Clinical Evaluation of the ButterfLife Device for Simultaneous Multiparameter Telemonitoring in Hospital and Home Settings
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuan, P.X.; Chan, W.K.; Fern Ying, D.K.; Rahman, M.A.A.; Peariasamy, K.M.; Lai, N.M.; Mills, N.L.; Anand, A. Efficacy of telemedicine for the management of cardiovascular disease: A systematic review and meta-analysis. Lancet Digit. Health 2022, 4, e676–e691. [Google Scholar] [CrossRef] [PubMed]
- Bashshur, R.L. On the definition and evaluation of telemedicine. Telemed. J. 1995, 1, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mishlanov, V.; Chuchalin, A.; Chereshnev, V.; Poberezhets, V.; Vitacca, M.; Nevzorova, V.; Aisanov, Z.; Vizel, A.; Shubin, I.; Nikitin, A.; et al. Scope and new horizons for implementation of m-Health/e-Health services in pulmonology in 2019. Monaldi Arch. Chest Dis. Arch. Monaldi Mal. Torace 2019, 89. [Google Scholar] [CrossRef]
- Bashshur, R.; Shannon, G.; Krupinski, E.; Grigsby, J. The taxonomy of telemedicine. Telemed. J. e-Health 2011, 17, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.B. Respiratory applications of telemedicine. Thorax 2009, 64, 189–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paré, G.; Jaana, M.; Sicotte, C. Systematic Review of Home Telemonitoring for Chronic Diseases: The Evidence Base. J. Am. Med. Inform. Assoc. 2007, 14, 269–277. [Google Scholar] [CrossRef]
- Ambrosino, N.; Makhabah, D.N.; Sutanto, Y.S. Tele-medicine in respiratory diseases. Multidiscip. Respir. Med. 2017, 12, 9. [Google Scholar] [CrossRef]
- Ambrosino, N.; Fracchia, C. The role of tele-medicine in patients with respiratory diseases. Expert Rev. Respir. Med. 2017, 11, 893–900. [Google Scholar] [CrossRef]
- Scalvini, S.; Vitacca, M.; Paletta, L.; Giordano, A.; Balbi, B. Telemedicine: A new frontier for effective healthcare services. Monaldi Arch. Chest Dis. Arch. Monaldi Mal. Torace 2004, 61, 226–233. [Google Scholar] [CrossRef]
- Kashem, A.; Cross, R.C.; Santamore, W.P.; Bove, A.A. Management of heart failure patients using telemedicine communication systems. Curr. Cardiol. Rep. 2006, 8, 171–179. [Google Scholar] [CrossRef]
- Hilty, D.M.; Alverson, D.C.; Alpert, J.E.; Tong, L.; Sagduyu, K.; Boland, R.J.; Mostaghimi, A.; Leamon, M.L.; Fidler, D.; Yellowlees, P.M. Virtual reality, telemedicine, web and data processing innovations in medical and psychiatric education and clinical care. Acad. Psychiatry 2006, 30, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Brignell, M.; Wootton, R.; Gray, L. The application of telemedicine to geriatric medicine. Age Ageing 2007, 36, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.; Gibson, O.J.; Tarassenko, L.; Neil, A. A systematic review of telemedicine interventions to support blood glucose self-monitoring in diabetes. Diabet. Med. 2005, 22, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, G.; Abboud, S. Telespirometry for home monitoring of pulmonary function. J. R. Soc. Med. 1999, 92, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Noel, H.C.; Vogel, D.C.; Erdos, J.J.; Cornwall, D.; Levin, F. Home telehealth reduces healthcare costs. Telemed. J. e-Health 2004, 10, 170–183. [Google Scholar] [CrossRef]
- Hersh, W.R.; Helfand, M.; Wallace, J.; Kraemer, D.; Patterson, P.; Shapiro, S.; Greenlick, M. Clinical outcomes resulting from telemedicine interventions: A systematic review. BMC Med. Inform. Decis. Mak. 2001, 1, 5. [Google Scholar] [CrossRef]
- MacKinnon, G.E.; Brittain, E.L. Mobile Health Technologies in Cardiopulmonary Disease. Chest 2020, 157, 654–664. [Google Scholar] [CrossRef]
- Hong, Z.; Li, N.; Li, D.; Li, J.; Li, B.; Xiong, W.; Lu, L.; Li, W.; Zhou, D. Telemedicine During the COVID-19 Pandemic: Experiences From Western China. J. Med. Internet Res. 2020, 22, e19577. [Google Scholar] [CrossRef]
- Lukas, H.; Xu, C.; Yu, Y.; Gao, W. Emerging Telemedicine Tools for Remote COVID-19 Diagnosis, Monitoring, and Management. ACS Nano 2020, 14, 16180–16193. [Google Scholar] [CrossRef]
- Liao CTe Chang, W.T.; Yu, W.L.; Toh, H.S. Utility of telemedicine in the COVID-19 era. Rev. Cardiovasc. Med. 2020, 21, 577–581. [Google Scholar]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Confalonieri, P.; Umberto Meduri, G.; Santus, P.; Harari, S.; Scala, R.; Lanini, S.; Vertui, V.; Oggionni, T.; Caminati, A.; et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect. Dis. 2020, 7, ofaa421. [Google Scholar] [CrossRef] [PubMed]
- Silven, A.V.; Petrus, A.H.J.; Villalobos-Quesada, M.; Dirikgil, E.; Oerlemans, C.R.; Landstra, C.P.; Boosman, H.; van Os, H.J.A.; Blanker, M.H.; Treskes, R.W.; et al. Telemonitoring for Patients with COVID-19: Recommendations for Design and Implementation. J. Med. Internet Res. 2020, 22, e20953. [Google Scholar] [CrossRef] [PubMed]
- Panicacci, S.; Donati, M.; Lubrano, A.; Vianello, A.; Ruiu, A.; Melani, L.; Tomei, A.; Fanucci, L. Telemonitoring in the COVID-19 Era: The Tuscany Region Experience. Healthcare 2021, 9, 516. [Google Scholar] [CrossRef]
- Radin, J.M.; Wineinger, N.E.; Topol, E.J.; Steinhubl, S.R. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: A population-based study. Lancet Digit. Healh 2020, 2, e85–e93. [Google Scholar] [CrossRef]
- Nessle, C.N.; Flora, C.; Sandford, E.; Choi, S.W.; Tewari, M. High-frequency temperature monitoring at home using a wearable device: A case series of early fever detection and antibiotic administration for febrile neutropenia with bacteremia. Pediatr. Blood Cancer 2022, 69, e29835. [Google Scholar] [CrossRef]
- Spender, A.; Bullen, C.; Altmann-Richer, L.; Cripps, J.; Duffy, R.; Falkous, C.; Farrell, M.; Horn, T.; Wigzell, J.; Yeap, W. Wearables and the internet of things: Considerations for the life and health insurance industry. Br. Actuar. J. 2019, 24, e22. [Google Scholar] [CrossRef]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
- Chow, E.J.; Tenforde, M.W.; Rolfes, M.A.; Lee, B.; Chodisetty, S.; Ramirez, J.A.; Fry, A.M.; Patel, M.M. Differentiating severe and non-severe lower respiratory tract illness in patients hospitalized with influenza: Development of the Influenza Disease Evaluation and Assessment of Severity (IDEAS) scale. PLoS ONE 2021, 16, e0258482. [Google Scholar] [CrossRef]
- Teo, J. Early Detection of Silent Hypoxia in COVID-19 Pneumonia Using Smartphone Pulse Oximetry. J. Med. Syst. 2020, 44, 134. [Google Scholar] [CrossRef]
- Levine, D.M.; Ouchi, K.; Blanchfield, B.; Diamond, K.; Licurse, A.; Pu, C.T.; Schnipper, J.L. Hospital-Level Care at Home for Acutely Ill Adults: A Pilot Randomized Controlled Trial. J. Gen. Intern. Med. 2018, 33, 729. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.P.; Pompilio, P.P.; Zanaboni, P.; Bergmo, T.S.; Prikk, K.; Malinovschi, A.; Montserrat, J.M.; Middlemass, J.; Šonc, S.; Munaro, G.; et al. Telemonitoring in Chronic Obstructive Pulmonary Disease (CHROMED). A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kum, H.C.; Morrisey, M.A.; Zheng, Q.; Lawley, M.A. Adherence to Telemonitoring Therapy for Medicaid Patients with Hypertension: Case Study. J. Med. Internet Res. 2021, 23, e29018. [Google Scholar] [CrossRef]
- Mira-Solves, J.J.; Orozco-Beltrán, D.; Sánchez-Molla, M.; Sánchez García, J.J. Evaluation of satisfaction with telemedicine devices and with the results of the care received among chronic patients. The ValCrònic program. Aten. Primaria 2014, 46 (Suppl. S3), 16–23. [Google Scholar] [CrossRef] [PubMed]
- Brekke, I.J.; Puntervoll, L.H.; Pedersen, P.B.; Kellett, J.; Brabrand, M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS ONE 2019, 14, e0210875. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, S.; Bhattacharya, T.; Tagde, P.; Chopra, H.; Akter, R.; Kaushik, D.; Rahman, M.H. Blockchain and artificial intelligence technology in e-Health. Environ. Sci. Pollut. Res. Int. 2021, 28, 52810–52831. [Google Scholar] [CrossRef]
- Turcian, D.; Stoicu-Tivadar, V. Artificial Intelligence in Primary Care: An Overview. Stud. Health Technol. Inform. 2022, 289, 208–211. [Google Scholar]
- Goudra, B.G.; Penugonda, L.C.; Speck, R.M.; Sinha, A.C.; Goudra, B.G.; Penugonda, L.C.; Speck, R.M.; Sinha, A.C. Comparison of Acoustic Respiration Rate, Impedance Pneumography and Capnometry Monitors for Respiration Rate Accuracy and Apnea Detection during GI Endoscopy Anesthesia. Open J. Anesthesiol. 2013, 3, 74–79. [Google Scholar] [CrossRef][Green Version]
- Granholm, A.; Pedersen, N.E.; Lippert, A.; Petersen, L.F.; Rasmussen, L.S. Respiratory rates measured by a standardised clinical approach, ward staff, and a wireless device. Acta Anaesthesiol. Scand. 2016, 60, 1444–1452. [Google Scholar] [CrossRef]
- Bawua, L.K.; Miaskowski, C.; Suba, S.; Badilini, F.; Mortara, D.; Hu, X.; Rodway, G.W.; Hoffmann, T.J.; Pelter, M.M. Agreement between respiratory rate measurement using a combined electrocardiographic derived method versus impedance from pneumography. J. Electrocardiol. 2022, 71, 16–24. [Google Scholar] [CrossRef]

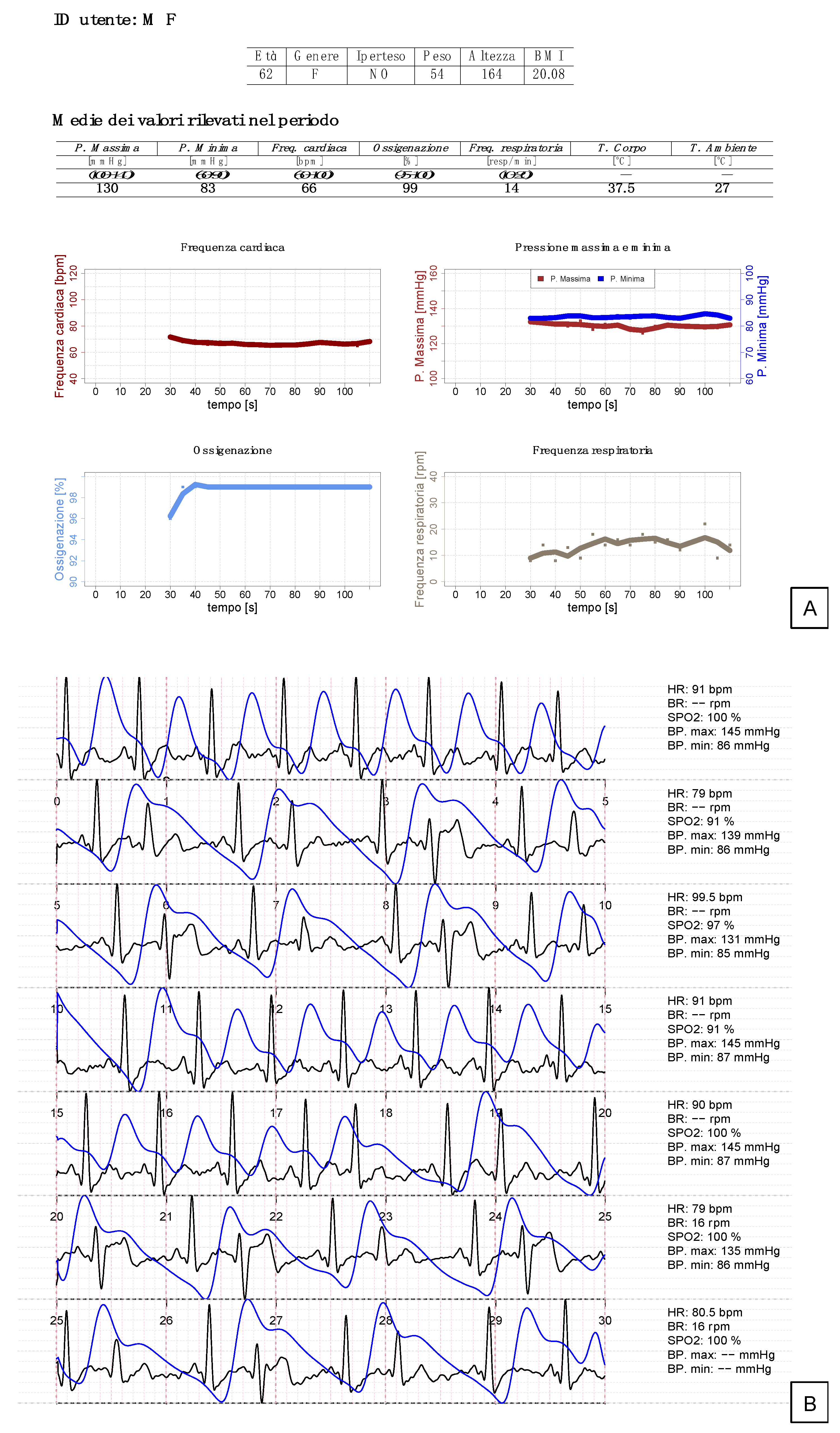
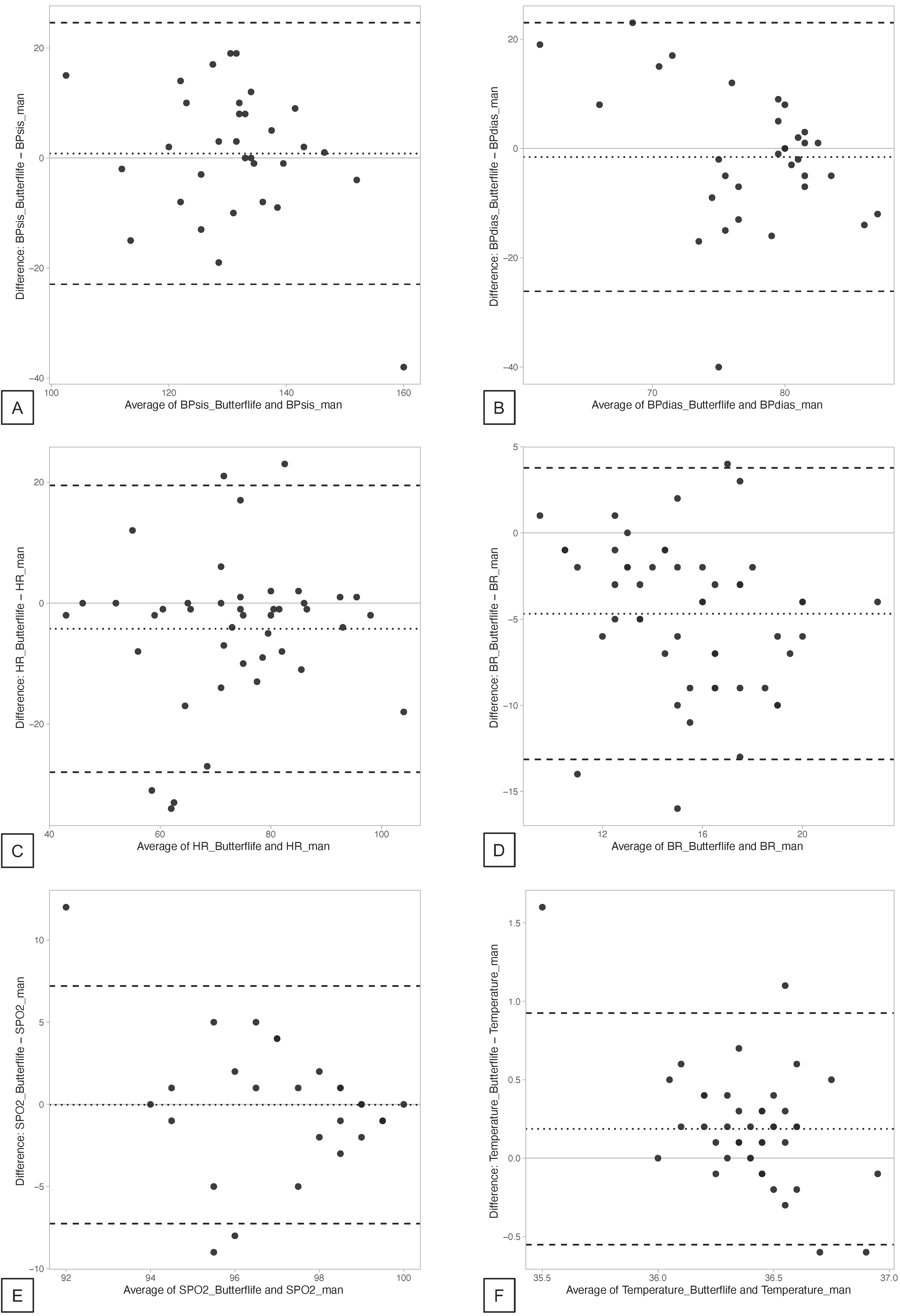
| ButterfLife, Mean (SD) | SoC, Mean (SD) | ΔButterfLife—SoC, Mean (Limits of Agreement 95% CI) * | Correlation ¶ | p-Value ¶ | |
|---|---|---|---|---|---|
| SBP (mmHg) | 131.72 (11.03) | 130.91 (14.31) | 0.81 (−3.63; 5.25) | 0.54 | <0.01 |
| DBP (mmHg) | 76.75 (6.55) | 78.31 (9.87) | −1.56 (−6.15; 3.03) | −0.18 | 0.33 |
| HR (bpm) | 71.56 (15.47) | 75.81 (14.18) | −4.26 (−8.02; −0.49) | 0.65 | <0.01 |
| RR (bpm) | 13.12 (3.18) | 17.80 (4.02) | −4.69 (−5.91; −3.46) | 0.33 | 0.02 |
| SpO2 (%) | 97.58 (2.27) | 97.61 (3.04) | −0.03 (−1.29; 1.24) | 0.20 | 0.24 |
| Temperature (°C) | 36.50 (0.71) | 36.25 (0.46) | 0.19 (0.07; 0.30) | 0.36 | 0.02 |
| Patient | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Age | 91 | 75 | 53 | 62 | 69 | 79 | 52 | 83 |
| Sex | F | M | M | F | M | F | F | F |
| BMI | 22.2 | 27.4 | 23.3 | 20.1 | 31.6 | 22.0 | 22.8 | 30.4 |
| Smoker | No | Yes | No | Yes | No | No | No | Former |
| Main disease | CRF (IPF) Type 2 DM CHF | Hypertension; Type 2 DM | Hypertension | Hypertension | Hypertension; Lung cancer Parkinsonism | CHF; MGUS | Asthma | CRF (COPD); kyphoscoliosis |
| Oxygen therapy | Yes | No | No | No | No | No | No | Yes |
| Baseline ECG abnormalities | None | None | None | None | None | None | None | None |
| Patient | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| No. Tests performed (% of test due) | 107 (89.2) | 21 (17.5) | 123 (102.5) | 46 (38.3) | 126 (105.0) | 86 (71.7) | 20 (16.7) | 118 (98.3) |
| No. of valid tests (% of tests performed) | 104 (97.2) | 20 (95.2) | 118 (95.9) | 42 (91.3) | 78 (61.9) | 85 (98.8) | 20 (100.0) | 112 (94.9) |
| Identified condition | None | None | Sinus tachycardia | Bigeminism | Hypertension | Isolated extrasystoles | RBBB; Isolated extrasystoles | Desaturation |
| Confirmed condition | None | None | Sinus tachycardia | Bigeminism | Hypertension | Isolated extrasystoles | Isolated extrasystoles | Desaturation |
| Need for hospitalization | No | No | No | No | No | No | No | No |
| Patient’s satisfaction (0–10) | 10 | 8 | 10 | 10 | 7 | 9 | 7 | 10 |
| Problems with the device | None | None | None | None | Parkinsonism | None | None | None |
| SBP, mmHg (median, IQR) | 132.0 (5.8) | 139.0 (3.0) | 123.0 (7.0) | 136.0 (8.0) | 146 (4.0) | 132.0 (3.0) | 127.0 (3.0) | 133.0 (6.0) |
| DBP, mmHg (median, IQR) | 82.0 (1.0) | 81.0 (0.2) | 80.0 (3.0) | 83.0 (1.0) | 81.0 (2.5) | 83.0 (0.0) | 73.0 (2.0) | 81.0 (1.0) |
| HR, bpm (median, IQR) | 77.0 (7.5) | 69.0 (7.0) | 68.0 (12.0) | 76.0 (15.2) | 71.0 (6.2) | 67.0 (5.2) | 77.0 (8.2) | 64.0 (5.5) |
| RR, bpm (median, IQR) | 15.5 (5.0) | 15.0 (2.0) | 12.0 (4.0) | 14.0 (3.0) | 12.0 (4.0) | 15.0 (2.5) | 13.0 (6.0) | 13.0 (4.0) |
| SpO2, % (median, IQR) | 99.0 (0.0) | 99.0 (0.0) | 99.0 (2.0) | 99.0 (1.2) | 98.0 (0.5) | 96.0 (5.0) | 98.0 (2.5) | 94.0 (3.0) |
| Temperature, °C (median, IQR) | 36.3 (0.0) | 36.3 (0.0) | 36.5 (0.2) | 36.5 (0.7) | 36.2 (0.2) | 36.4 (0.2) | 36.6 (0.0) | 36.4 (0.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salton, F.; Kette, S.; Confalonieri, P.; Fonda, S.; Lerda, S.; Hughes, M.; Confalonieri, M.; Ruaro, B. Clinical Evaluation of the ButterfLife Device for Simultaneous Multiparameter Telemonitoring in Hospital and Home Settings. Diagnostics 2022, 12, 3115. https://doi.org/10.3390/diagnostics12123115
Salton F, Kette S, Confalonieri P, Fonda S, Lerda S, Hughes M, Confalonieri M, Ruaro B. Clinical Evaluation of the ButterfLife Device for Simultaneous Multiparameter Telemonitoring in Hospital and Home Settings. Diagnostics. 2022; 12(12):3115. https://doi.org/10.3390/diagnostics12123115
Chicago/Turabian StyleSalton, Francesco, Stefano Kette, Paola Confalonieri, Sergio Fonda, Selene Lerda, Michael Hughes, Marco Confalonieri, and Barbara Ruaro. 2022. "Clinical Evaluation of the ButterfLife Device for Simultaneous Multiparameter Telemonitoring in Hospital and Home Settings" Diagnostics 12, no. 12: 3115. https://doi.org/10.3390/diagnostics12123115
APA StyleSalton, F., Kette, S., Confalonieri, P., Fonda, S., Lerda, S., Hughes, M., Confalonieri, M., & Ruaro, B. (2022). Clinical Evaluation of the ButterfLife Device for Simultaneous Multiparameter Telemonitoring in Hospital and Home Settings. Diagnostics, 12(12), 3115. https://doi.org/10.3390/diagnostics12123115










