Ultrasound Imaging of Brachial and Antebrachial Fasciae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Ultrasonography Imaging Measurements
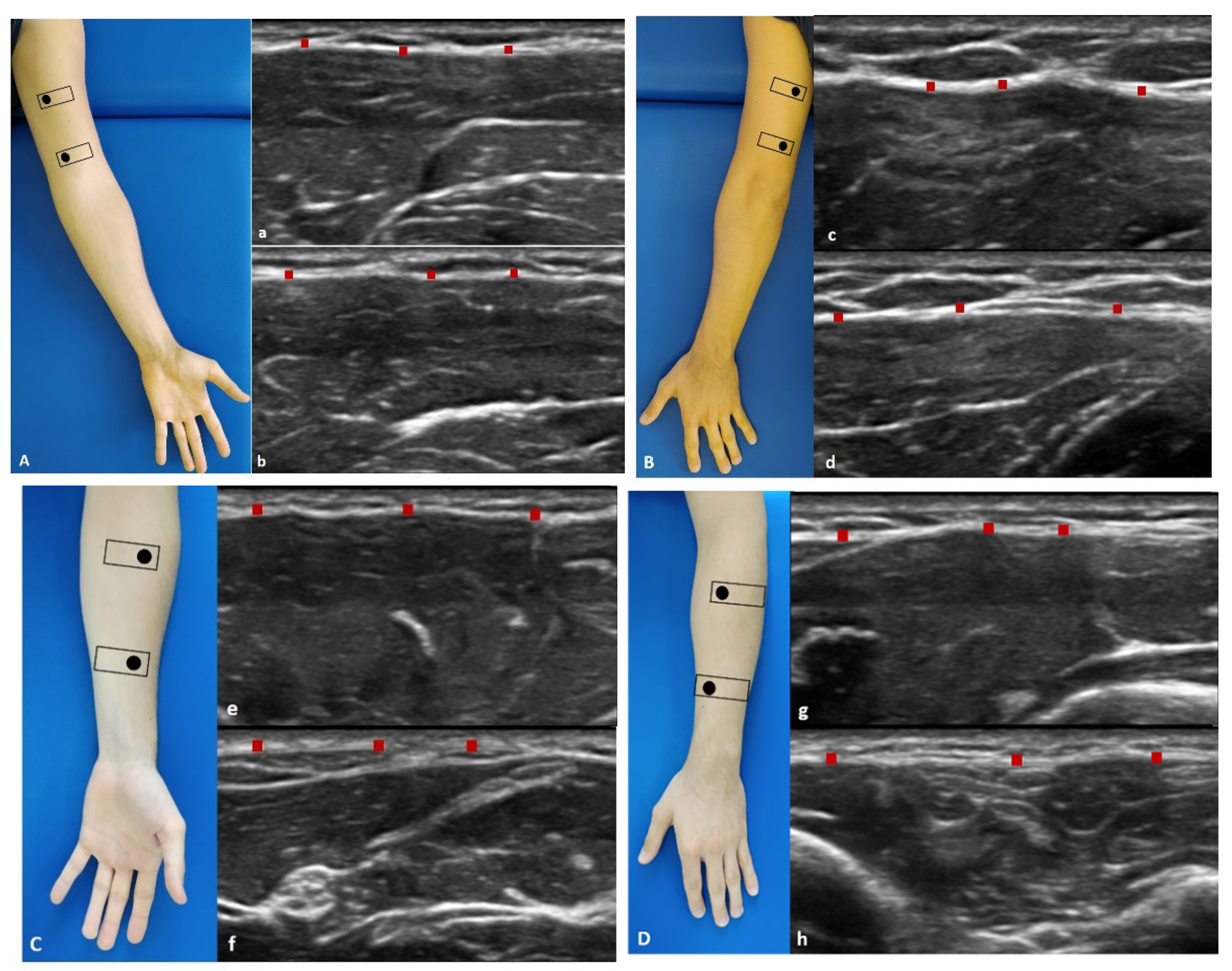
2.3.1. Arm
- Anterior region (Figure 1A): the patient was in a relaxed supine position with the upper limb in a neutral position.
- Anterior 1: the probe is placed axially, over the proximal half of the anterior arm. The median nerve and brachial artery lie medially between the brachialis and triceps muscles (Figure 1(Aa)).
- Anterior 2: the probe was axially moved downwards following the median nerve and brachial artery. The brachialis muscle can be seen deep to the biceps muscle (Figure 1(Ab)).
- Posterior region (Figure 1B): the patient was prone with the upper limb in a neutral position.
- Posterior 1: the probe is placed axially, over the proximal half of the posterior arm. Each belly of the triceps muscle can be seen separately. The median nerve, ulnar, and radial nerves can be seen in close proximity. The brachial artery and vein should be taken into consideration with the nerves as landmarks (Figure 1(Bc)).
- Posterior 2: the probe was axially moved downwards following the landmarks (Figure 1(Bd)).
2.3.2. Forearm
- Anterior region (Figure 1C): the patient was in a relaxed supine position with the upper limb in a neutral position and the forearm in supination position.
- Anterior 1: the probe was axially placed on the anterior proximal half of the forearm. The pronator teres lies lateral to the flexor carpi radialis (FCR) and flexor digitorum superficialis (FDS). The median nerve can be seen between the two heads of the pronator teres muscle (Figure 1(Ce)).
- Anterior 2: the probe was axially moved downwards, over the distal half of the anterior forearm. Flexor digitorum profundus (FDP) is seen deep in the FDS, flexor carpi ulnaris (FCU), and palmaris longus. These structures lay over the interosseus membrane (Figure 1(Cf)).
- Posterior region (Figure 1D): the patient was prone with the upper limb in a neutral position and the forearm is in pronation position.
- Posterior 1: place the probe axially over the dorsal aspect of the proximal half of the forearm. At this level extensor digitorum (ED) lies between extensor carpi radialis brevis (ECRB) and extensor digiti minimi (EDM) over the supinator muscle. The brachioradialis muscle can be observed lying medial to the extensor carpi radialis longus (ECRL) and brevis (ECRB) muscles. Adjacent branches of the radial nerve and artery can be seen (Figure 1(Dg)).
- Posterior 2: the probe was axially moved downwards, over the distal dorsal half of the forearm. ED becomes smaller, while EDM becomes larger. The abductor pollicis longus (APL) lies adjacent to radius bone, and the extensor pollicis longus (EPL) lies adjacent to ulna (U). The posterior interosseus nerve is located between the extensor digitorum superficially. The extensor carpi ulnaris (ECU) is the most medially located extensor muscle lying over the ulna (U), medial to the extensor digiti minimi (EDM) and EPL and APL deeply. The extensor pollicis brevis (EPB) and the extensor indicis (EI) can already be seen (Figure 1(Dh)).
2.4. Statistical Analysis
3. Results
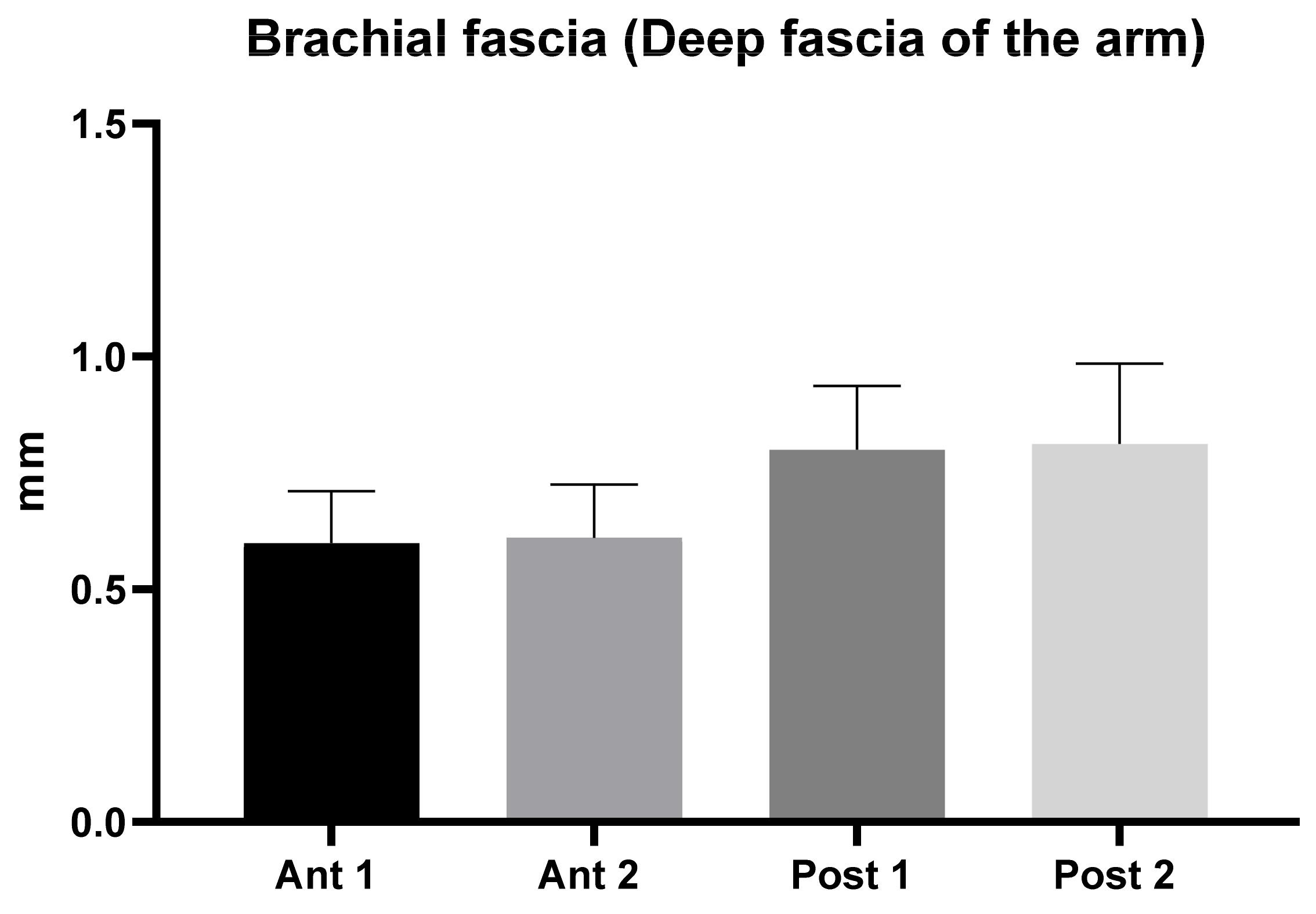
3.1. Ultrasound Measurements of the Brachial Fascia (Deep Fascia of the Arm)
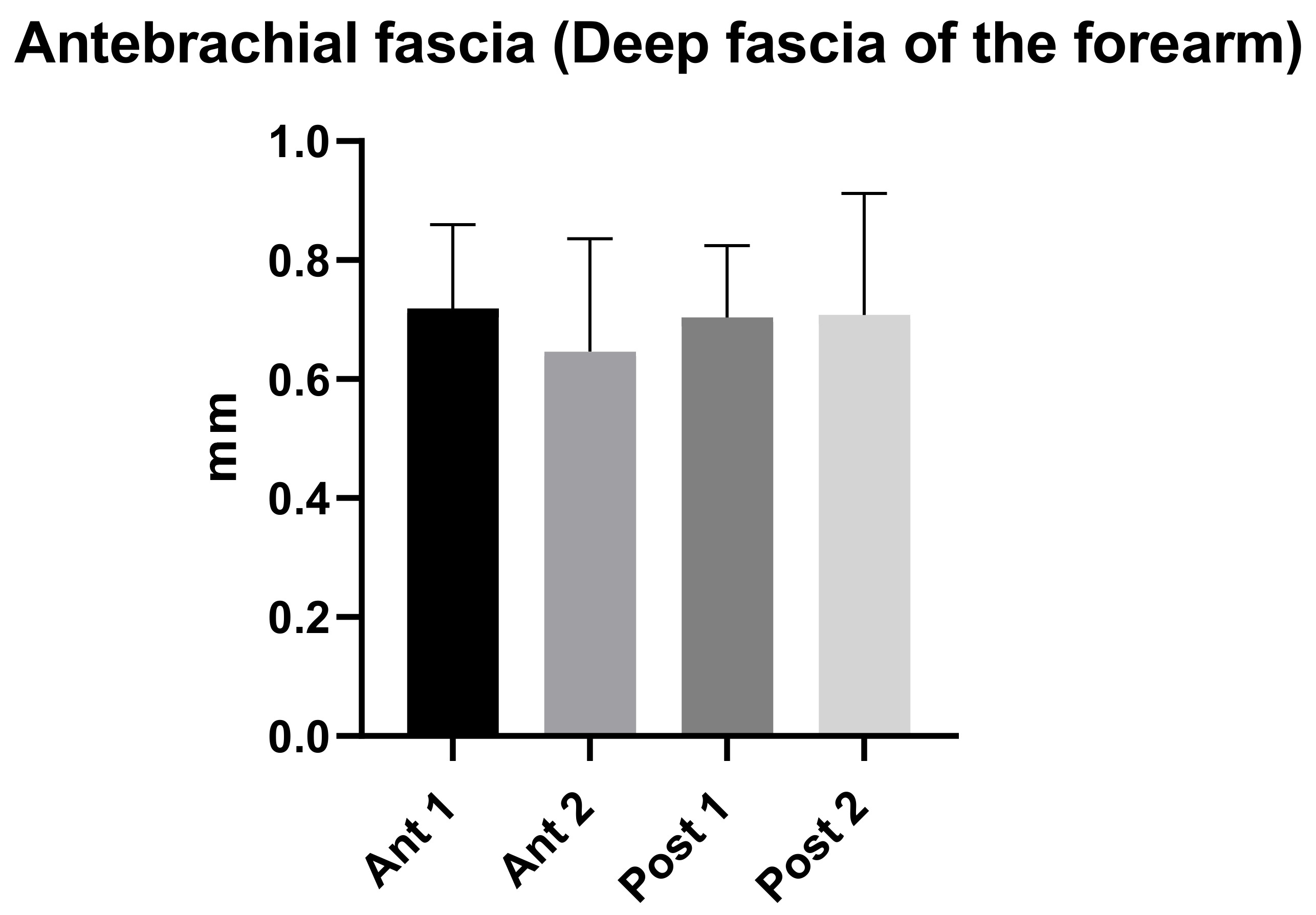
3.2. Ultrasound Measurements of the Antebrachial Fascia (Deep Fascia of the Forearm)
3.3. Ultrasound Measurements Comparison between the Brachial Fascia and the Antebrachial Fascia
3.4. Intra-Rater Reliability
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotta, K. Fascial plane blocks: Anatomical structures that affect the spread of local anesthetic. J. Anesth. 2019, 33, 493–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Miguel, S.; Miguel-Pérez, M.; Navarro, J.; Möller, I.; Pérez-Bellmunt, A.; Agullo, J.L.; Ortiz-Sagristà, J.; Blasi, J.; Martinoli, C. Compartments of the antebrachial fascia of the forearm: Clinically relevant ultrasound, anatomical and histological findings. Surg. Radiol. Anat. 2021, 43, 1569–1579. [Google Scholar] [CrossRef]
- Gourgiotis, S.; Villias, C.; Germanos, S.; Foukas, A.; Ridolfini, M.P. Acute limb compartment syndrome: A review. J. Surg. Educ. 2007, 64, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Ruyer, J.; Rutka, V.; Garret, J.; Rizzo, C.; Guigal, V. Endoscopic fasciotomy for chronic exertional compartment syndrome of the forearm: Clinical results of a new technique using an endoscopic carpal tunnel release device. Hand Surg. Rehabil. 2020, 39, 154–158. [Google Scholar] [CrossRef]
- Özçakar, L.; Kara, M.; Chang, K.V.; Çarl, A.B.; Akkaya, N.; Tok, F.; Chen, W.S.; Wang, T.G.; Tekin, L.; Ulaşl, A.M.; et al. Nineteen reasons why physiatrists should do musculoskeletal ultrasound: EURO-MUSCULUS/USPRM recommendations. Am. J. Phys. Med. Rehabil. 2015, 94, e45–e49. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, C.; Fede, C.; Macchi, V.; Özçakar, L. Ultrasound Imaging of the Fascial Layers: You See (Only) What You Know. J. Ultrasound Med. 2020, 39, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Fede, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. An anatomical comparison of the fasciae of the thigh: A macroscopic, microscopic and ultrasound imaging study. J. Anat. 2021, 238, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Fede, C.; Stecco, A.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Ultrasound Imaging of Crural Fascia and Epimysial Fascia Thicknesses in Basketball Players with Previous Ankle Sprains Versus Healthy Subjects. Diagnostics 2021, 11, 177. [Google Scholar] [CrossRef]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomised clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Wilke, J.; Macchi, V.; De Caro, R.; Stecco, C. Fascia thickness, aging and flexibility: Is there an association? J. Anat. 2019, 234, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Langevin, H.M.; Rizzo, D.M.; Fox, J.R.; Badger, G.J.; Wu, J.; Konofagou, E.; Stevens-Tuttle, D.; Bouffard, N.A.; Krag, M.H. Dynamic morphometric characterization of local connective tissue network structure in humans using ultrasound. BMC Syst. Biol. 2007, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirri, C.; Stecco, C.; Fede, C.; De Caro, R.; Özçakar, L. Dynamic ultrasonography for assessing the nerve-fasciae relationship in entrapment syndromes. Med. Ultrason. 2021, 23, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Gaudreault, N.; Fan, C.; Macchi, V.; De Caro, R.; Stecco, C. Morphometric and dynamic measurements of muscular fascia in healthy individuals using ultrasound imaging: A summary of the discrepancies and gaps in the current literature. Surg. Radiol. Anat. 2018, 40, 1329–1341. [Google Scholar] [CrossRef]
- Lee, K.J.; Jin, W.; Kim, G.Y.; Rhee, S.J.; Park, S.Y.; Park, J.S.; Ryu, K.N. Sonographic features of superficial-type nodular fasciitis in the musculoskeletal system. J. Ultrasound Med. 2015, 34, 1465–1471. [Google Scholar] [CrossRef]
- Stecco, C.; Tiengo, C.; Stecco, A.; Porzionato, A.; Macchi, V.; Stern, R.; De Caro, R. Fascia redefined: Anatomical features and technical relevance in fascial flap surgery. Surg. Radiol. Anat. 2013, 35, 369–376. [Google Scholar] [CrossRef]
- Stecco, C.; Porzionato, A.; Macchi, V.; Stecco, A.; Vigato, E.; Parenti, A.; Delmas, V.; Aldegheri, R.; De Caro, R. The expansions of the pectoral girdle muscles onto the brachial fascia: Morphological aspects and spatial disposition. Cells Tissues Organs 2008, 188, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C. Functional Atlas of the Human Fascial System, 1st ed.; Churchill Livingstone Elsevier: Edinburgh, UK, 2015. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
- Derchi, L.; Rizzatto, G. Technical requirements. In Ultrasound of the Musculoskeletal System; Bianchi, S., Martinoli, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–16. [Google Scholar]
- Ahn, A.C.; Kaptchuk, T.J. Spatial anisotropy analyses of subcutaneous tissue layer: Potential insights into its biomechanical characteristics. J. Anat. 2011, 219, 515–524. [Google Scholar] [CrossRef]
- Cohen, J. Things I have learned (so far). Am. Psychol. 1990, 45, 1304–1312. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting Intraclass Correlation Coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecco, A.; Macchi, V.; Stecco, C.; Porzionato, A.; Ann Day, J.; Delmas, V.; De Caro, R. Anatomical study of myofascial continuity in the anterior region of the upper limb. J. Bodyw. Mov. Ther. 2009, 13, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Lancerotto, L.; Porzionato, A.; Macchi, V.; Tiengo, C.; Parenti, A.; Sanudo, J.R.; De Caro, R. The palmaris longus muscle and its relations with the antebrachial fascia and the palmar aponeurosis. Clin. Anat. 2009, 22, 221–229. [Google Scholar] [CrossRef]
- Stecco, C.; Macchi, V.; Lancerotto, L.; Tiengo, C.; Porzionato, A.; De Caro, R. Comparison of transverse carpal ligament and flexor retinaculum terminology for the wrist. J. Hand Surg. Am. 2010, 35, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Todros, S.; Fede, C.; Pianigiani, S.; Fan, C.; Foti, C.; Stecco, C.; Pavan, P. Inter-rater reliability and variability of ultrasound measurements of abdominal muscles and fasciae thickness. Clin. Anat. 2019, 32, 948–960. [Google Scholar] [CrossRef]
- Fan, C.; Fede, C.; Pirri, C.; Guidolin, D.; Biz, C.; Macchi, V.; De Caro, R.; Stecco, C. Quantitative Evaluation of the Echo Intensity of Paraneural Area and Myofascial Structure around Median Nerve in Carpal Tunnel Syndrome. Diagnostics 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Kolny, M.; Stasiowski, M.J.; Zuber, M.; Marciniak, R.; Chabierska, E.; Pluta, A.; Jałowiecki, P.; Byrczek, T. Randomized, comparative study of the effectiveness of three different techniques of interscalene brachial plexus block using 0.5% ropivacaine for shoulder arthroscopy. Anaesthesiol. Intensive Ther. 2017, 49, 47–52. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Kolny, M.; Zuber, M.; Marciniak, R.; Chabierska, E.; Jałowiecki, P.; Pluta, A.; Możdżyński, B. Randomised controlled trial of analgesic effectiveness of three different techniques of single-shot interscalene brachial plexus block using 20 mL of 0.5% ropivacaine for shoulder arthroscopy. Anaesthesiol. Intensive Ther. 2017, 49, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Descriptive Statistics | Age | BMI | Height | Weight |
|---|---|---|---|---|
| Number of values | 25 | 25 | 25 | 25 |
| Minimum | 20 | 15.79 | 158 | 43 |
| Maximum | 60 | 31.6 | 183 | 87 |
| Range | 40 | 15.81 | 25 | 44 |
| Mean | 32.72 | 23.61 | 171.1 | 69.5 |
| Std. Deviation | 13.48 | 3.594 | 7.224 | 13.06 |
| Coefficient of variation | 41.19% | 15.23% | 4.222% | 18.79% |
| Descriptive Statistics | Ant 1 | Ant 2 | Post 1 | Post 2 |
|---|---|---|---|---|
| Number of values | 50 | 50 | 50 | 50 |
| Minimum | 0.43 | 0.4 | 0.47 | 0.5 |
| Maximum | 1.11 | 0.9 | 1.2 | 1.26 |
| Range | 0.68 | 0.5 | 0.73 | 0.76 |
| Mean | 0.60 | 0.61 | 0.80 | 0.81 |
| Std. Deviation | 0.11 | 0.11 | 0.14 | 0.20 |
| Std. Error of Mean | 0.02 | 0.02 | 0.02 | 0.02 |
| Coefficient of variation | 18.62% | 18.72% | 17.16% | 21.27% |
| Type of Comparison | Mean Diff. | 95.00% CI of Diff. | Significant? | Summary | Adjusted p Value |
|---|---|---|---|---|---|
| Ant 1 vs. Ant 2 | −0.0114 | −0.08196 to 0.05916 | No | ns | 0.9752 |
| Ant 1 vs. Post 1 | −0.2006 | −0.2712 to −0.1300 | Yes | **** | <0.0001 |
| Ant 1 vs. Post 2 | −0.213 | −0.2836 to −0.1424 | Yes | **** | <0.0001 |
| Ant 2 vs. Post 1 | −0.1892 | −0.2598 to −0.1186 | Yes | **** | <0.0001 |
| Ant 2 vs. Post 2 | −0.2016 | −0.2722 to −0.1310 | Yes | **** | <0.0001 |
| Post 1 vs. Post 2 | −0.0124 | −0.08296 to 0.05816 | No | ns | 0.9685 |
| Descriptive Statistics | Ant 1 | Ant 2 | Post 1 | Post 2 |
|---|---|---|---|---|
| Number of values | 50 | 50 | 50 | 50 |
| Minimum | 0.44 | 0.34 | 0.49 | 0.51 |
| Maximum | 1.1 | 1.04 | 1.1 | 1.1 |
| Range | 0.66 | 0.7 | 0.61 | 1.049 |
| Mean | 0.72 | 0.70 | 0.70 | 0.71 |
| Std. Deviation | 0.14 | 0.20 | 0.12 | 0.20 |
| Std. Error of Mean | 0.02 | 0.03 | 0.02 | 0.03 |
| Coefficient of variation | 19.58% | 29.35% | 17.19% | 28.81% |
| Type of Comparison | Mean Diff. | 95.00% CI of Diff. | Significant? | Summary | Adjusted p Value |
|---|---|---|---|---|---|
| Ant 1 vs. Ant 2 | 0.0726 | −0.01414 to 0.1593 | No | ns | 0.1356 |
| Ant 1 vs. Post 1 | 0.0156 | −0.07114 to 0.1023 | No | ns | 0.9664 |
| Ant 1 vs. Post 2 | 0.01078 | −0.07596 to 0.09752 | No | ns | 0.9884 |
| Ant 2 vs. Post1 | −0.057 | −0.1437 to 0.02974 | No | ns | 0.325 |
| Ant 2 vs. Post 2 | −0.06182 | −0.1486 to 0.02492 | No | ns | 0.2547 |
| Post 1 vs. Post 2 | −0.00482 | −0.09156 to 0.08192 | No | ns | 0.9989 |
| Type of Comparison | Mean Diff. | 95.00% CI of Diff. | Significant? | Summary | Adjusted p Value |
|---|---|---|---|---|---|
| Ant 1 arm vs. Ant 1 forearm | −0.12 | −0.213 to −0.027 | Yes | ** | 0.0025 |
| Ant 1 arm vs. Ant 2 forearm | −0.05 | −0.140 to 0.045 | No | ns | 0.7776 |
| Ant 1 arm vs. Post 1 forearm | −0.11 | −0.197 to −0.011 | Yes | * | 0.0157 |
| Ant 1 arm vs. Post 2 forearm | −0.11 | −0.202 to −0.016 | Yes | ** | 0.0092 |
| Ant 2 arm vs. Ant 1 forearm | −0.11 | −0.201 to −0.015 | Yes | ** | 0.0098 |
| Ant 2 arm vs. Ant 2 forearm | −0.04 | −0.129 to 0.056 | No | ns | 0.9373 |
| Ant 2 arm vs. Post 1 forearm | −0.09 | −0.186 to −1.345 | Yes | * | 0.0499 |
| Ant 2 arm vs. Post 2 forearm | −0.10 | −0.191 to −0.005 | Yes | * | 0.0312 |
| Post 1 arm vs. Ant 1 forearm | 0.08 | −0.012 to 0.174 | No | ns | 0.1444 |
| Post 1 arm vs. Ant 2 forearm | 0.15 | 0.060 to 0.246 | Yes | **** | <0.0001 |
| Post 1 arm vs. Post 1 forearm | 0.10 | 0.003 to 0.20 | Yes | * | 0.0367 |
| Post 1 arm vs. Post 2 forearm | 0.09 | −0.001 to 0.184 | No | ns | 0.0581 |
| Post 2 arm vs. Ant 1 forearm | 0.09 | 1.345 to 0.20 | Yes | * | 0.0499 |
| Post 2 arm vs. Ant 2 forearm | 0.17 | 0.072 to 0.260 | Yes | **** | <0.0001 |
| Post 2 arm vs. Post1 forearm | 0.11 | 0.020 to 0.205 | Yes | ** | 0.0098 |
| Post 2 arm vs. Post 2 forearm | 0.10 | 0.011 to 0.20 | Yes | * | 0.0168 |
| Type of Fascia | Region | ICC |
|---|---|---|
| Brachial fascia | Anterior | 0.88 (0.85–0.90) |
| Brachial fascia | Posterior | 0.88 (0.85–0.90) |
| Antebrachial fascia | Anterior | 0.89 (0.85–0.92) |
| Antebrachial fascia | Posterior | 0.88 (0.85–0.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Guidolin, D.; Fede, C.; Macchi, V.; De Caro, R.; Stecco, C. Ultrasound Imaging of Brachial and Antebrachial Fasciae. Diagnostics 2021, 11, 2261. https://doi.org/10.3390/diagnostics11122261
Pirri C, Guidolin D, Fede C, Macchi V, De Caro R, Stecco C. Ultrasound Imaging of Brachial and Antebrachial Fasciae. Diagnostics. 2021; 11(12):2261. https://doi.org/10.3390/diagnostics11122261
Chicago/Turabian StylePirri, Carmelo, Diego Guidolin, Caterina Fede, Veronica Macchi, Raffaele De Caro, and Carla Stecco. 2021. "Ultrasound Imaging of Brachial and Antebrachial Fasciae" Diagnostics 11, no. 12: 2261. https://doi.org/10.3390/diagnostics11122261
APA StylePirri, C., Guidolin, D., Fede, C., Macchi, V., De Caro, R., & Stecco, C. (2021). Ultrasound Imaging of Brachial and Antebrachial Fasciae. Diagnostics, 11(12), 2261. https://doi.org/10.3390/diagnostics11122261











