An Extremely Rare Case of Disseminated Peritoneal Leiomyomatosis with a Pelvic Leiomyosarcoma and Omental Metastasis after Laparoscopic Morcellation: Systematic Review of the Literature
Abstract
1. Introduction
2. Case Presentations
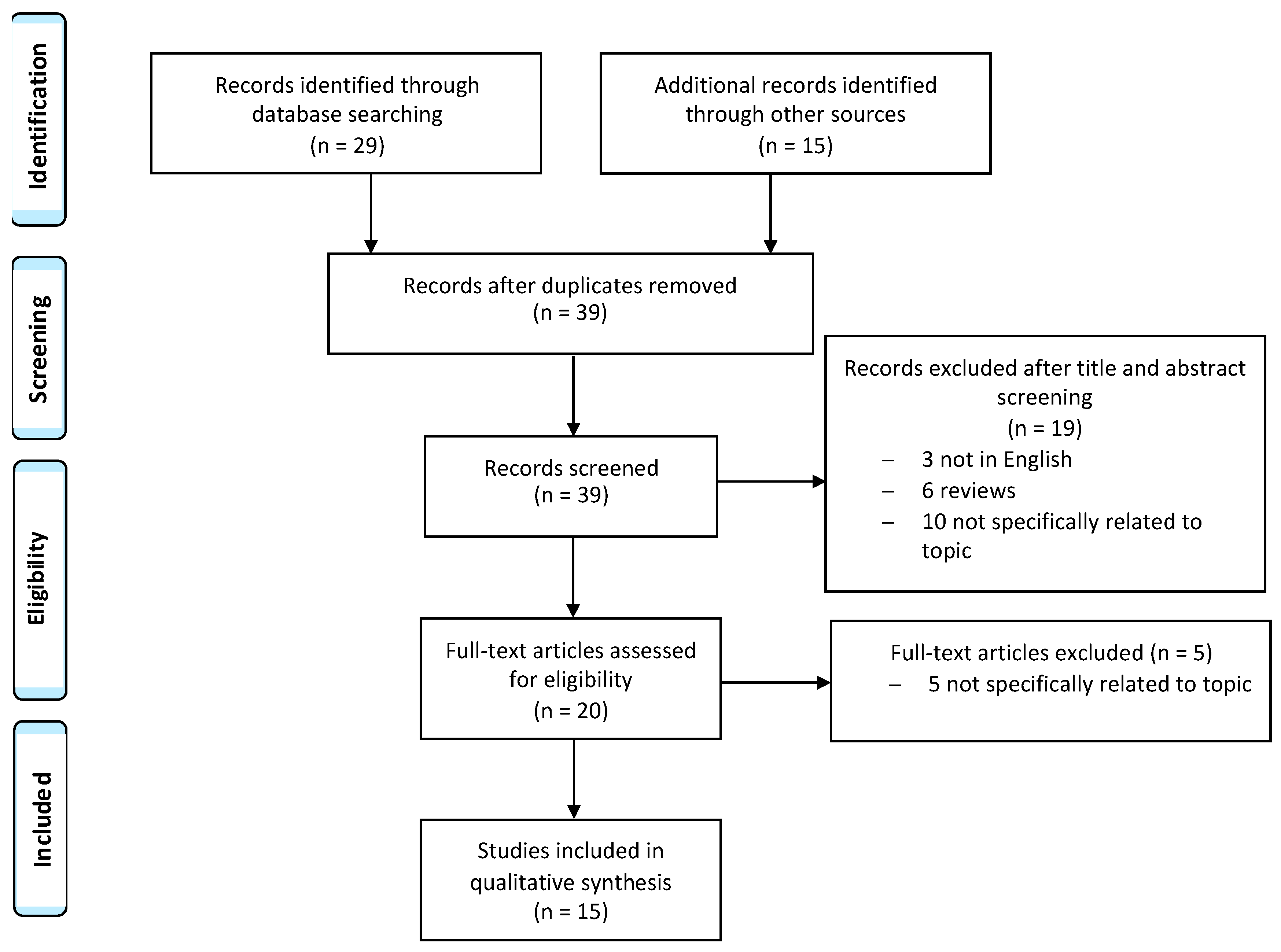
3. Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baird, D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Gynecology. Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obs. Gynecol. 2021, 137, e100–e115. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. Uterine Morcellation for Presumed Leiomyomas: ACOG Committee Opinion, Number 822. Obs. Gynecol. 2021, 137, e63–e74. [Google Scholar] [CrossRef] [PubMed]
- Bafunno, D.; Romito, F.; Lagattolla, F.; Delvino, V.A.; Minoia, C.; Loseto, G.; Dellino, M.; Guarini, A.; Catino, A.; Montrone, M.; et al. Psychological well-being in cancer outpatients during COVID-19. J. BUON Off. J. Balk. Union Oncol. 2021, 26, 1127–1134. [Google Scholar]
- Daniele, A.; Divella, R.; Pilato, B.; Tommasi, S.; Pasanisi, P.; Patruno, M.; Digennaro, M.; Minoia, C.; Dellino, M.; Pisconti, S.; et al. Can harmful lifestyle, obesity and weight changes increase the risk of breast cancer in BRCA 1 and BRCA 2 mutation carriers? A Mini review. Hered. Cancer Clin. Pract. 2021, 19, 45. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Wu, M.-Y.; Li, C.-H.; Huang, S.-C.; Yiang, G.-T.; Yen, H.-S.; Liu, W.-L.; Li, C.-J.; Kao, W.-Y. Epithelial-Mesenchymal Transition with Malignant Transformation Leading Multiple Metastasis from Disseminated Peritoneal Leiomyomatosis. J. Clin. Med. 2018, 7, 207. [Google Scholar] [CrossRef]
- Żyła, M.M.; Dzieniecka, M.; Kostrzewa, M.; Stetkiewicz, T.; Wilamowska, A.; Księżakowska-Łakoma, K.; Wilczyński, J.R. Leiomyomatosis peritonealis disseminata of unusual course with malignant transformation: Case report. Acta Obstet. Gynecol. Scand. 2015, 94, 220–223. [Google Scholar] [CrossRef]
- Sharma, P.; Chaturvedi, K.U.; Gupta, R.; Nigam, S. Leiomyomatosis peritonealis disseminata with malignant change in a post-menopausal woman. Gynecol. Oncol. 2004, 95, 742–745. [Google Scholar] [CrossRef]
- Fulcher, A.S.; Szucs, R.A. Leiomyomatosis peritonealis disseminata complicated by sarcomatous transformation and ovarian torsion: Presentation of two cases and review of the literature. Abdom. Imaging 1998, 23, 640–644. [Google Scholar] [CrossRef]
- Lamarca, M.; Rubio, P.; Andres, P.; Rodrigo, C. Leiomyomatosis peritonealis disseminata with malignant degeneration. A case report. Eur. J. Gynaecol. Oncol. 2011, 32, 702–704. [Google Scholar]
- Akkersdijk, G.J.; Flu, P.K.; Giard, R.W.; Van Lent, M.; Wallenhurg, H.C. Malignant leiomyomatosis peritonealis disseminata. Am. J. Obstet. Gynecol. 1990, 163, 591–593. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Quattrone, P.; Grosso, G.; Cobellis, L.; Di Re, E. Malignant Degeneration in Leiomyomatosis Peritonealis Disseminata. Gynecol. Oncol. 1996, 61, 272–274. [Google Scholar] [CrossRef]
- Morizaki, A.; Hayashi, H.; Ishikawa, M. Leiomyomatosis peritonealis disseminata with malignant transformation. Int. J. Gynecol. Obstet. 1999, 66, 43–45. [Google Scholar] [CrossRef]
- Xu, S.; Qian, J. Leiomyomatosis Peritonealis Disseminata with Sarcomatous Transformation: A Rare Case Report and Literature Review. Case Rep. Obstet. Gynecol. 2019, 2019, 3684282. [Google Scholar] [CrossRef]
- Tun, A.M.; Tun, N.M.; Zin Thein, K.; Naing, E.E.; Giashuddin, S.; Shulimovich, M. A Rare Concurrence of Leiomyomatosis Peritonealis Disseminata, Leiomyosarcoma of the Pelvis and Leiomyomatous Nodule of the Liver. Case Rep. Oncol. Med. 2016, 2016, 3025432. [Google Scholar] [CrossRef]
- Wen, C.Y.; Lee, H.S.; Lin, J.T.; Yu, C.C. Disseminated peritoneal leiomyomatosis with malignant transformation involving right ureter: A case report. World J. Clin. Cases 2022, 10, 1639–1644. [Google Scholar] [CrossRef]
- Rosati, A.; Vargiu, V.; Angelico, G.; Zannoni, G.F.; Ciccarone, F.; Scambia, G.; Fanfani, F. Disseminated peritoneal leiomyomatosis and malignant transformation: A case series in a single referral center. Eur. J. Obs. Gynecol. Reprod. Biol. 2021, 262, 21–27. [Google Scholar] [CrossRef]
- Rubin, S.C.; Wheeler, J.E.; Mikuta, J.J. Malignant leiomyomatosis peritonealis disseminata. Obs. Gynecol. 1986, 68, 126–130. [Google Scholar]
- Syed, M.; Parida, B.; Mankeshwar, T.; Patil, A. Imaging Findings in a Rare Case of Leiomyomatosis Peritonealis Disseminata with Malignant Transformation. Pol. J. Radiol. 2017, 82, 426–430. [Google Scholar] [CrossRef]
- Abulafia, O.; Angel, C.; Sherer, D.M.; Fultz, P.J.; Bonfiglio, T.A.; DuBeshter, B. Computed tomography of leiomyomatosis peritonealis disseminata with malignant transformation. Am. J. Obs. Gynecol. 1993, 169, 52–54. [Google Scholar] [CrossRef]
- Kotani, Y.; Tobiume, T.; Fujishima, R.; Shigeta, M.; Takaya, H.; Nakai, H.; Suzuki, A.; Tsuji, I.; Mandai, M.; Matsumura, N. Recurrence of uterine myoma after myomectomy: Open myomectomy versus laparoscopic myomectomy. J. Obs. Gynaecol. Res. 2018, 44, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Ushijima, K.; Yanai, H.; Mikami, Y.; Ohishi, Y.; Kobayashi, H.; Tashiro, H.; Mikami, M.; Miyamoto, S.; Katabuchi, H. A critical review of “uterine leiomyoma” with subsequent recurrence or metastasis: A multicenter study of 62 cases. J. Obs. Gynaecol. Res. 2022, 48, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Vasta, F.M.; Dellino, M.; Bergamini, A.; Gargano, G.; Paradiso, A.; Loizzi, V.; Bocciolone, L.; Silvestris, E.; Petrone, M.; Cormio, G.; et al. Reproductive Outcomes and Fertility Preservation Strategies in Women with Malignant Ovarian Germ Cell Tumors after Fertility Sparing Surgery. Biomedicines 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Zhou, J.; Gou, J.; Li, N.; Nie, D.; Xue, L.; Li, Z. A prognostic index model for predicting long-term recurrence of uterine leiomyoma after myomectomy. PLoS ONE 2021, 16, e0254142. [Google Scholar] [CrossRef] [PubMed]
- Cascardi, E.; Cazzato, G.; Daniele, A.; Silvestris, E.; Cormio, G.; Di Vagno, G.; Malvasi, A.; Loizzi, V.; Scacco, S.; Pinto, V.; et al. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology 2022, 11, 1114. [Google Scholar] [CrossRef]
- Vimercati, A.; Dellino, M.; Crupano, F.M.; Gargano, G.; Cicinelli, E. Ultrasonic assessment of cesarean section scar to vesicovaginal fold distance: An instrument to estimate pre-labor uterine rupture risk. J. Matern. Fetal Neonatal. Med. 2022, 35, 4370–4374. [Google Scholar] [CrossRef]
- Dellino, M.; Gargano, G.; Tinelli, R.; Carriero, C.; Minoia, C.; Tetania, S.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Casamassima, P.; et al. A strengthening the reporting of observational studies in epidemiology (STROBE): Are HE4 and CA 125 suitable to detect a Paget disease of the vulva? Medicine 2021, 100, e24485. [Google Scholar] [CrossRef]
- Lagana, A.S.; Vitagliano, A.; Casarin, J.; Garzon, S.; Uccella, S.; Franchi, M.; Cromi, A.; Ghezzi, F. Transvaginal versus Port-Site Specimen Retrieval after Laparoscopic Myomectomy: A Systematic Review and Meta-Analysis. Gynecol. Obs. Investig. 2022, 87, 177–183. [Google Scholar] [CrossRef]
- Lagana, A.S.; Casarin, J.; Uccella, S.; Garzon, S.; Cromi, A.; Guerrisi, R.; Flamminio, F.D.; Ghezzi, F. Outcomes of in-bag transvaginal extraction in a series of 692 laparoscopic myomectomies: Results from a large retrospective analysis. J. Minim. Invasive Gynecol. 2022. online ahead of printing. [Google Scholar] [CrossRef]
- Vilos, G.A.; Allaire, C.; Laberge, P.Y.; Leyland, N.; Special, C. The management of uterine leiomyomas. J. Obs. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef]
- Moch, H. Female Genital Tumours: WHO Classification of Tumours; WHO: Geneva, Switzerland, 2020; Volume 4. [Google Scholar]
- Devereaux, K.A.; Schoolmeester, J.K. Smooth Muscle Tumors of the Female Genital Tract. Surg. Pathol. Clin. 2019, 12, 397–455. [Google Scholar] [CrossRef]
- Croce, S.; Young, R.H.; Oliva, E. Uterine leiomyomas with bizarre nuclei: A clinicopathologic study of 59 cases. Am. J. Surg. Pathol. 2014, 38, 1330–1339. [Google Scholar] [CrossRef]
- Bennett, J.A.; Weigelt, B.; Chiang, S.; Selenica, P.; Chen, Y.B.; Bialik, A.; Bi, R.; Schultheis, A.M.; Lim, R.S.; Ng, C.K.Y.; et al. Leiomyoma with bizarre nuclei: A morphological, immunohistochemical and molecular analysis of 31 cases. Mod. Pathol. 2017, 30, 1476–1488. [Google Scholar] [CrossRef]
- Ubago, J.M.; Zhang, Q.; Kim, J.J.; Kong, B.; Wei, J.J. Two Subtypes of Atypical Leiomyoma: Clinical, Histologic, and Molecular Analysis. Am. J. Surg. Pathol. 2016, 40, 923–933. [Google Scholar] [CrossRef]
- Zhang, Q.; Ubago, J.; Li, L.; Guo, H.; Liu, Y.; Qiang, W.; Kim, J.J.; Kong, B.; Wei, J.J. Molecular analyses of 6 different types of uterine smooth muscle tumors: Emphasis in atypical leiomyoma. Cancer 2014, 120, 3165–3177. [Google Scholar] [CrossRef]
- Downes, K.A.; Hart, W.R. Bizarre leiomyomas of the uterus: A comprehensive pathologic study of 24 cases with long-term follow-up. Am. J. Surg. Pathol. 1997, 21, 1261–1270. [Google Scholar] [CrossRef]
- Mathew, R.P.; Francis, S.; Jayaram, V.; Anvarsadath, S. Uterine leiomyomas revisited with review of literature. Abdom. Radiol. 2021, 46, 4908–4926. [Google Scholar] [CrossRef]
- Gadducci, A.; Zannoni, G.F. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol. Oncol. 2019, 154, 631–637. [Google Scholar] [CrossRef]
- Gupta, M.; Laury, A.L.; Nucci, M.R.; Quade, B.J. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): A clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology 2018, 73, 284–298. [Google Scholar] [CrossRef]
- Croce, S.; Ducoulombier, A.; Ribeiro, A.; Lesluyes, T.; Noel, J.C.; Amant, F.; Guillou, L.; Stoeckle, E.; Devouassoux-Shisheboran, M.; Penel, N.; et al. Genome profiling is an efficient tool to avoid the STUMP classification of uterine smooth muscle lesions: A comprehensive array-genomic hybridization analysis of 77 tumors. Mod. Pathol. 2018, 31, 816–828. [Google Scholar] [CrossRef]
- Croce, S.; Ribeiro, A.; Brulard, C.; Noel, J.C.; Amant, F.; Stoeckle, E.; Devouassoux-Shisheborah, M.; Floquet, A.; Arnould, L.; Guyon, F.; et al. Uterine smooth muscle tumor analysis by comparative genomic hybridization: A useful diagnostic tool in challenging lesions. Mod. Pathol. 2015, 28, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Ahvenainen, T.V.; Makinen, N.M.; von Nandelstadh, P.; Vahteristo, M.E.A.; Pasanen, A.M.; Butzow, R.C.; Vahteristo, P.M. Loss of ATRX/DAXX expression and alternative lengthening of telomeres in uterine leiomyomas. Cancer 2018, 124, 4650–4656. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Aavikko, M.; Heikkinen, T.; Taipale, M.; Taipale, J.; Koivisto-Korander, R.; Butzow, R.; Vahteristo, P. Exome Sequencing of Uterine Leiomyosarcomas Identifies Frequent Mutations in TP53, ATRX, and MED12. PLoS Genet. 2016, 12, e1005850. [Google Scholar] [CrossRef] [PubMed]
- Pisacane, A.; Cascardi, E.; Berrino, E.; Polidori, A.; Sarotto, I.; Casorzo, L.; Panero, M.; Boccaccio, C.; Verginelli, F.; Benvenuti, S.; et al. Real-world histopathological approach to malignancy of undefined primary origin (MUO) to diagnose cancers of unknown primary (CUPs). Virchows Arch. 2022, 1–13. [Google Scholar] [CrossRef]
- Dellino, M.; Carriero, C.; Silvestris, E.; Capursi, T.; Paradiso, A.; Cormio, G. Primary vaginal carcinoma arising on cystocele mimicking vulvar cancer. J. Obstet. Gynaecol. Can. 2020, 42, 1543–1545. [Google Scholar] [CrossRef]
- Rush, D.S.; Tan, J.; Baergen, R.N.; Soslow, R.A. h-Caldesmon, a novel smooth muscle-specific antibody, distinguishes between cellular leiomyoma and endometrial stromal sarcoma. Am. J. Surg. Pathol. 2001, 25, 253–258. [Google Scholar] [CrossRef]
- Limongelli, L.; Cascardi, E.; Capodiferro, S.; Favia, G.; Corsalini, M.; Tempesta, A.; Maiorano, E. Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics 2020, 10, 424. [Google Scholar] [CrossRef]
- Cazzato, G.; Cascardi, E.; Colagrande, A.; Belsito, V.; Lospalluti, L.; Foti, C.; Arezzo, F.; Dellino, M.; Casatta, N.; Lupo, C.; et al. PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience. Diagnostics 2022, 12, 2197. [Google Scholar] [CrossRef]
- Cazzato, G.; Cascardi, E.; Colagrande, A.; Lettini, T.; Resta, L.; Bizzoca, C.; Arezzo, F.; Loizzi, V.; Dellino, M.; Cormio, G.; et al. The Thousand Faces of Malignant Melanoma: A Systematic Review of the Primary Malignant Melanoma of the Esophagus. Cancers 2022, 14, 3725. [Google Scholar] [CrossRef]
- Bennett, J.A.; Oliva, E. Perivascular epithelioid cell tumors (PEComa) of the gynecologic tract. Genes Chromosom. Cancer 2021, 60, 168–179. [Google Scholar] [CrossRef]
- Bennett, J.A.; Braga, A.C.; Pinto, A.; Van de Vijver, K.; Cornejo, K.; Pesci, A.; Zhang, L.; Morales-Oyarvide, V.; Kiyokawa, T.; Zannoni, G.F.; et al. Uterine PEComas: A Morphologic, Immunohistochemical, and Molecular Analysis of 32 Tumors. Am. J. Surg. Pathol. 2018, 42, 1370–1383. [Google Scholar] [CrossRef]
- Schaefer, I.M.; Hornick, J.L.; Sholl, L.M.; Quade, B.J.; Nucci, M.R.; Parra-Herran, C. Abnormal p53 and p16 staining patterns distinguish uterine leiomyosarcoma from inflammatory myofibroblastic tumour. Histopathology 2017, 70, 1138–1146. [Google Scholar] [CrossRef]
- Chiang, S.; Samore, W.; Zhang, L.; Sung, Y.S.; Turashvili, G.; Murali, R.; Soslow, R.A.; Hensley, M.L.; Swanson, D.; Dickson, B.C.; et al. PGR Gene Fusions Identify a Molecular Subset of Uterine Epithelioid Leiomyosarcoma with Rhabdoid Features. Am. J. Surg. Pathol. 2019, 43, 810–818. [Google Scholar] [CrossRef]
- Arias-Stella, J.A., 3rd; Benayed, R.; Oliva, E.; Young, R.H.; Hoang, L.N.; Lee, C.H.; Jungbluth, A.A.; Frosina, D.; Soslow, R.A.; Antonescu, C.R.; et al. Novel PLAG1 Gene Rearrangement Distinguishes a Subset of Uterine Myxoid Leiomyosarcoma from Other Uterine Myxoid Mesenchymal Tumors. Am. J. Surg. Pathol. 2019, 43, 382–388. [Google Scholar] [CrossRef]
- Abeler, V.M.; Royne, O.; Thoresen, S.; Danielsen, H.E.; Nesland, J.M.; Kristensen, G.B. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009, 54, 355–364. [Google Scholar] [CrossRef]
- Friedman, C.F.; Hensley, M.L. Options for Adjuvant Therapy for Uterine Leiomyosarcoma. Curr. Treat. Options Oncol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Ayhan, A.; Gungorduk, K.; Khatib, G.; Firat Cuylan, Z.; Boran, N.; Gokcu, M.; Celik, H.; Ozgul, N.; Akbayir, O.; Simsek, T.; et al. Prognostic factors and survival outcomes of women with uterine leiomyosarcoma: A Turkish Uterine Sarcoma Group Study-003. Curr. Probl. Cancer 2021, 45, 100712. [Google Scholar] [CrossRef]
- Zivanovic, O.; Jacks, L.M.; Iasonos, A.; Leitao, M.M., Jr.; Soslow, R.A.; Veras, E.; Chi, D.S.; Abu-Rustum, N.R.; Barakat, R.R.; Brennan, M.F.; et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer 2012, 118, 660–669. [Google Scholar] [CrossRef]
- Sampath, S.; Gaffney, D.K. Role of radiotherapy treatment of uterine sarcoma. Best Pract. Res. Clin. Obs. Gynaecol. 2011, 25, 761–772. [Google Scholar] [CrossRef]
- Reed, N.S.; Mangioni, C.; Malmstrom, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef]
- Patel, D.; Handorf, E.; von Mehren, M.; Martin, L.; Movva, S. Adjuvant Chemotherapy in Uterine Leiomyosarcoma: Trends and Factors Impacting Usage. Sarcoma 2019, 2019, 3561501. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Floquet, A.; Gladieff, L.; Bompas, E.; Ray-Coquard, I.; Piperno-Neumann, S.; Selle, F.; Guillemet, C.; Weber, B.; Largillier, R.; et al. A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group. Ann. Oncol. 2013, 24, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Ishill, N.; Soslow, R.; Larkin, J.; Abu-Rustum, N.; Sabbatini, P.; Konner, J.; Tew, W.; Spriggs, D.; Aghajanian, C.A. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecol. Oncol. 2009, 112, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Wathen, J.K.; Maki, R.G.; Araujo, D.M.; Sutton, G.; Priebat, D.A.; George, S.; Soslow, R.A.; Baker, L.H. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: Results of a phase 2 trial (SARC 005). Cancer 2013, 119, 1555–1561. [Google Scholar] [CrossRef]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 1997, 350, 1647–1654. [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2008, 113, 573–581. [Google Scholar] [CrossRef]
- Hensley, M.L.; Maki, R.; Venkatraman, E.; Geller, G.; Lovegren, M.; Aghajanian, C.; Sabbatini, P.; Tong, W.; Barakat, R.; Spriggs, D.R. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J. Clin. Oncol. 2002, 20, 2824–2831. [Google Scholar] [CrossRef]
- Giuntoli, R.L., 2nd; Garrett-Mayer, E.; Bristow, R.E.; Gostout, B.S. Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma. Gynecol. Oncol. 2007, 106, 82–88. [Google Scholar] [CrossRef]
- Leitao, M.M., Jr.; Zivanovic, O.; Chi, D.S.; Hensley, M.L.; O'Cearbhaill, R.; Soslow, R.A.; Barakat, R.R. Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecol. Oncol. 2012, 125, 409–413. [Google Scholar] [CrossRef]
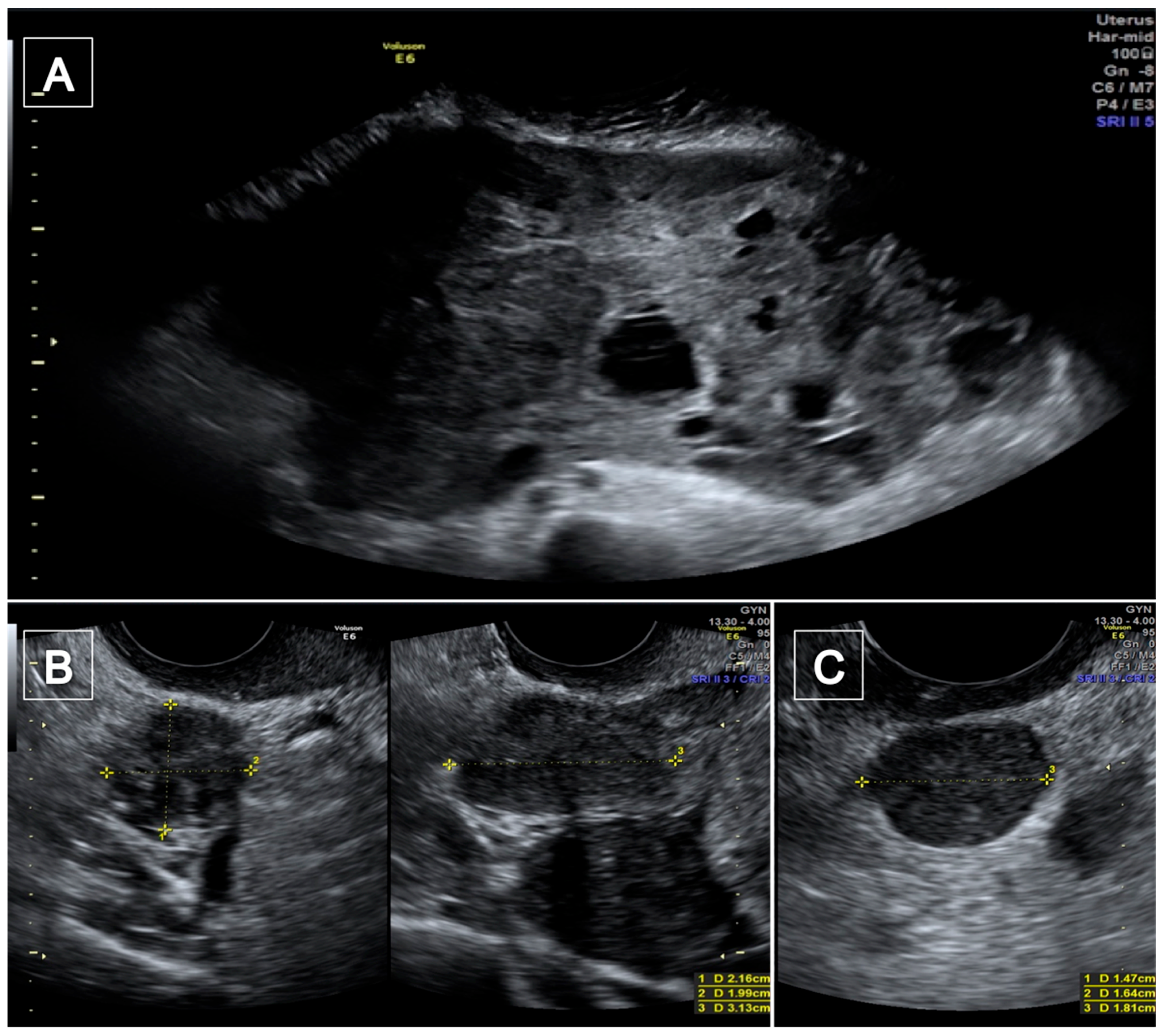
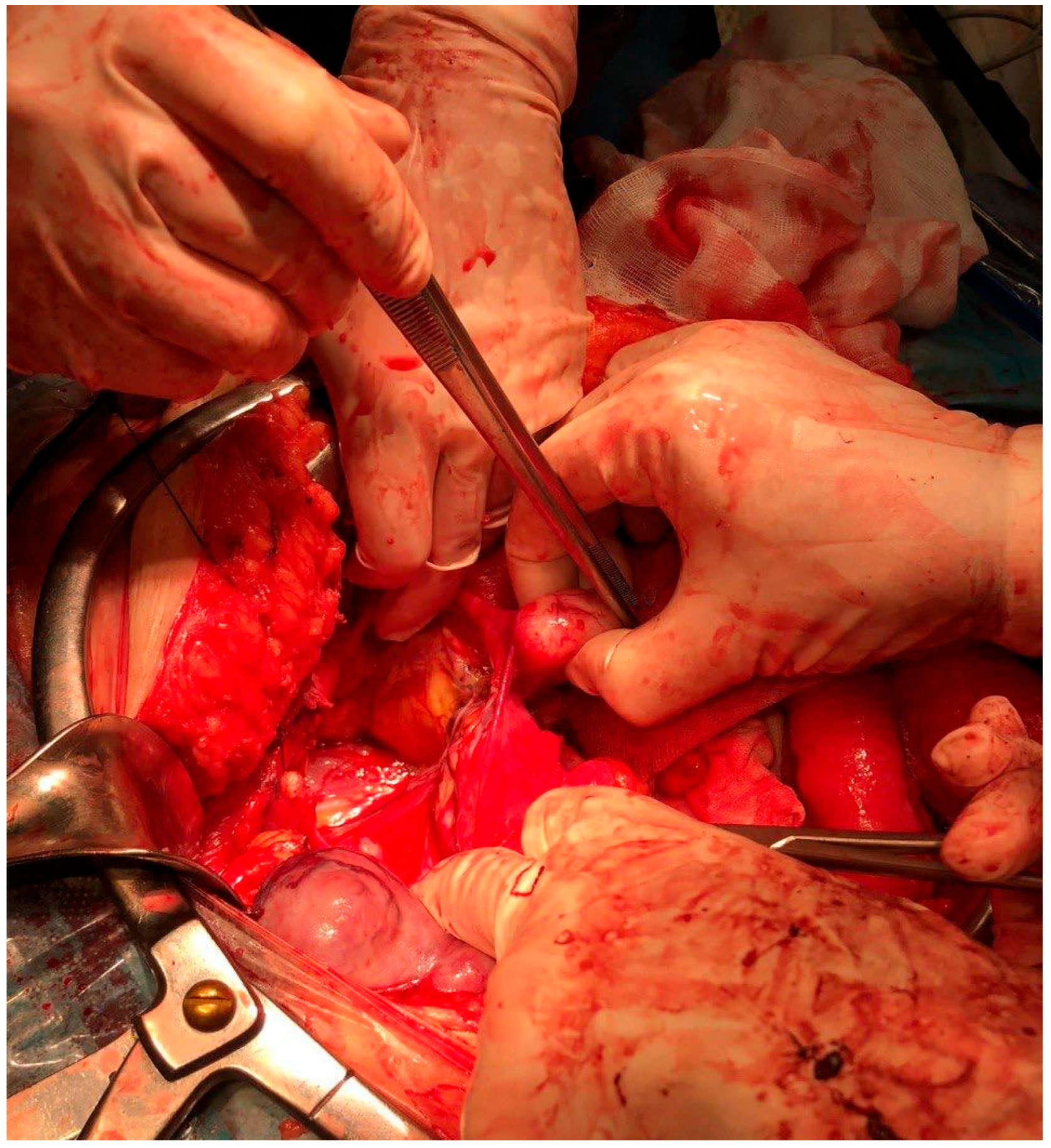

| Author and Year | Age | Number of DPL | Location | Type of Sarcoma | Pre-Operative Examinations | Pre-Operative Diagnosis |
|---|---|---|---|---|---|---|
| Chiu et al. 2018 [6] | 61 | 3 | Retroperitoneal pelvic cavity | high-grade leiomyosarcoma with peritoneal carcinomatosis and pulmonary and hepatic metastases | TV ultrasound CT whole body scan Chest X ray | yes |
| Rubin et al. 1986 [18] | 27 | ND | Pelvis | Small spindle cell sarcoma and diffuse bone metastases | No | No, found at cesarean section |
| Zyla et al. 2015 [7] | 26 | ND (numerous) | Pelvis Peritoneal cavity Omentum Retroperitoneal space | Low-grade Endometrial stromal sarcoma | No | No |
| Sharma et al. 2004 [8] | 55 | ND (multiple) | Omentum Mesentery | Leiomyosarcoma | TV Ultrasound | Yes |
| Fulcher et al. 1998 [9] | 48 | ND (more than four) | Pelvis Subdiaphragmatic peritoneum | Moderate-grade Leiomyosarcoma | Renal sonography | No |
| Lamarca et al. 2011 [10] | 37 | ND (multiple) | Peritoneal cavity | Leiomyosarcoma | “Imaging techniques” | Yes |
| Akkersdijk et al. 1990 [11] | 25 | ND (multiple) | Omentum Colon Small intestine | High-grade Leiomyosarcoma | TV * Ultrasound | No |
| Raspagliesi et al. 1996 [12] | 26 | 4 | Adnex Mesosigmoid Sigmoid serosa | High-grade Leiomyosarcoma | Ultrasound | No |
| Morizaki et al. 1999 [13] | 33 | ND (multiple) | Peritoneum Mesentery Descending Colon Pelvis | Leiomyosarcoma and Fibrosarcoma | “Serial examinations” | No |
| Xu et al. 2019 [14] | 47 | ND (multiple) | Surface of retroperitoneum sigmoid colon urinary bladder | Leiomyosarcoma | 3D TV Ultrasound MRI * | No |
| Tun et al. 2016 [15] | 56 | ND (multiple) | Pelvis Peritoneum Omentum Liver | Leiomyosarcoma with lung and liver metastases | “Imaging studies” | No |
| Syed et al. 2017 [19] | 40 | ND (multiple) | Peritoneum Recto-uterine pouch Prevesical space Left rectus Abdominis muscle | Leiomyosarcoma | Ultrasound Contrast-enhanced CT scan MRI | Yes |
| Abulafia et al. 1993 [20] | 20 | ND (multiple) | Pelvis Omentum | Low grade Leiomyosarcoma | Ultrasound CT scan | No |
| Rosati et al. 2021 [17] | 49 | ND (multiple) | Pelvis | Low grade Leiomyosarcoma | All patients underwent CT scan or MRI and abdominal/transvaginal ultrasound | Not available |
| 36 | ND (multiple) | Diaphragm Liver | Low grade Leiomyosarcoma | Not available | ||
| 31 | ND | Peritoneum | Spindle cell Sarcoma | Not available | ||
| 48 | ND (multiple) | Abdominal wal Bowel serosa | High grade Leiomyosarcoma | Not available | ||
| 46 | ND (multiple) | Peritoneum Bowel’s serosa | Low grade Leiomyosarcoma | Not available | ||
| Wen et al. 2022 [16] | 72 | multiple | Rectus Sigmoid colon Bilateral inguinal areas Right ureter | Myxoid leiomyosarcoma | CT scan | No |
| Vimercati et al. 2022 * | 47 | 6 | Pelvis | High-grade Leiomyosarcoma with two omental metastases | TV ultrasound CT scan | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vimercati, A.; Santarsiero, C.M.; Esposito, A.; Putino, C.; Malvasi, A.; Damiani, G.R.; Laganà, A.S.; Vitagliano, A.; Marinaccio, M.; Resta, L.; et al. An Extremely Rare Case of Disseminated Peritoneal Leiomyomatosis with a Pelvic Leiomyosarcoma and Omental Metastasis after Laparoscopic Morcellation: Systematic Review of the Literature. Diagnostics 2022, 12, 3219. https://doi.org/10.3390/diagnostics12123219
Vimercati A, Santarsiero CM, Esposito A, Putino C, Malvasi A, Damiani GR, Laganà AS, Vitagliano A, Marinaccio M, Resta L, et al. An Extremely Rare Case of Disseminated Peritoneal Leiomyomatosis with a Pelvic Leiomyosarcoma and Omental Metastasis after Laparoscopic Morcellation: Systematic Review of the Literature. Diagnostics. 2022; 12(12):3219. https://doi.org/10.3390/diagnostics12123219
Chicago/Turabian StyleVimercati, Antonella, Carla Mariaflavia Santarsiero, Angela Esposito, Carmela Putino, Antonio Malvasi, Gianluca Raffaello Damiani, Antonio Simone Laganà, Amerigo Vitagliano, Marco Marinaccio, Leonardo Resta, and et al. 2022. "An Extremely Rare Case of Disseminated Peritoneal Leiomyomatosis with a Pelvic Leiomyosarcoma and Omental Metastasis after Laparoscopic Morcellation: Systematic Review of the Literature" Diagnostics 12, no. 12: 3219. https://doi.org/10.3390/diagnostics12123219
APA StyleVimercati, A., Santarsiero, C. M., Esposito, A., Putino, C., Malvasi, A., Damiani, G. R., Laganà, A. S., Vitagliano, A., Marinaccio, M., Resta, L., Cicinelli, E., Cazzato, G., Cascardi, E., & Dellino, M. (2022). An Extremely Rare Case of Disseminated Peritoneal Leiomyomatosis with a Pelvic Leiomyosarcoma and Omental Metastasis after Laparoscopic Morcellation: Systematic Review of the Literature. Diagnostics, 12(12), 3219. https://doi.org/10.3390/diagnostics12123219













