.; Nagarajappa, H.; Tighezza, A.M.; Albaqami, M.D.; Sillanpää, M. Electrochemical Polymerisation of Glutamic Acid on the Surface of Graphene Paste Electrode for the Detection and Quantification of Rutin in Food and Medicinal Samples. Diagnostics 2022, 12, 3113. https://doi.org/10.3390/diagnostics12123113
Amrutha BM, Manjunatha JG, Nagarajappa H, Tighezza AM, Albaqami MD, Sillanpää M. Electrochemical Polymerisation of Glutamic Acid on the Surface of Graphene Paste Electrode for the Detection and Quantification of Rutin in Food and Medicinal Samples. Diagnostics. 2022; 12(12):3113. https://doi.org/10.3390/diagnostics12123113
Chicago/Turabian StyleAmrutha, Balliamada M., Jamballi G. Manjunatha, Hareesha Nagarajappa, Ammar M. Tighezza, Munirah D. Albaqami, and Mika Sillanpää. 2022. "Electrochemical Polymerisation of Glutamic Acid on the Surface of Graphene Paste Electrode for the Detection and Quantification of Rutin in Food and Medicinal Samples" Diagnostics 12, no. 12: 3113. https://doi.org/10.3390/diagnostics12123113
APA StyleAmrutha, B. M., Manjunatha, J. G., Nagarajappa, H., Tighezza, A. M., Albaqami, M. D., & Sillanpää, M. (2022). Electrochemical Polymerisation of Glutamic Acid on the Surface of Graphene Paste Electrode for the Detection and Quantification of Rutin in Food and Medicinal Samples. Diagnostics, 12(12), 3113. https://doi.org/10.3390/diagnostics12123113










