Quantification of Significant Aortic Stenosis by Echocardiography versus Four-Dimensional Cardiac Computed Tomography: A Multi-Modality Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Computed Tomography
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; de Bonis, M.; de Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Magne, J.; Pibarot, P. Low-gradient aortic stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamvakidou, A.; Annabi, M.-S.; Pibarot, P.; Plonska-Gosciniak, E.; Almeida, A.G.; Guzzetti, E.; Dahou, A.; Burwash, I.G.; Koschutnik, M.; Bartko, P.E.; et al. Clinical Value of Stress Transaortic Flow Rate During Dobutamine Echocardiography in Reduced Left Ventricular Ejection Fraction, Low-Gradient Aortic Stenosis: A Multicenter Study. Circ. Cardiovasc. Imaging 2021, 14, e012809. [Google Scholar] [CrossRef]
- Sato, K.; Sankaramangalam, K.; Kandregula, K.; Bullen, J.A.; Kapadia, S.R.; Krishnaswamy, A.; Mick, S.; Rodriguez, L.L.; Grimm, R.A.; Menon, V.; et al. Contemporary Outcomes in Low-Gradient Aortic Stenosis Patients Who Underwent Dobutamine Stress Echocardiography. J. Am. Heart Assoc. 2019, 8, e011168. [Google Scholar] [CrossRef] [Green Version]
- Magne, J.; Donal, E.; Davin, L.; O’Connor, K.; Szymanski, C.; Piérard, L.A.; Lancellotti, P. 194 Clinical outcome in asymptomatic severe aortic stenosis. Insights from the new proposed aortic stenosis grading classification. Arch. Cardiovasc. Dis. Suppl. 2012, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Levy, F.; Laurent, M.; Monin, J.L.; Maillet, J.M.; Pasquet, A.; Le Tourneau, T.; Petit-Eisenmann, H.; Gori, M.; Jobic, Y.; Bauer, F.; et al. Aortic Valve Replacement for Low-Flow/Low-Gradient Aortic Stenosis: Operative Risk Stratification and Long-Term Outcome: A European Multicenter Study. J. Am. Coll. Cardiol. 2008, 51, 1466–1472. [Google Scholar] [CrossRef] [Green Version]
- Ueyama, H.; Kuno, T.; Harrington, M.; Takagi, H.; Krishnamoorthy, P.; Sharma, S.K.; Kini, A.; Stamatios, L. Impact of Surgical and Transcatheter Aortic Valve Replacement in Low-Gradient Aortic Stenosis: A Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 1481–1492. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Malouf, J.; Messika-Zeitoun, D.; Araoz, P.A.; Michelena, H.I.; Enriquez-Sarano, M. Aortic Valve Area Calculation in Aortic Stenosis by CT and Doppler Echocardiography. JACC Cardiovasc. Imaging 2015, 8, 248–257. [Google Scholar] [CrossRef]
- Ng, A.C.; Delgado, V.; van der Kley, F.; Shanks, M.; van de Veire, N.R.; Bertini, M.; Nucifora, G.; van Bommel, R.J.; Tops, L.F.; de Weger, A.; et al. Comparison of Aortic Root Dimensions and Geometries Before and After Transcatheter Aortic Valve Implantation by 2- and 3-Dimensional Transesophageal Echocardiography and Multislice Computed Tomography. Circ. Cardiovasc. Imaging 2010, 3, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, B.; Schoenhagen, P.; Kapadia, S.R.; Svensson, L.G.; Rodriguez, L.; Griffin, B.P.; Tuzcu, E.M.; Desai, M.Y. Integration of 3D imaging data in the assessment of aortic stenosis: Impact on classification of disease severity. Circ Cardiovasc. Imaging 2011, 4, 566–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamperidis, V.; van Rosendael, P.; Katsanos, S.; Van Der Kley, F.; Regeer, M.; Al Amri, I.; Sianos, G.; Marsan, N.A.; Delgado, V.; Bax, J.J. Low gradient severe aortic stenosis with preserved ejection fraction: Reclassification of severity by fusion of Doppler and computed tomographic data. Eur. Heart J. 2015, 36, 2087–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monin, J.L.; Quere, J.P.; Monchi, M.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation 2003, 108, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messika-Zeitoun, D.; Serfaty, J.-M.; Brochet, E.; Ducrocq, G.; Lepage, L.; Detaint, D.; Hyafil, F.; Himbert, D.; Pasi, N.; Laissy, J.-P.; et al. Multimodal Assessment of the Aortic Annulus Diameter: Implications for Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2010, 55, 186–194. [Google Scholar] [CrossRef]
- Doherty, J.U.; Kort, S.; Mehran, R.; Schoenhagen, P.; Soman, P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2017, 70, 1647–1672. [Google Scholar]
- Sato, K.; Wang, T.K.M.; Desai, M.Y.; Kapadia, S.R.; Krishnaswamy, A.; Rodriguez, L.L.; Grimm, R.A.; Griffin, B.P.; Popović, Z.B. Physical and physiological effects of dobutamine stress echocardiography in low-gradient aortic stenosis. Am. J. Physiol. Circ. Physiol. 2022, 322, H94–H104. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Clavel, M.A.; Messika-Zeitoun, D.; Pibarot, P.; Aggarwal, S.R.; Malouf, J.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; Capoulade, R.; et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler echocardiographic and computed tomographic study. J. Am. Coll. Cardiol. 2013, 62, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Clavel, M.-A.; Pibarot, P.; Messika-Zeitoun, D.; Capoulade, R.; Malouf, J.; Aggarwal, S.R.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014, 64, 1202–1213. [Google Scholar] [CrossRef] [Green Version]
- Pawade, T.; Clavel, M.-A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; Macron, L.; et al. Computed Tomography Aortic Valve Calcium Scoring in Patients with Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [CrossRef] [PubMed]
- Cueff, C.; Serfaty, J.-M.; Cimadevilla, C.; Laissy, J.-P.; Himbert, D.; Tubach, F.; Duval, X.; Iung, B.; Enriquez-Sarano, M.; Vahanian, A.; et al. Measurement of aortic valve calcification using multislice computed tomography: Correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2010, 97, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Doris, M.K.; Jenkins, W.; Robson, P.; Pawade, T.; Andrews, J.P.; Bing, R.; Cartlidge, T.; Shah, A.; Pickering, A.; Williams, M.C.; et al. Computed tomography aortic valve calcium scoring for the assessment of aortic stenosis progression. Heart 2020, 106, 1906–1913. [Google Scholar] [CrossRef]
- Eberhard, M.; Mastalerz, M.; Frauenfelder, T.; Tanner, F.; Maisano, F.; Nietlispach, F.; Seifert, B.; Alkadhi, H.; Nguyen-Kim, T. Quantification of aortic valve calcification on contrast-enhanced CT of patients prior to transcatheter aortic valve implantation. EuroIntervention 2017, 13, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Cartlidge, T.R.; Bing, R.; Kwiecinski, J.; Guzzetti, E.; A Pawade, T.; Doris, M.K.; Adamson, P.D.; Massera, D.; Lembo, M.; Peeters, F.E.C.M.; et al. Contrast-enhanced computed tomography assessment of aortic stenosis. Heart 2021, 107, 1905–1911. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2019, 13, 1–20. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, T.; Adawi, S.; Sachner, R.; Asmer, I.; Ganaeem, M.; Rubinshtein, R.; Shiran, A. Three-Dimensional Imaging of the Left Ventricular Outflow Tract: Impact on Aortic Valve Area Estimation by the Continuity Equation. J. Am. Soc. Echocardiogr. 2012, 25, 749–757. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Yamamoto, H.; Horiguchi, J.; Kunita, E.; Okada, T.; Yamazato, R.; Hidaka, T.; Kihara, Y. Underestimation of aortic valve area in calcified aortic valve disease: Effects of left ventricular outflow tract ellipticity. Int. J. Cardiol. 2012, 157, 347–353. [Google Scholar] [CrossRef]
- Saitoh, T.; Shiota, M.; Izumo, M.; Gurudevan, S.V.; Tolstrup, K.; Siegel, R.J.; Shiota, T. Comparison of Left Ventricular Outflow Geometry and Aortic Valve Area in Patients with Aortic Stenosis by 2-Dimensional Versus 3-Dimensional Echocardiography. Am. J. Cardiol. 2012, 109, 1626–1631. [Google Scholar] [CrossRef]
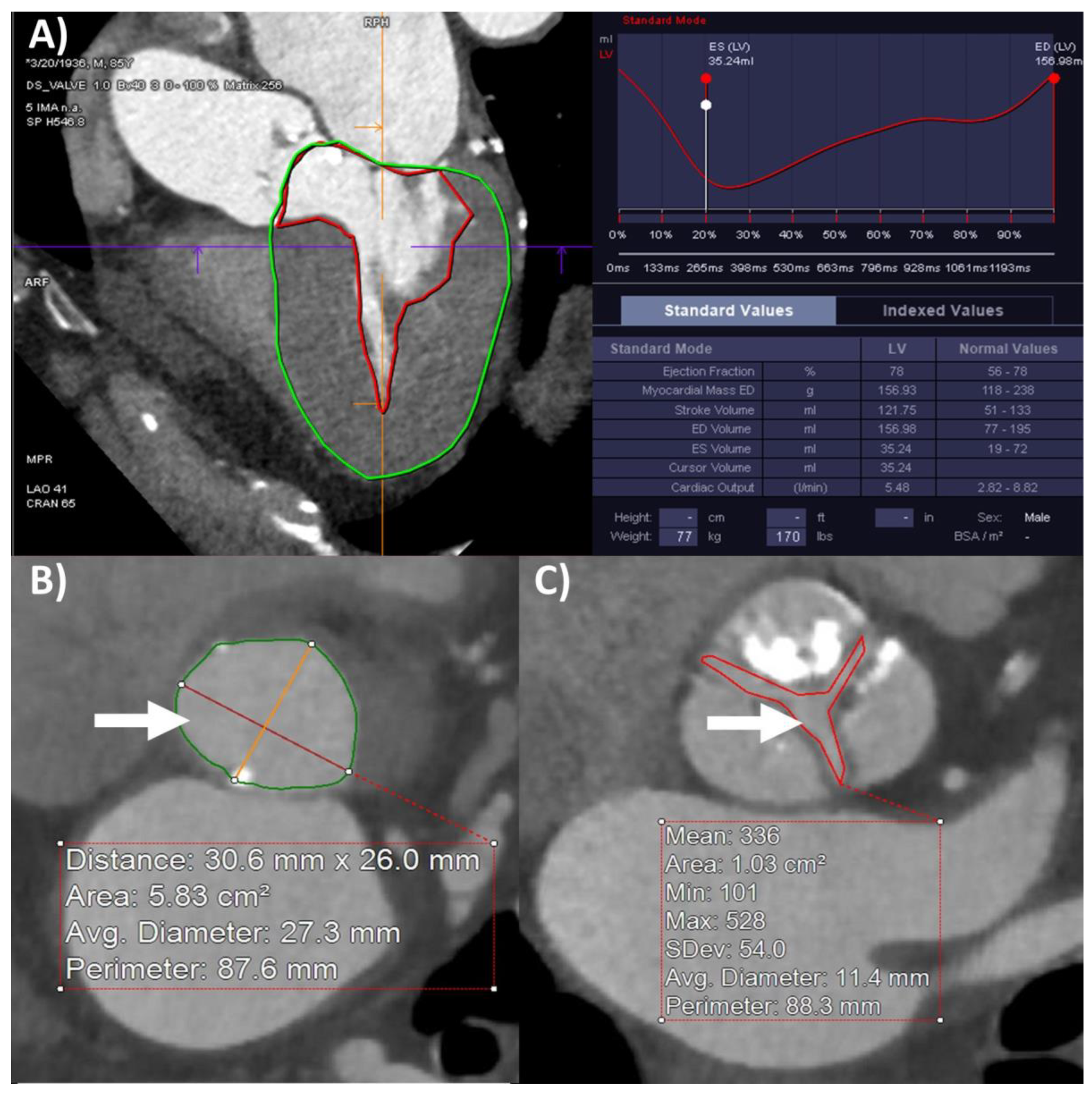
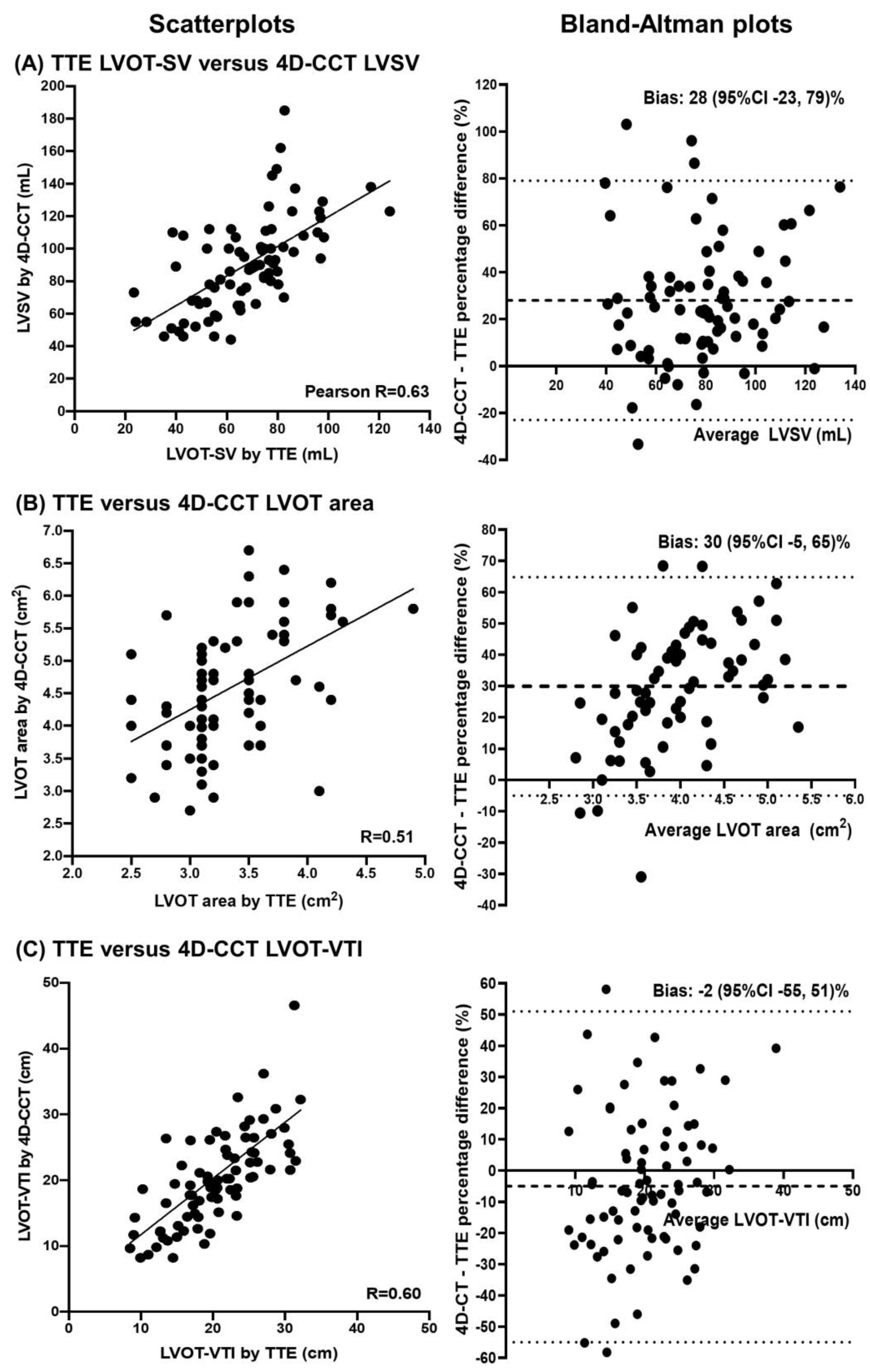
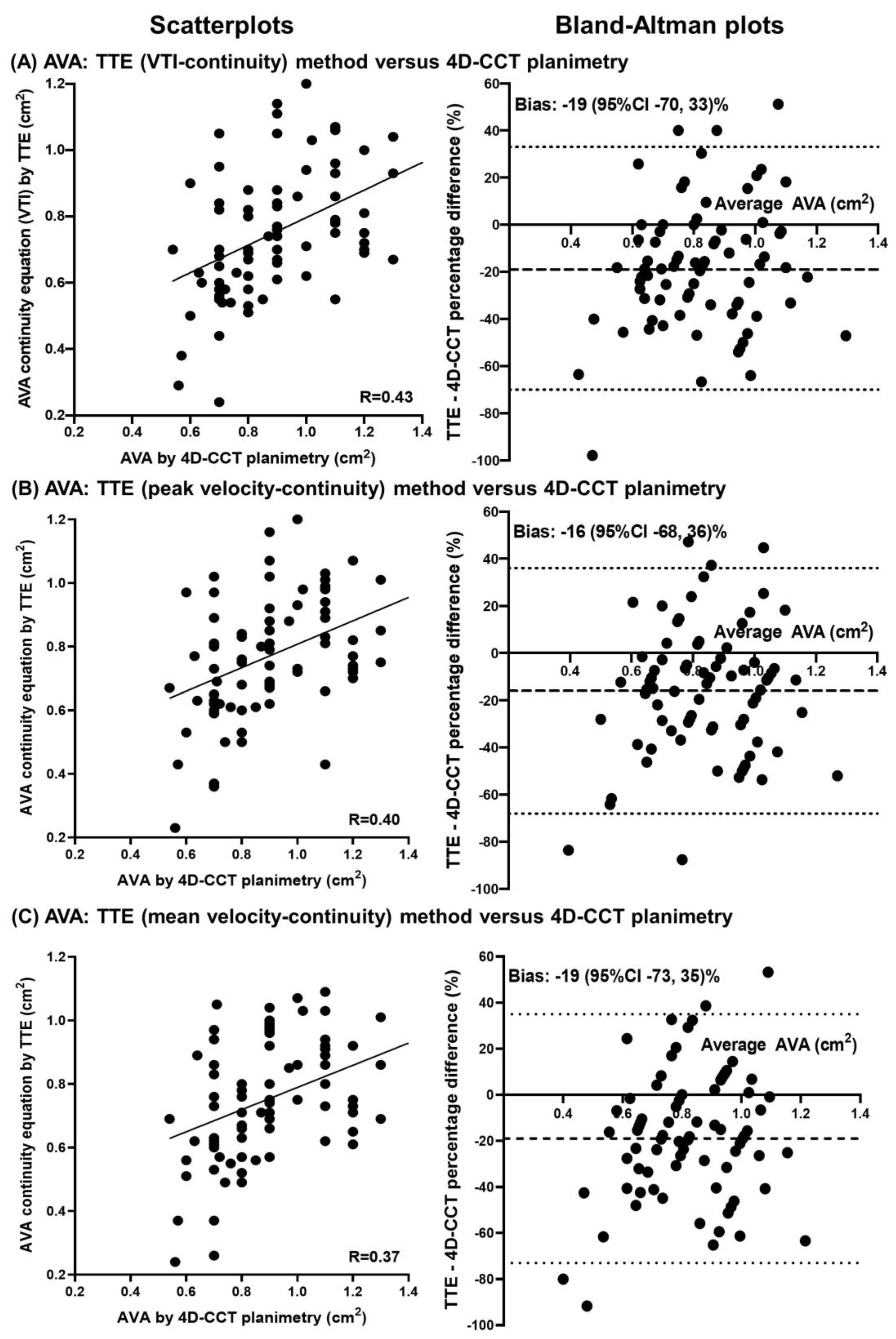

| Total | HG-AS | CLFLG-AS | PLFLG-AS | NFLG-AS | p-Value | |
|---|---|---|---|---|---|---|
| Number of patients | 80 | 20 | 20 | 20 | 20 | |
| Demographics | ||||||
| Age (years) | 81 ± 9 | 79 ± 10 | 81 ± 9 | 82 ± 7 | 81 ± 12 | |
| Male | 57 (71%) | 10 (50%) | 16 980%) | 17 (85%) | 14 (70%) | 0.071 |
| Caucasian | 76 (95%) | 17 (85%) | 19 (95%) | 20 (100%) | 20 (100%) | 0.421 |
| Past medical history | ||||||
| Hypertension | 68 (85%) | 19 (95%) | 17 (85%) | 17 (85%) | 15 (75%) | 0.371 |
| Diabetes | 33 (41%) | 8 (40%) | 9 (45%) | 10 (50%) | 6 (30%) | 0.614 |
| Atrial fibrillation | 36 (45%) | 4 (20%) | 10 (50%) | 14 (70%) | 8 (40%) | 0.015 |
| Coronary artery disease | 58 (73%) | 10 (50%) | 18 (90%) | 14 (70%) | 16 (80%) | 0.061 |
| Myocardial infarction | 23 (29%) | 5 (25%) | 4 (20%) | 8 (40%) | 6 (30%) | 0.545 |
| Coronary artery bypass grafting | 26 (33%) | 5 (25%) | 7 (35%) | 7 (35%) | 7 (35%) | 0.877 |
| Percutaneous coronary intervention | 25 (31%) | 3 (15%) | 8 (40%) | 7 (35%) | 7 (35%) | 0.330 |
| Cardiac implantable electronic device | 15 (19%) | 1 (5%) | 6 (30%) | 4 (20%) | 4 (20%) | 0.242 |
| Stroke | 14 (18%) | 5 (25%) | 2 (10%) | 5 (25%) | 2 (10%) | 0.374 |
| Peripheral arterial disease | 28 (35%) | 6 (30%) | 8 (40%) | 5 (25%) | 9 (45%) | 0.532 |
| Chronic kidney disease | 17 (21%) | 3 (15%) | 5 (25%) | 2 (10%) | 7 (35%) | 0.221 |
| Dialysis | 2 (3%) | 0 (0%) | 1 (5%) | 0 (0%) | 1 (5%) | 0.562 |
| Chronic lung disease | 10 (13%) | 2 (10%) | 2 (10%) | 4 (20%) | 2 (10%) | 0.712 |
| Cirrhosis | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (10%) | 0.104 |
| STS score (%) | 6.3 ± 4.6% | 4.8 ± 1.9% * | 9.1 ± 6.9% * | 5.7 ± 3.7% | 5.5 ± 3.4% |
| Total | HG-AS | CLFLG-AS | PLFLG-AS | NFLG-AS | |
|---|---|---|---|---|---|
| 80 | 20 | 20 | 20 | 20 | |
| Transthoracic echocardiography | |||||
| Left ventricular end-diastolic volume (mL) | 101 ± 39 | 96 ± 30 * | 135 ± 43 *^’ | 84 ± 19 ^ | 88 ± 35 ’ |
| Left ventricular end-systolic volume (mL) | 49 ± 32 | 38 ± 13 * | 88 ± 38 *^’ | 36 ± 12 ^ | 37 ± 18 ’ |
| Left ventricular stroke volume (mL) | 51 ± 17 | 59 ± 20 | 48 ± 15 | 48 ± 11 | 51 ± 20 |
| Left ventricular outflow tract stroke volume (mL) | 68 ± 20 | 79 ± 16 *’ | 50 ± 15 *^ | 60 ± 12 ’# | 81 ± 14 ^# |
| Let ventricular ejection fraction (%) | 54 ± 13% | 61 ± 6% * | 37 ± 10% *^’ | 58 ± 8% ^ | 59 ± 9% ’ |
| Left ventricular mass (g) | 207 ± 64 | 193 ± 62 | 240 ± 68 | 203 ± 58 | 191 ± 60 |
| Left ventricular outflow tract diameter (cm) | 2.1 ± 0.1 | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Left ventricular outflow tract area (cm2) | 3.4 ± 0.5 | 3.3 ± 0.6 | 3.4 ± 0.5 | 3.4 ± 0.4 | 3.3 ± 0.5 |
| Left ventricular velocity-time integral (cm) | 20 ± 6 | 24 ± 5 *’ | 14 ± 4 *^ | 18 ± 4 ’# | 25 ± 4 ^# |
| Aortic valve velocity-time integral (cm) | 91 ± 22 | 114 ± 24 *^’ | 81 ± 13 * | 80 ± 11 ^ | 89 ± 16 ’ |
| Aortic valve mean velocity (cm/s) | 279 ± 47 | 341 ± 48 *^’ | 254 ± 27 * | 257 ± 22 ^ | 265 ± 14 ’ |
| Aortic valve peak velocity (cm/s) | 388 ± 55 | 462 ± 55 *^’ | 358 ± 29 * | 360 ± 26 ^ | 371 ± 19 ’ |
| Aortic valve mean gradient (mmHg) | 32 ± 12 | 47 ± 14 *^’ | 26 ± 5 * | 27 ± 5 ^ | 28 ± 3 ’ |
| Aortic valve peak gradient (mmHg) | 62 ± 19 | 87 ± 21 *^’ | 51 ± 9 * | 52 ± 8 ^ | 56 ± 8 ’ |
| Dimensionless index | 0.23 ± 0.06 | 0.22 ± 0.06 * | 0.18 ± 0.04 ^’ | 0.23 ± 0.05 ^# | 0.29 ± 0.06 *’# |
| Aortic valve area (velocity time integral continuity method cm2) | 0.76 ± 0.20 | 0.72 ± 0.17 * | 0.61 ± 0.14 ^’ | 0.76 ± 0.16 ^# | 0.93 ± 0.20 *’# |
| Computed tomography | |||||
| Left ventricular end-diastolic volume (mL) | 160 ± 55 | 147 ± 36 * | 216 ± 69 *^’ | 132 ± 27 ^ | 147 ± 37 ’ |
| Left ventricular end-systolic volume (mL) | 70 ± 55 | 46 ± 17 * | 145 ± 63 *^’ | 45 ± 13 ^ | 45 ± 12 ’ |
| Left ventricular stroke volume (mL) | 90 ± 28 | 100 ± 26 * | 71 ± 20 *^ | 87 ± 24 | 102 ± 32 ^ |
| Let ventricular ejection fraction (%) | 59 ± 17% | 69 ± 9%* | 35 ± 10% *^’ | 65 ± 9% ^ | 69 ± 9% ’ |
| Left ventricular mass (g) | 154 ± 34 | 160 ± 33 | 170 ± 38 | 142 ± 29 | 145 ± 32 |
| Left ventricular outflow tract diameter (cm) | 2.5 ± 0.2 | 2.4 ± 0.3 * | 2.6 ± 0.2 *^ | 2.5 ± 0.2 | 2.4 ± 0.2 ^ |
| Left ventricular outflow tract area (cm2) | 4.6 ± 0.9 | 4.4 ± 1.1 * | 5.2 ± 0.7 *^ | 4.8 ± 0.7 | 4.0 ± 0.8 ^ |
| Left ventricular velocity-time integral (cm) | 21 ± 8 | 23 ± 5 * | 14 ± 4 *^ | 19 ± 5 ’ | 26 ± 11 ^’ |
| Aortic valve area (planimetry cm2) | 0.90 ± 0.21 | 0.86 ± 0.24 | 0.80 ± 0.20 * | 0.96 ± 0.18 | 0.99 ± 0.18 * |
| Parameter | Echocardiography | Computed Tomography | Difference (Paired t-Test) (95% CI, p-Value) | Correlation r (p-Value) |
|---|---|---|---|---|
| Left ventricular end-diastolic volume (mL) | 101 ± 39 | 160 ± 55 | 60 (51, 68; <0.001) | 0.734 (<0.001) |
| Left ventricular end-systolic volume (mL) | 49 ± 32 | 70 ± 55 | 21 (14, 28; <0.001) | 0.878 (<0.00) |
| Left ventricular stroke volume (mL) | 51 ± 17 | 90 ± 28 | 39 (33, 45; <0.001) | 0.327 (0.003) |
| Left ventricular outflow tract stroke volume (mL) | 68 ± 20 | 23 (18, 27; <0.001) | 0.631 (<0.001) | |
| Let ventricular ejection fraction (%) | 54 ± 13% | 59 ± 17% | 6 (3, 8; <0.001) | 0.759 (<0.001) |
| Left ventricular mass (g) | 207 ± 64 | 154 ± 34 | −53 (−65, −41; <0.001) | 0.513 (<0.001) |
| Left ventricular outflow tract diameter (cm) | 2.1 ± 0.1 | 2.5 ± 0.2 | 0.43 (0.38, 0.47; <0.001) | 0.518 (<0.001) |
| Left ventricular outflow tract area (cm2) | 3.4 ± 0.5 | 4.6 ± 0.9 | 1.2 (1.1, 1.4; <0.001) | 0.507 (<0.001) |
| Left ventricular outflow tract velocity-time integral (cm) | 20 ± 6 | 21 ± 8 | 0.2 (−1.3, 1.7; 0.771) | 0.603 (<0.001) |
| AVA (cm2) | Measurement | % of Patients AVA ≤ 1.0 cm2 | Difference (95% CI, p-Value) Versus CT Planimetry | Correlation (p-Value) Versus CT Planimetry |
|---|---|---|---|---|
| CT: valve planimetry | 0.90 ± 0.21 | 57 (71%) | ||
| TTE: LVOT area, LVOT-VTI, AV-VTI | 0.76 ± 0.20 | 70 (88%) | −0.15 (−0.10, −0.19; <0.001) | 0.432 (<0.001) |
| TTE: LVOT area, LVOT-Vmax, AV-Vmax | 0.77 ± 0.19 | 70 (88%) | −0.13 (−0.08, −0.18; <0.001) | 0.401 (<0.001) |
| TTE: LVOT area, LVOT-Vmean, AV-Vmean | 0.75 ± 0.20 | 71 (89%) | −0.15 (−0.10, −0.20; <0.001) | 0.369 (0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.K.M.; Abou Hassan, O.K.; Popović, Z.B.; Griffin, B.P.; Rodriguez, L.L. Quantification of Significant Aortic Stenosis by Echocardiography versus Four-Dimensional Cardiac Computed Tomography: A Multi-Modality Imaging Study. Diagnostics 2022, 12, 3106. https://doi.org/10.3390/diagnostics12123106
Wang TKM, Abou Hassan OK, Popović ZB, Griffin BP, Rodriguez LL. Quantification of Significant Aortic Stenosis by Echocardiography versus Four-Dimensional Cardiac Computed Tomography: A Multi-Modality Imaging Study. Diagnostics. 2022; 12(12):3106. https://doi.org/10.3390/diagnostics12123106
Chicago/Turabian StyleWang, Tom Kai Ming, Ossama K. Abou Hassan, Zoran B. Popović, Brian P. Griffin, and Luis Leonardo Rodriguez. 2022. "Quantification of Significant Aortic Stenosis by Echocardiography versus Four-Dimensional Cardiac Computed Tomography: A Multi-Modality Imaging Study" Diagnostics 12, no. 12: 3106. https://doi.org/10.3390/diagnostics12123106
APA StyleWang, T. K. M., Abou Hassan, O. K., Popović, Z. B., Griffin, B. P., & Rodriguez, L. L. (2022). Quantification of Significant Aortic Stenosis by Echocardiography versus Four-Dimensional Cardiac Computed Tomography: A Multi-Modality Imaging Study. Diagnostics, 12(12), 3106. https://doi.org/10.3390/diagnostics12123106






