Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors
Abstract
:1. Introduction
2. Materials and Methods
3. Diagnosis
4. Management
5. Recurrence, Prognosis, Metastasis and Follow-Up
6. Other Ovarian Malignant Germ Cell Tumors, Differentiation and Management
- ▪
- dysgerminomas;
- ▪
- immature teratomas;
- ▪
- yolk sac tumors;
- ▪
- polyembryomas;
- ▪
- mixed germ cell tumors;
- ▪
- embryonic carcinomas;
- ▪
- choriocarcinomas;
- ▪
- struma ovaries.
6.1. Yolk Sac Tumors
6.2. Immature Teratomas
6.2.1. Malignant Struma Ovarii
6.2.2. Malignant Carcinoid Tumors
6.3. Nongestational Choriocarcinoma
6.4. Embryonal Carcinoma, Polyembrioma and Mixed Germ Cell Tumors
6.5. Ovotestis with Seminoma
6.6. Extragonadal Germ Cell Tumor
7. Epigenetics, a Scientific Reality?
8. Coherent Management Guidelines—A Necessity
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsutsumi, M.; Miura, H.; Inagaki, H.; Shinkai, Y.; Kato, A.; Kato, T.; Hamada-Tsutsumi, S.; Tanaka, M.; Kudo, K.; Yoshikawa, T.; et al. An aggressive systemic mastocytosis preceded by ovarian dysgerminoma. BMC Cancer 2020, 20, 1162. [Google Scholar] [CrossRef]
- Michael, K.K.; Wampler, K.; Underwood, J.; Hansen, C. Ovarian Dysgerminoma: A Case Study. J. Diagn. Med. Sonogr. 2015, 31, 327–330. [Google Scholar] [CrossRef] [Green Version]
- Tîrnovanu, M.C.; Florea, I.D.; Tănase, A.; Toma, B.F.; Cojocaru, E.; Ungureanu, C.; Lozneanu, L. Uncommon Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature. Medicina 2021, 57, 534. [Google Scholar] [CrossRef] [PubMed]
- Esin, S.; Baser, E.; Kucukozkan, T.; Magden, H.A. Ovarian gonadoblastoma with dysgerminoma in a 15-year-old girl with 46, XX karyotype: Case report and review of the literature. Arch. Gynecol. Obstet. 2012, 285, 447–451. [Google Scholar] [CrossRef]
- Sato, Y.; Hayashi, T.; Yamamoto, H.; Niina, I.; Kuroki, N.; Iwamura, T.; Onishi, J. Late Recurrence in Ovarian Dysgerminoma Presenting as a Primary Retroperitoneal Tumor: A Case Report and Review of the Literature. Case Rep. Pathol. 2020, 2020, 4737606. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Liu, Z.; Lu, Y.S.; Pan, M.; Huang, H. True hermaphroditism with dysgerminoma: A case report. Medicine 2020, 99, e20472. [Google Scholar] [CrossRef] [PubMed]
- Husaini, H.A.L.; Soudy, H.; Darwish, A.E.D.; Ahmed, M.; Eltigani, A.; Mubarak, M.A.L.; Abu Sabaa, A.; Edesa, W.; A L-Tweigeri, T.; Al-Badawi, I.A. Pure dysgerminoma of the ovary: A single institutional experience of 65 patients. Med. Oncol. 2012, 29, 2944–2948. [Google Scholar] [CrossRef]
- Keskin, M.; Savaş-Erdeve, Ş.; Kurnaz, E.; Çetinkaya, S.; Karaman, A.; Apaydın, S.; Aycan, Z. Gonadoblastoma in a patient with 46, XY complete gonadal dysgenesis. Turk. J. Pediatr. 2016, 58, 538–540. [Google Scholar] [CrossRef]
- Arndt, M.; Taube, T.; Deubzer, H.; Calaminus, K.; Sehouli, J.; Pietzner, K. Management of malignant dysgerminoma of the ovary. Eur. J. Gynaecol. Oncol. 2022, 43, 353–362. [Google Scholar] [CrossRef]
- Gupta, M.; Jindal, R.; Saini, V. An Incidental Finding of Bilateral Dysgerminoma during Cesarean Section: Dilemmas in Management. J. Clin. Diagn. Res. 2016, 10, QD04–QD05. [Google Scholar] [CrossRef]
- Ajao, M.; Vachon, T.; Snyder, P. Ovarian dysgerminoma: A case report and literature review. Mil. Med. 2013, 178, e954–e955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, Y.; Han, C.; Tian, W.; Yang, W.; Wang, Y.; Xue, F. Ovarian dysgerminoma in pregnancy: A case report and literature review. Cancer Biol. Ther. 2018, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, A.; Maddy, B.; DeWitt, M.; Cliby, W.; Dow, M. Dysfunctional labor and hemoperitoneum secondary to an incidentally discovered dysgerminoma: A case report. BMC Pregnancy Childbirth 2021, 21, 611. [Google Scholar] [CrossRef] [PubMed]
- Seilanian Toosi, F.; Hasanzadeh, M.; Maftouh, M.; Tavassoli, A. Cutaneous Metastasis in a Previously Known Case of Ovarian Dysgerminoma: A Case Report. Int. J. Cancer Manag. 2021, 14, e104715. [Google Scholar] [CrossRef]
- De Jesus Escano, M.R.; Mejia Sang, M.E.; Reyes-Mugica, M.; Colaco, M.; Fox, J. Ovotesticular Disorder of Sex Development: Approach and Management of an Index Case in the Dominican Republic. Cureus 2021, 13, e18512. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Radhakrishnan, A.P.; Saaya, M.I. An Unusual Case of Ovarian Dysgerminoma Associated with Secondary Hemophagocytic Lymphohistiocytosis (HLH). Open Access Library J. 2022, 9, 1–6. [Google Scholar] [CrossRef]
- Bandala-Jacques, A.; Estrada-Rivera, F.; Cantu, D.; Prada, D.; Montalvo-Esquivel, G.; González-Enciso, A.; Barquet-Munoz, S.A. Role of optimal cytoreduction in patients with dysgerminoma. Int. J. Gynecol. Cancer 2019, 29, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sun, F.; Bao, L.; Chu, C.; Li, H.; Yin, Q.; Guan, W.; Wang, D. Pure dysgerminoma of the ovary: CT and MRI features with pathological correlation in 13 tumors. J. Ovarian Res. 2020, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Kilic, C.; Cakir, C.; Yuksel, D.; Kilic, F.; Kayikcioglu, F.; Koc, S.; Korkmaz, V.; Kimyon Comert, G.; Turkmen, O.; Boran, N.; et al. Ovarian Dysgerminoma: A Tertiary Center Experience. J. Adolesc. Young Adult Oncol. 2021, 10, 303–308. [Google Scholar] [CrossRef]
- Kota, S.K.; Gayatri, K.; Pani, J.P.; Kota, S.K.; Meher, L.K.; Modi, K.D. Dysgerminoma in a female with turner syndrome and Y chromosome material: A case-based review of literature. Indian J. Endocrinol. Metab. 2012, 16, 436–440. [Google Scholar] [CrossRef]
- Khare, M.; Gupta, M.K.; Airun, A.; Sharma, U.B.; Garg, K. A Case of True Hemaphroditism Presenting with Dysgerminoma. J. Clin. Diagn. Res. 2017, 11, ED07–ED09. [Google Scholar] [CrossRef]
- Tsuboyama, T.; Hori, Y.; Hori, M.; Onishi, H.; Tatsumi, M.; Sakane, M.; Ota, T.; Tomiyama, N. Imaging findings of ovarian dysgerminoma with emphasis on multiplicity and vascular architecture: Pathogenic implications. Abdom. Radiol. 2018, 43, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Cacioppa, L.M.; Crusco, F.; Marchetti, F.; Duranti, M.; Renzulli, M.; Golfieri, R. Magnetic resonance imaging of pure ovarian dysgerminoma: A series of eight cases. Cancer Imaging 2021, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Changchien, Y.C.; Haltrich, I.; Micsik, T.; Kiss, E.; Fónyad, L.; Papp, G.; Sápi, Z. Gonadoblastoma: Case report of two young patients with isochromosome 12p found in the dysgerminoma overgrowth component in one case. Pathol. Res. Pract. 2012, 208, 628–632. [Google Scholar] [CrossRef]
- Batool, A.; Karimi, N.; Wu, X.N.; Chen, S.R.; Liu, Y.X. Testicular germ cell tumor: A comprehensive review. Cell Mol. Life Sci. 2019, 76, 1713–1727. [Google Scholar] [CrossRef]
- Govindaraj, S.K.; Muralidhar, L.; Venkatesh, S.; Saxena, R.K. Disorder of Sexual Development with Sex Chromosome Mosaicism 46 XY and 47 XXY. Int. J. Infertil. Fetal Med. 2013, 4, 34–37. [Google Scholar] [CrossRef]
- Cormio, G.; Seckl, M.J.; Loizzi, V.; Resta, L.; Cicinelli, E. Increased human Chorionic Gonadotropin levels five years before diagnosis of an ovarian dysgerminoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 138–139. [Google Scholar] [CrossRef]
- Adekunle, O.; Zayyan, M.; Kolawole, A.; Ahmed, S. Case report: A rare case of dysgerminoma presenting with skin and breast metastasis. Case Rep. Clin. Med. 2013, 2, 170–172. [Google Scholar] [CrossRef] [Green Version]
- Milewicz, T.; Mrozińska, S.; Szczepański, W.; Białas, M.; Kiałka, M.; Doroszewska, K.; Kabzińska-Turek, M.; Wojtyś, A.; Ludwin, A.; Chmura, Ł. Dysgerminoma and gonadoblastoma in the course of Swyer syndrome. Pol. J. Pathol. 2016, 67, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Guida, M.; Gentile, A.; De Fazio, M.; Cramarossa, A.; Sabatelli, A.; Colucci, G. Mediastinal mass following successful chemotherapy for ovary dysgerminoma: Benign process or disease relapse? A case report. J. Pediatr. Adolesc. Gynecol. 2013, 26, e13-6. [Google Scholar] [CrossRef]
- Sharma, S.; Mukul, B.; Geet, M. Extragonadal dysgerminoma presenting as neck metastasis and masquerading as a thyroid swelling. Clin. Cancer Investig. J. 2017, 5, 43–45. [Google Scholar] [CrossRef]
- Talukdar, S.; Kumar, S.; Bhatla, N.; Mathur, S.; Thulkar, S.; Kumar, L. Neo-adjuvant chemotherapy in the treatment of advanced malignant germ cell tumors of ovary. Gynecol. Oncol. 2014, 132, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Curioni-Fontecedro, A.; Allmann, V.; Beyer, J.; Tischler, V.; Sulser, T.; Moch, H.; Bode, P.K. Frequent PD-L1 expression in testicular germ cell tumors. Br. J. Cancer 2015, 113, 411–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, J.; Yu, M.; Cao, D.; Zhang, Y.; Zong, X.; Shen, K. Ovarian yolk sac tumor in postmenopausal females: A case series and a literature review. Medicine 2018, 97, e11838. [Google Scholar] [CrossRef]
- Yao, X.D.; Hong, Y.P.; Ye, D.W.; Wang, C.F. Primary yolk sac tumor of seminal vesicle: A case report and literature review. World J. Surg. Oncol. 2012, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Nasioudis, D.; Chapman-Davis, E.; Frey, M.K.; Caputo, T.A.; Holcomb, K. Management and prognosis of ovarian yolk sac tumors; an analysis of the National Cancer Data Base. Gynecol. Oncol. 2017, 147, 296–301. [Google Scholar] [CrossRef]
- Hasdemir, P.; Guvenal, T.; Menekse, S.; Solmaz, U.; Kandiloglu, A.; Koyuncu, F.; Ayhan, A. Ovarian Immature Teratoma Detected During Pregnancy. Med. Sci. Discov. 2017, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Stolnicu, S.; Szekely, E.; Molnar, C.; Molnar, C.V.; Barsan, I.; D’Alfonso, V.; Moldovan, C.; Zheng, G.; Ronnett, B.M.; Soslow, R.A. Mature and Immature Solid Teratomas Involving Uterine Corpus, Cervix, and Ovary. Int. J. Gynecol. Pathol. 2017, 36, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Alwazzan, A.B.; Popowich, S.; Dean, E.; Robinson, C.; Lotocki, R.; Altman, A.D. Pure Immature Teratoma of the Ovary in Adults: Thirty-Year Experience of a Single Tertiary Care Center. Int. J. Gynecol. Cancer 2015, 25, 1616–1622. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jiang, S.; Zhang, Y.; Jiang, C.; Xia, F.; Lyu, W.; Ma, X. The application of 18F-FDG PET/CT in ovarian immature teratomas when pathological examination results contradict clinical observations: A case report. Medicine 2017, 96, e9171. [Google Scholar] [CrossRef]
- Frazer, J.L.; Hook, C.E.; Addley, H.C.; Jackson, C.R.; Latimer, J.A.; Nicholson, J.C.; Murray, M.J. Recurrent ovarian immature teratoma in a 12-year-old girl: Implications for management. Gynecol. Oncol. 2019, 154, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Cang, W.; Zhu, S.; Jia, C.; Cao, D.; Yang, J.; Xiang, Y. Oncological and Reproductive Outcomes in Patients With Advanced-Stage Ovarian Immature Teratoma: Experience From a Tertiary Center. Front. Oncol. 2022, 12, 822341. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Mizoguchi, C.; Nishida, M.; Sato, S.; Nasu, K.; Narahara, H. Recurrent immature teratoma of the ovary with long-term disease-free interval. J. Obstet. Gynaecol. Res. 2014, 40, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.S.; Maqsood, H.; Younus, S.; Anjum, S.; Fatima, M. A Rare Case of Non-Gestational Metastatic Ovarian Choriocarcinoma: Case Report and Literature Review with a Special Emphasis on Imaging. Cureus 2021, 13, e13121. [Google Scholar] [CrossRef]
- Adow, M.T.; Gebresilasie, S.F.; Abebe, N.A. Primary Ovarian Choriocarcinoma: Rare Entity. Case Rep. Obstet. Gynecol. 2021, 2021, 4545375. [Google Scholar] [CrossRef]
- Exman, P.; Takahashi, T.K.; Gattás, G.F.; Cantagalli, V.D.; Anton, C.; Nalesso, F.; Diz, M.D.P.E. Primary ovary choriocarcinoma: Individual DNA polymorphic analysis as a strategy to confirm diagnosis and treatment. Rare Tumors 2013, 5, 89–92. [Google Scholar] [CrossRef] [Green Version]
- De Lucia, D.R.; Castaldo, A.; D'Agostino, V.; Ascione, R.; Pesce, I.; Coppola, L.; Catelli, A.; Radice, L. Metastatic choriocarcinoma with hemorrhagic complications and spontaneous ovarian hyperstimulation syndrome: A case report. Radiol. Case Rep. 2021, 16, 3868–3874. [Google Scholar] [CrossRef]
- Sakurai, S.; Asano, R.; Furugori, M.; Shigeta, H. A rare case of gestational ovarian choriocarcinoma coexistent with intrauterine pregnancy. Taiwan J. Obstet. Gynecol. 2022, 61, 708–712. [Google Scholar] [CrossRef]
- Heo, E.J.; Choi, C.H.; Park, J.M.; Lee, J.W.; Bae, D.S.; Kim, B.G. Primary ovarian choriocarcinoma mimicking ectopic pregnancy. Obstet. Gynecol. Sci. 2014, 57, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, X.; Pang, Y.; Ma, Y.; Zhang, X.; Liu, P. Clinicopathological factors and prognosis analysis of 39 cases of non-gestational ovarian choriocarcinoma. Arch. Gynecol. Obstet. 2020, 301, 901–912. [Google Scholar] [CrossRef]
- Rana, S.; Gill, M.K.; Kalhan, S.; Satarkar, R.N.; Sangwaiya, A.; Singh, P. Immature Teratoma with Embryonal Carcinoma; a Rare Malignant Mixed Germ Cell Tumor in a 13-Year-Old Girl. Iran. J. Pathol. 2016, 11, 66–70. [Google Scholar] [PubMed]
- Samantray, S.R.; Mohapatra, I. Ovotesticular Disorder with Seminoma. Cureus 2020, 12, e12130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Peng, Y.; Chen, R.; Ge, P.; Wang, J. 46, XX Ovotesticular disorder of sex development (true hermaphroditism) with seminoma: A case report. Medicine 2020, 99, e22530. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, E.; Binay, Ç.; Demiral, M.; Tokar, B.; Kabukçuoğlu, S.; Üstün, M. Gonadoblastoma and Papillary Tubal Hyperplasia in Ovotesticular Disorder of Sexual Development. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 351–355. [Google Scholar] [CrossRef]
- Alam, S.; Boro, H.; Goyal, A.; Khadgawat, R. 46, XY complete gonadal dysgenesis with pubertal virilisation due to dysgerminoma/gonadoblastoma. BMJ Case Rep. 2020, 13, e235501. [Google Scholar] [CrossRef]
- Raafey, M.A.; Abdulwaasey, M.; Fatima, S.S.; Uddin, Z.; Tariq, M.U. Bilateral Gonadoblastoma with Dysgerminoma in a Phenotypically Normal Female With 46XX Karyotype: Report of a Rare Case and Literature Review. Cureus 2020, 12, e8990. [Google Scholar] [CrossRef]
- Yüce, Ö.; Döğer, E.; Çelik, N.; Emeksiz, H.C.; Çamurdan, M.O.; Bideci, A.; Cinaz, P. Gonadoblastoma with Dysgerminoma in a Phenotypically Turner-Like Girl with 45,X/46,XY Karyotype. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 336–339. [Google Scholar] [CrossRef]
- Ronchi, A.; Cozzolino, I.; Montella, M.; Panarese, I.; Zito Marino, F.; Rossetti, S.; Chieffi, P.; Accardo, M.; Facchini, G.; Franco, R. Extragonadal germ cell tumors: Not just a matter of location. A review about clinical, molecular and pathological features. Cancer Med. 2019, 8, 6832–6840. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; Jerónimo, C.; Henrique, R.; Looijenga, L.H.J. Human Germ Cell Tumors are Developmental Cancers: Impact of Epigenetics on Pathobiology and Clinic. Int. J. Mol. Sci. 2019, 20, 258. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.J.; Liu, Z.C.; Wei, R.J.; Li, L. An Analysis of Prognostic Factors in Patients with Ovarian Malignant Germ Cell Tumors Who Are Treated with Fertility-Preserving Surgery. Gynecol. Obstet. Investig. 2016, 81, 1–9. [Google Scholar] [CrossRef]
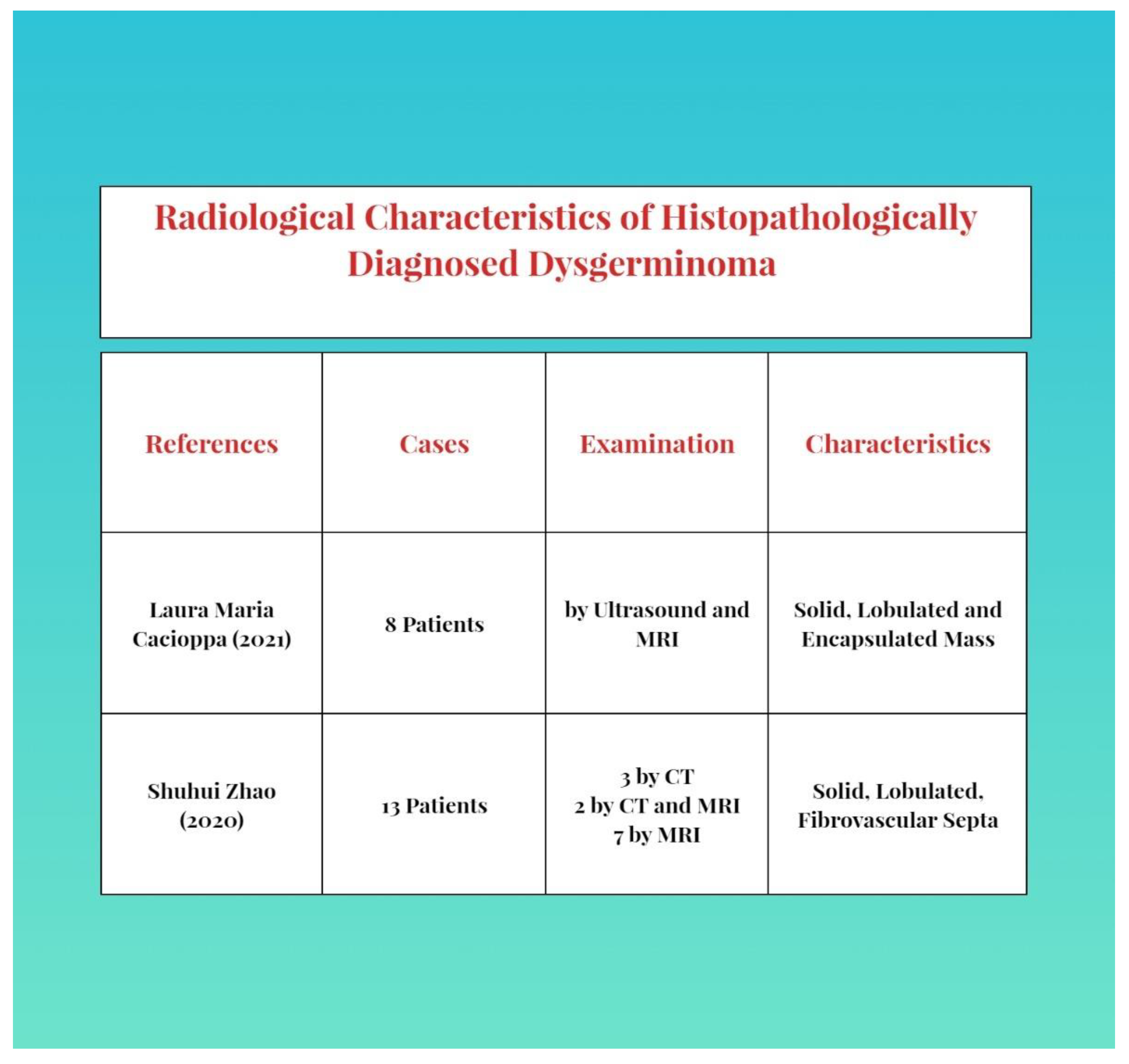
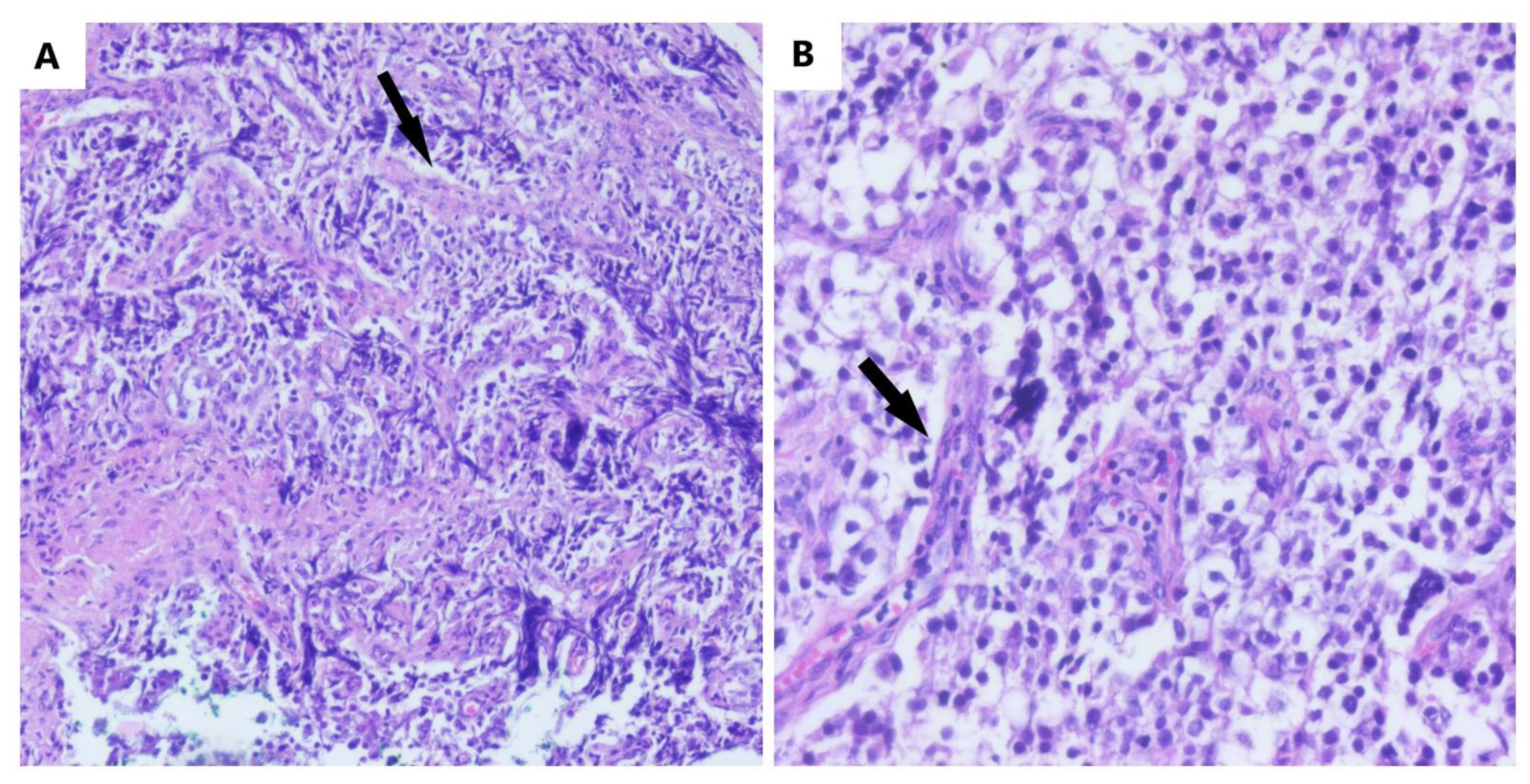
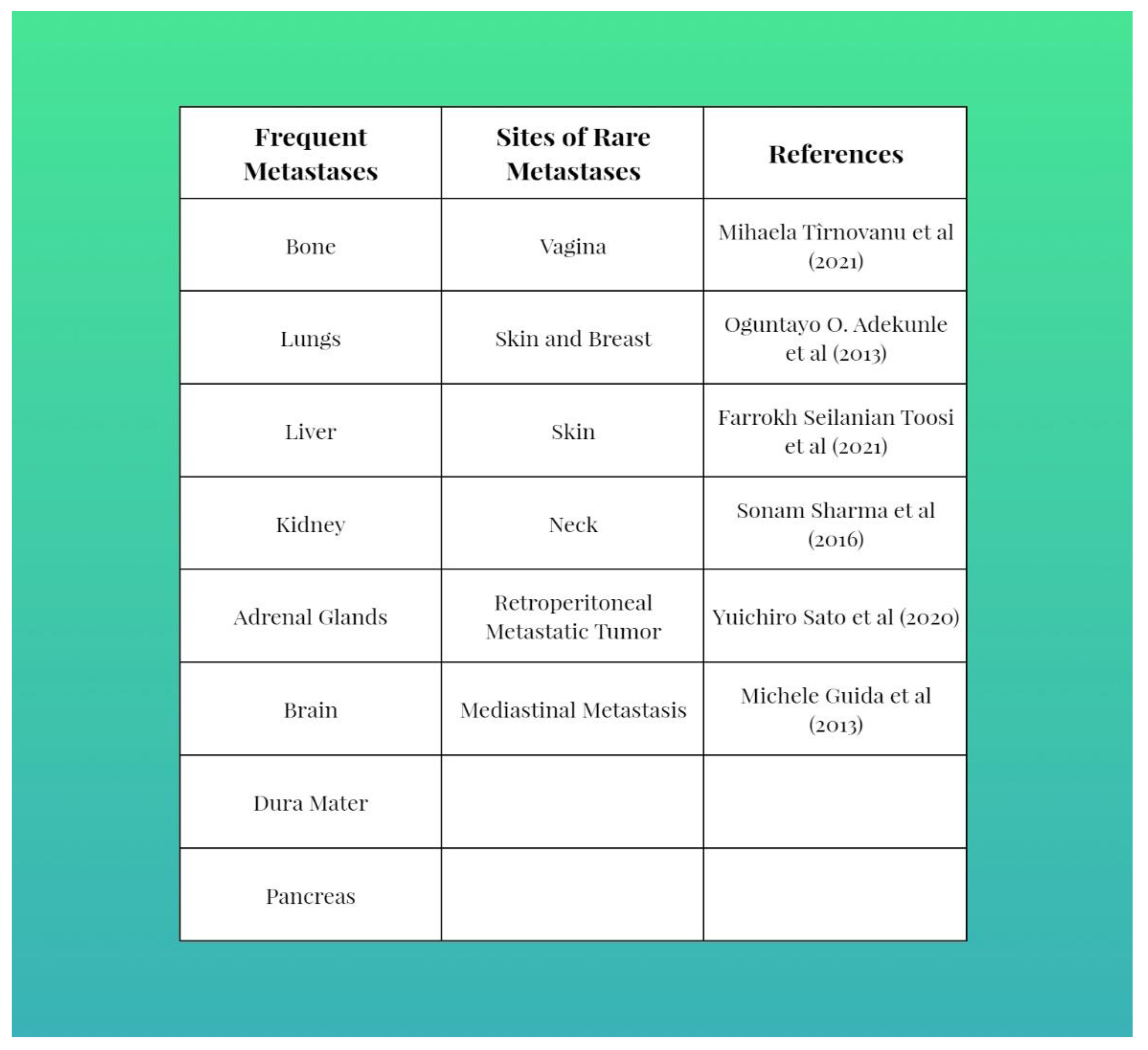
| All Tumors Diagnosed by Histology | |||||||
|---|---|---|---|---|---|---|---|
| References | Stage | Main Symptom | Therapeutic Management | Relapse | Five Year DFS (Disease Free Survival) | Five Year OS (Overall Survival) | Fertility Preservation |
| Hamid Al-Huseini et al., 2012 [7]–65 patients | ▪ 21 pt. I A (32.3%); ▪ 12 pt. I C (18.5%); ▪ 2 pt. II A/IIB (3%); ▪ 4 pt. III A/IIIB (6.2%); ▪ 18 pt. III C (27.6%); ▪ 4 pt. IV (6.2%) | Pelvic pain (most frequent) 44 pt. (67.7%); Asymptomatic 4 pt. (6.2%) | Surgery BO 4 pt. (6.2%); UO 50 pt. (76.9%) Post-Surgery Adjuvant Therapy ▪ CHT 40 cases (61.5%); ▪ RT 4 cases (6.2%); ▪ NT 21 cases (32.3%) | 6 pt. (9.2%) | 88% | 100% | Out of 50 patients treated with fertility-sparing surgery, 16 patients (32%) became pregnant |
| Antonio Bandala-Jacquer et al., 2019 [17]–180 patients ▪ 166 pure dysgerminoma (92.1%); ▪ rest presented mixed histology | ▪ 80 pt. (44.4%) unstaged; ▪ 54 pt. (30%)–I; ▪ 9 pt. (5%)–II; ▪ 29 pt. (16%)–III; ▪ 8 pt. (4.4%)–IV; | Pelvic pain 112 pt. (62.2%) | Surgery 71 pt. (39.4%) from which 51 (28.3%) TAH.; 14 pt. (7.8%) BO; 32 pt. (17.77%) LnD; Adjuvant therapy 135 pt. (75%) ▪ CHT 125 cases (69.3%); ▪ RT 13 cases (7.2%) | 4 cases (2.2%) pelvic; 6 cases (3.3%) nodal; 3 cases (1.7%) distant | 92.8% | 97.9% | 37 pt. (20.6%) became pregnant |
| Cigen Killie et al., 2021 [19]–18 patients | ▪ 6 pt.-I A; (33%) ▪ 4 pt.-I C; (22%) ▪ 1 pt.-II A; (5%) ▪ 1 pt.-II B; (5%) ▪ 1 pt.-III A; (5%) ▪ 4 pt.-IIIC; (22%) | Pelvic pain 7 pt. (38%) | Surgery UO 13 pt. (72%); BO 1 pt. (5%) THBSO 4 pt. (22%) LnD 18 pt. (100%) Adjuvant Therapy ▪ CHT 8 pt. (44%); ▪ RT 1 pt. (5%) ▪ CHT and RT 2 pt. (11%) | 3 pt. (16%) | Not Applicable (Follow-up in progress) | Not Applicable (Follow-up in progress) | 2 cases (11%) |
| Family History | Personal Medical History | Symptoms | Clinical Diagnosis | Imaging US, MRI, CT, PET Chest X-Ray | Biomarkers | Histopathology | Management | Prognosis | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Family cancers are inversely linked with this type of tumor | NAD | NAD | NAD | NAD | β-HCG | Microscopic changes | Fertility-sparing surgery | Histopathology | Every 3 months/ 3 years |
| Pelvic pain | Tumoral mass | Tumoral mass | AFP | Chromosome mapping | Surgery (unilateral oophorectomy) | Age | Every 6 months/ 2 years | ||
| Nausea | Bleeding | Calcifications | CEA | Buccal swab | BEP (chemotherapy) | Staging | Annually/ 10 years | ||
| Bleeding | Casexia | Vascular changes | LDH | Peritoneal washing cytology and Biopsy | Bilateral oophorectomy and hormone replacement therapy | Grading | Duration of long-term follow-up is not established | ||
| Amenorrhea | Metastases | Low-resistance flow (Doppler) | Total hysterectomy | Assoc. with other germinal tumors | |||||
| Hermaphroditism | Hemorrhage | Omentectomy | Genetic anomalies | ||||||
| Necrosis | CA-125 | Lymphadenectomy | Biomarker levels | ||||||
| Ascitis (rare) | Radiotherapy | Residual tumors | |||||||
| Pleuresia (rare) | Treatment outside reference centers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitranovici, M.-I.; Chiorean, D.M.; Mureșan, M.C.; Buicu, C.-F.; Moraru, R.; Moraru, L.; Cotoi, T.C.; Cotoi, O.S.; Toru, H.S.; Apostol, A.; et al. Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors. Diagnostics 2022, 12, 3105. https://doi.org/10.3390/diagnostics12123105
Mitranovici M-I, Chiorean DM, Mureșan MC, Buicu C-F, Moraru R, Moraru L, Cotoi TC, Cotoi OS, Toru HS, Apostol A, et al. Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors. Diagnostics. 2022; 12(12):3105. https://doi.org/10.3390/diagnostics12123105
Chicago/Turabian StyleMitranovici, Melinda-Ildiko, Diana Maria Chiorean, Maria Cezara Mureșan, Corneliu-Florin Buicu, Raluca Moraru, Liviu Moraru, Titiana Cornelia Cotoi, Ovidiu Simion Cotoi, Havva Serap Toru, Adrian Apostol, and et al. 2022. "Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors" Diagnostics 12, no. 12: 3105. https://doi.org/10.3390/diagnostics12123105
APA StyleMitranovici, M.-I., Chiorean, D. M., Mureșan, M. C., Buicu, C.-F., Moraru, R., Moraru, L., Cotoi, T. C., Cotoi, O. S., Toru, H. S., Apostol, A., Turdean, S. G., Mărginean, C., Petre, I., Oală, I. E., Simon-Szabo, Z., Ivan, V., & Pușcașiu, L. (2022). Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors. Diagnostics, 12(12), 3105. https://doi.org/10.3390/diagnostics12123105










