Diagnostic Value of CEUS Prompting Liver Biopsy: Histopathological Correlation of Hepatic Lesions with Ambiguous Imaging Characteristics
Abstract
1. Introduction
2. Materials and Methods
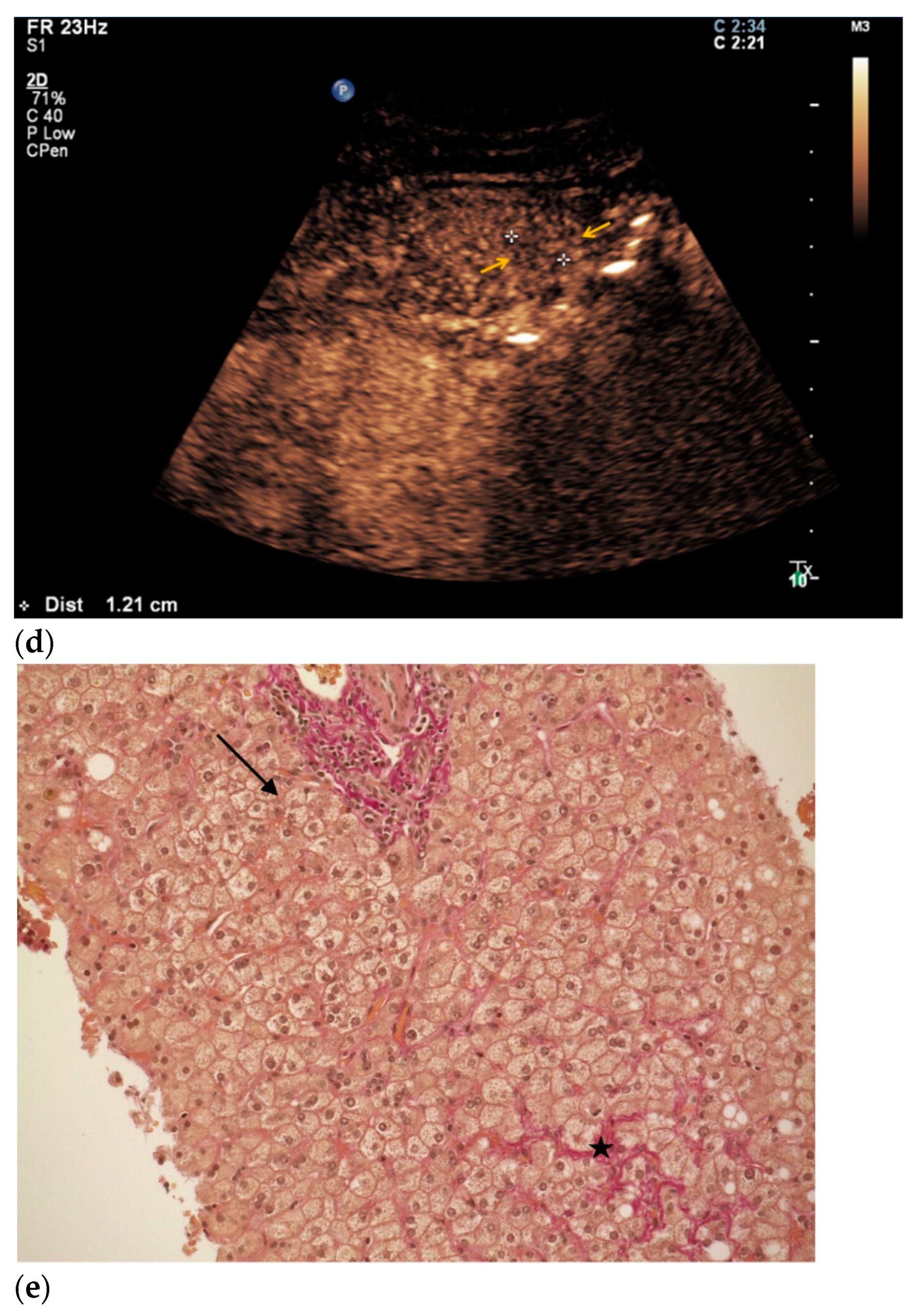
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.M.; Sirlin, C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part I. Development, growth, and spread: Key pathologic and imaging aspects. Radiology 2014, 272, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.M.; Sirlin, C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef]
- Rübenthaler, J.; Bogner, F.; Reiser, M.; Clevert, D.A. Contrast-Enhanced Ultrasound (CEUS) of the Kidneys by Using the Bosniak Classification. Ultraschall Med. 2016, 37, 234–251. [Google Scholar] [CrossRef]
- Rübenthaler, J.; Paprottka, K.; Marcon, J.; Hameister, E.; Hoffmann, K.; Joiko, N.; Reiser, M.; Clevert, D.-A. Comparison of magnetic resonance imaging (MRI) and contrast-enhanced ultrasound (CEUS) in the evaluation of unclear solid renal lesions. Clin. Hemorheol. Microcirc. 2016, 64, 757–763. [Google Scholar] [CrossRef]
- Rübenthaler, J.; Reiser, M.; Clevert, D.-A. Diagnostic vascular ultrasonography with the help of color Doppler and contrast-enhanced ultrasonography. Ultrasonography 2016, 35, 289–301. [Google Scholar] [CrossRef]
- Greis, C. Ultrasound contrast agents as markers of vascularity and microcirculation. Clin. Hemorheol. Microcirc. 2009, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clevert, D.A.; D’Anastasi, M.; Jung, E.M. Contrast-enhanced ultrasound and microcirculation: Efficiency through dynamics—Current developments. Clin. Hemorheol. Microcirc. 2013, 53, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Gassert, F.; Schnitzer, M.; Kim, S.H.; Kunz, W.G.; Ernst, B.P.; Clevert, D.-A.; Nörenberg, D.; Rübenthaler, J.; Froelich, M.F. Comparison of Magnetic Resonance Imaging and Contrast-Enhanced Ultrasound as Diagnostic Options for Unclear Cystic Renal Lesions: A Cost-Effectiveness Analysis. Ultraschall Med. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Bolondi, L. The safety of Sonovue in abdominal applications: Retrospective analysis of 23,188 investigations. Ultrasound Med. Biol. 2006, 32, 1369–1375. [Google Scholar] [CrossRef]
- Mueller-Peltzer, K.; Rübenthaler, J.; Reiser, M.; Clevert, D.-A. Contrast-enhanced ultrasound (CEUS) of the liver: Critical evaluation of use in clinical routine diagnostics. Radiology 2017, 57, 348–355. [Google Scholar]
- Kaltenbach, T.E.; Engler, P.; Kratzer, W.; Oeztuerk, S.; Seufferlein, T.; Haenle, M.M.; Graeter, T. Prevalence of benign focal liver lesions: Ultrasound investigation of 45,319 hospital patients. Abdom. Radiol. 2016, 41, 25–32. [Google Scholar] [CrossRef]
- Strobel, D.; Seitz, K.; Blank, W.; Schuler, A.; Dietrich, C.; Von Herbay, A.; Friedrich-Rust, M.; Kunze, G.; Becker, D.; Will, U.; et al. Contrast-enhanced Ultrasound for the Characterization of Focal Liver Lesions—Diagnostic Accuracy in Clinical Practice (DEGUM multicenter trial). Ultraschall Med. 2008, 29, 499–505. [Google Scholar] [CrossRef]
- Clevert, D.A.; Jung, E.; Stock, K.; Weckbach, S.; Feuerbach, S.; Reiser, M.; Jung, F. Evaluation of malignant liver tumors: Biphasic MS-CT versus quantitative contrast harmonic imaging ultrasound. Z. Gastroenterol. 2009, 47, 1195–1202. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Kono, Y.; Cosgrove, D.O.; Jang, H.-J.; Kim, T.K.; Piscaglia, F.; Sirlin, C.B.; Willmann, J.K.; Vezeridis, A.; Wilson, S.R.; et al. Contrast Enhanced Ultrasound: Liver Imaging Reporting and Data System (CEUS LI-RADS). Ultrasound Med. Biol. 2017, 43, S38–S39. [Google Scholar] [CrossRef]
- Seitz, K.; Strobel, D.; Bernatik, T.; Blank, W.; Friedrich-Rust, M.; Von Herbay, A.; Dietrich, C.F.; Strunk, H.; Kratzer, W.; Schuler, A. Contrast-Enhanced Ultrasound (CEUS) for the Characterization of Focal Liver Lesions—Prospective Comparison in Clinical Practice: CEUS vs. CT (DEGUM Multicenter Trial) Parts of this Manuscript were presented at the Ultrasound Dreiländertreffen 2008, Davos. Ultraschall Med. 2009, 30, 383–389. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C. Liver Tumor Characterization—Comments and Illustrations Regarding Guidelines. Ultraschall Med. 2012, 33 (Suppl. 1), S22–S30. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Cui, X.W.; Barreiros, A.P.; Hocke, M.; Ignee, A. EFSUMB Guidelines 2011: Comment on Emergent Indications and Visions. Ultraschall Med. 2012, 33 (Suppl. 1), S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Cui, X.W.; Boozari, B.; Hocke, M.; Ignee, A. Contrast-Enhanced Ultrasound (CEUS) in the Diagnostic Algorithm of Hepatocellular and Cholangiocellular Carcinoma, Comments on the AASLD Guidelines. Ultraschall Med. 2012, 33 (Suppl. 1), S57–S66. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Cui, X.W.; Schreiber-Dietrich, D.G.; Ignee, A. EFSUMB Guidelines 2011: Comments and Illustrations. Ultraschall Med. 2012, 33 (Suppl. 1), S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Badea, R.; Martie, A.; Dumitru, E.; Ioaniţescu, S.; Șirli, R.; Socaciu, M.A.; Popescu, A.; Danilă, M.; Voiculescu, M. Contrast Enhanced Ultrasound for the evaluation of focal liver lesions in daily practice. A multicentre study. Med Ultrason. 2012, 14, 95–100. [Google Scholar]
- Rübenthaler, J.; Paprottka, K.; Hameister, E.; Hoffmann, K.; Joiko, N.; Reiser, M.; Clevert, D. Vascular complications in liver transplantation: Beneficial role of contrast-enhanced ultrasound (CEUS) in the postoperative phase. Clin. Hemorheol. Microcirc. 2016, 64, 475–482. [Google Scholar] [CrossRef]
- Rübenthaler, J.; Paprottka, K.; Hameister, E.; Hoffmann, K.; Joiko, N.; Reiser, M.; Clevert, D.-A. Malignancies after liver transplantation: Value of contrast-enhanced ultrasound (CEUS). Clin. Hemorheol. Microcirc. 2016, 64, 467–473. [Google Scholar] [CrossRef]
- De Figueiredo, G.N.; Mueller-Peltzer, K.; Zengel, P.; Armbrüster, M.; Rübenthaler, J.; Clevert, D.-A. Diagnostic performance of contrast-enhanced ultrasound (CEUS) for the evaluation of gallbladder diseases1. Clin. Hemorheol. Microcirc. 2018, 69, 83–91. [Google Scholar] [CrossRef]
- Palmieri, V.O.; Santovito, D.; Marano, G.; Minerva, F.; Ricci, L.; D’Alitto, F.; Angelelli, G.; Palasciano, G. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. Radiol. Med. 2015, 120, 627–633. [Google Scholar] [CrossRef]
- Schwarze, V.; Marschner, C.; De Figueiredo, G.N.; Rübenthaler, J.; Clevert, D.-A. Single-Center Study: Evaluating the Diagnostic Performance and Safety of Contrast-Enhanced Ultrasound (CEUS) in Pregnant Women to Assess Hepatic Lesions. Ultraschall Med. 2019, 41, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, V.; Marschner, C.; Völckers, W.; Grosu, S.; De Figueiredo, G.N.; Rübenthaler, J.; Clevert, D.-A. Diagnostic value of contrast-enhanced ultrasound versus computed tomography for hepatocellular carcinoma: A retrospective, single-center evaluation of 234 patients. J. Int. Med Res. 2020, 48, 300060520930151. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, V.; Marschner, C.; Völckers, W.; De Figueiredo, G.N.; Rübenthaler, J.; Clevert, D.-Á. The diagnostic performance of contrast-enhanced ultrasound (CEUS) for evaluating hepatocellular carcinoma (HCC) juxtaposed to MRI findings; a retrospective single-center analysis of 292 patients. Clin. Hemorheol. Microcirc. 2020, 76, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, E.; Jang, H.J. Imaging findings of mimickers of hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 326–343. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, T.K.; Burns, P.N.; Wilson, S.R. Enhancement Patterns of Hepatocellular Carcinoma at Contrast-enhanced US: Comparison with Histologic Differentiation. Radiology 2007, 244, 898–906. [Google Scholar] [CrossRef]
- Brown, K.T.; Gandhi, R.T.; Covey, A.M.; Brody, L.A.; Getrajdman, G.I. Pylephlebitis and liver abscess mimicking hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2003, 2, 221–225. [Google Scholar]
- Mörk, H.; Ignee, A.; Schuessler, G.; Ott, M.; Dietrich, C.F. Analysis of neuroendocrine tumour metastases in the liver using contrast enhanced ultrasonography. Scand. J. Gastroenterol. 2007, 42, 652–662. [Google Scholar] [CrossRef]
- Ran, L.; Zhao, W.; Zhao, Y.; Bu, H. Value of contrast-enhanced ultrasound in differential diagnosis of solid lesions of pancreas (SLP): A systematic review and a meta-analysis. Medicine 2017, 96, e7463. [Google Scholar] [CrossRef]
- Schwarze, V.; Rübenthaler, J.; Marschner, C.; Fabritius, M.P.; Rueckel, J.; Fink, N.; Puhr-Westerheide, D.; Gresser, E.; Froelich, M.F.; Schnitzer, M.L.; et al. Advanced Fusion Imaging and Contrast-Enhanced Imaging (CT/MRI–CEUS) in Oncology. Cancers 2020, 12, 2821. [Google Scholar] [CrossRef]
- Seitz, K.; Strobel, D. A Milestone: Approval of CEUS for Diagnostic Liver Imaging in Adults and Children in the USA. Ultraschall Med. 2016, 37, 229–232. [Google Scholar] [CrossRef]
- Schwarze, V.; Froelich, M.F.; Marschner, C.; Knösel, T.; Rübenthaler, J.; Clevert, D.-A. Safe and pivotal approaches using contrast-enhanced ultrasound for the diagnostic workup of non-obstetric conditions during pregnancy, a single-center experience. Arch. Gynecol. Obstet. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
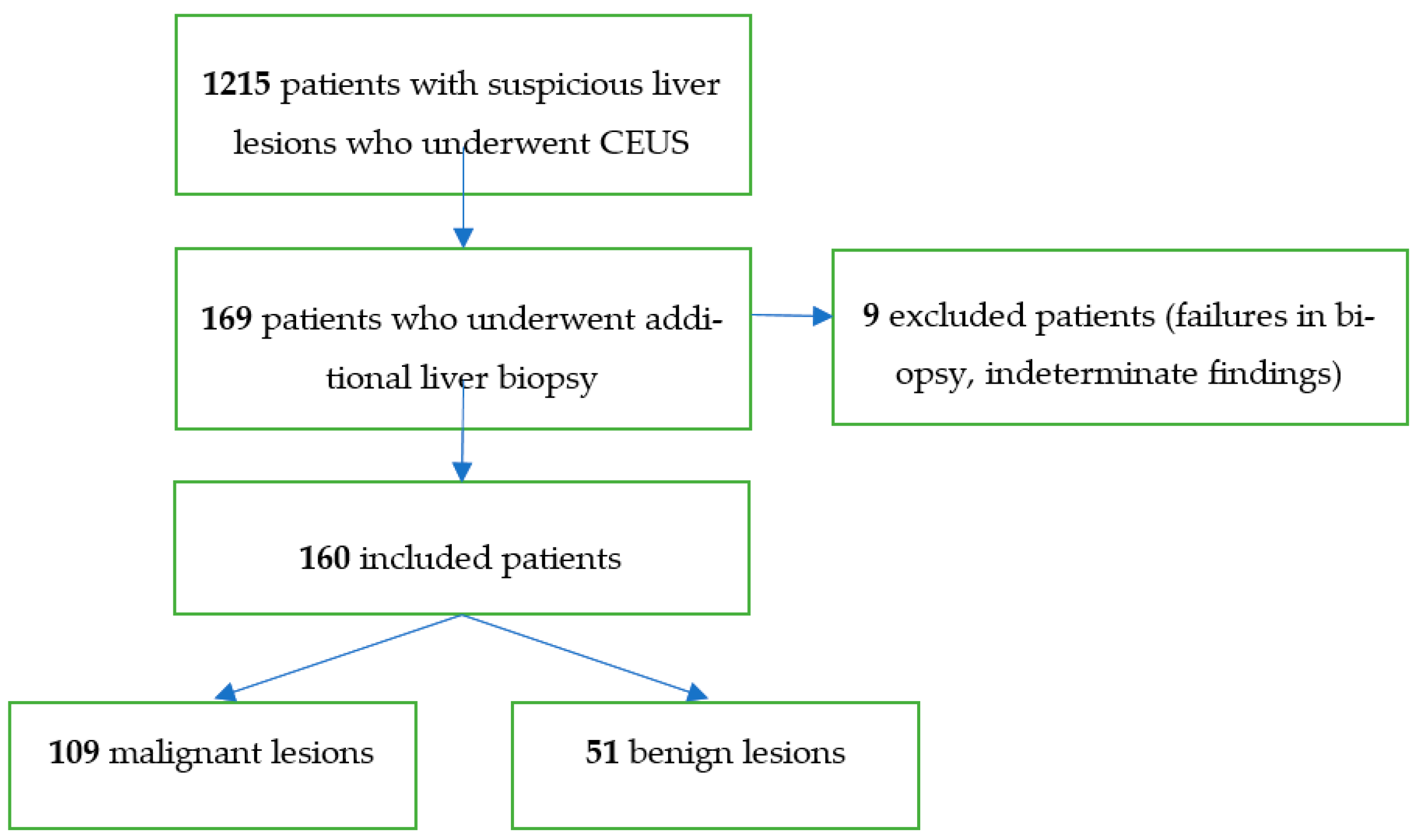
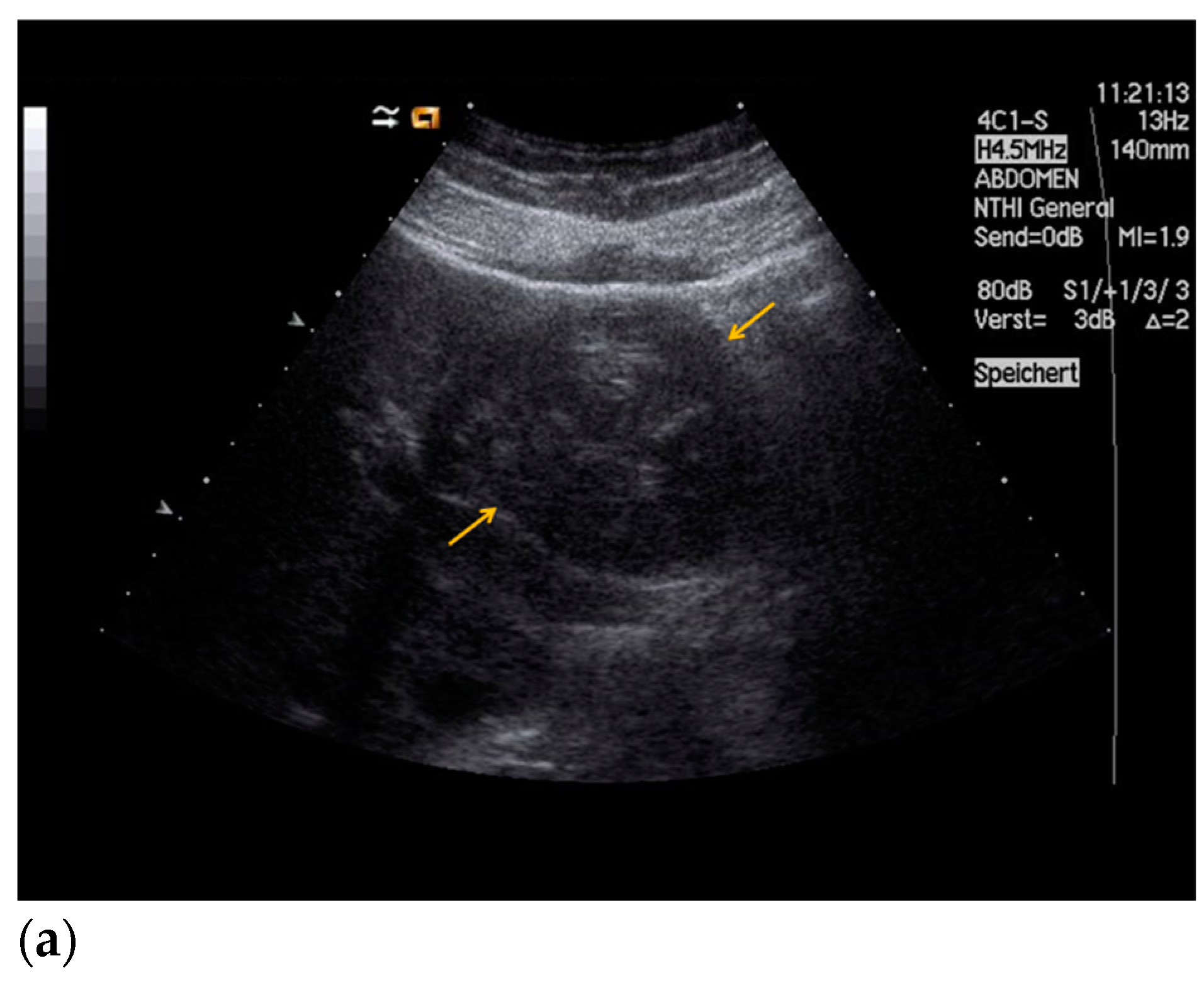
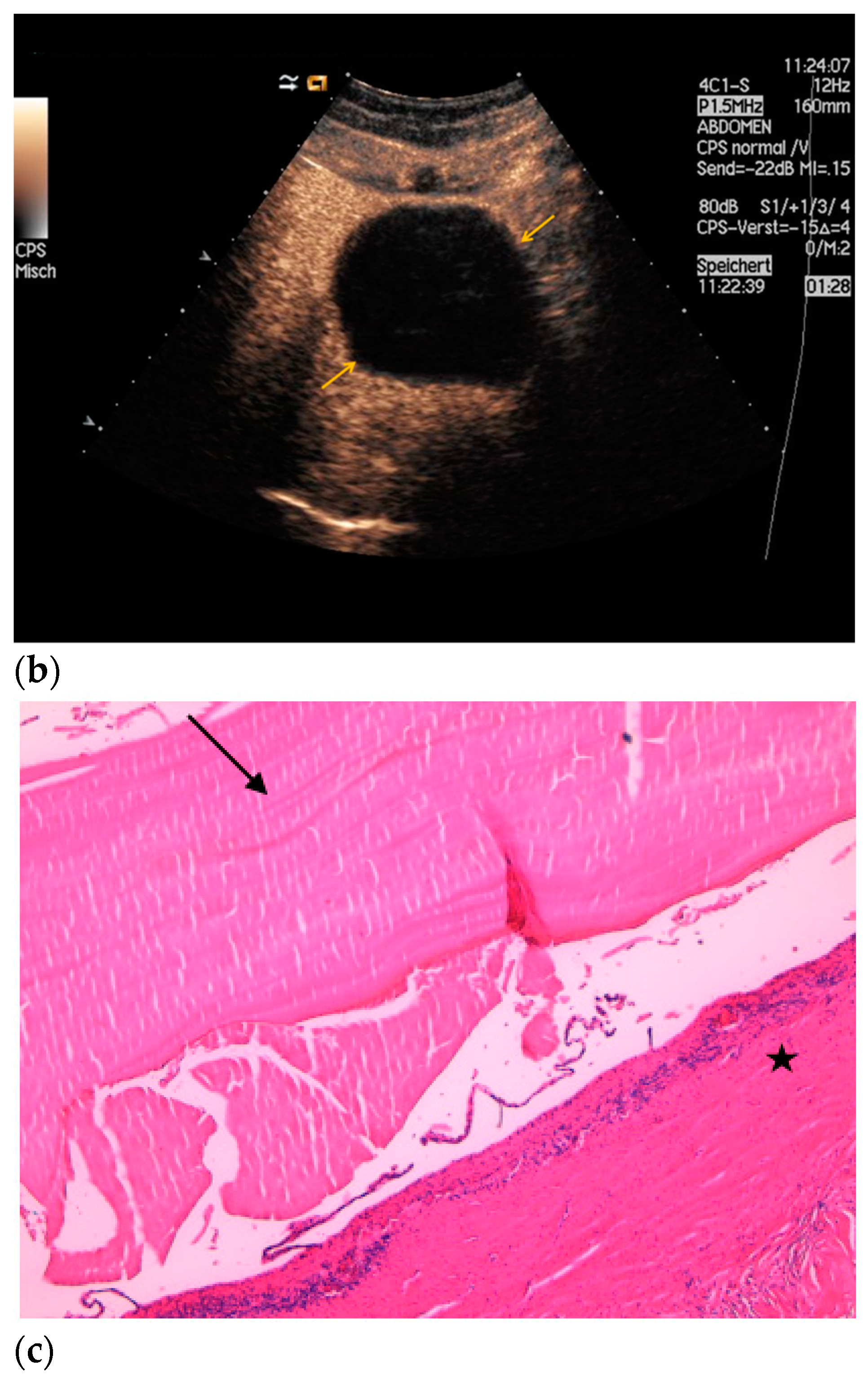
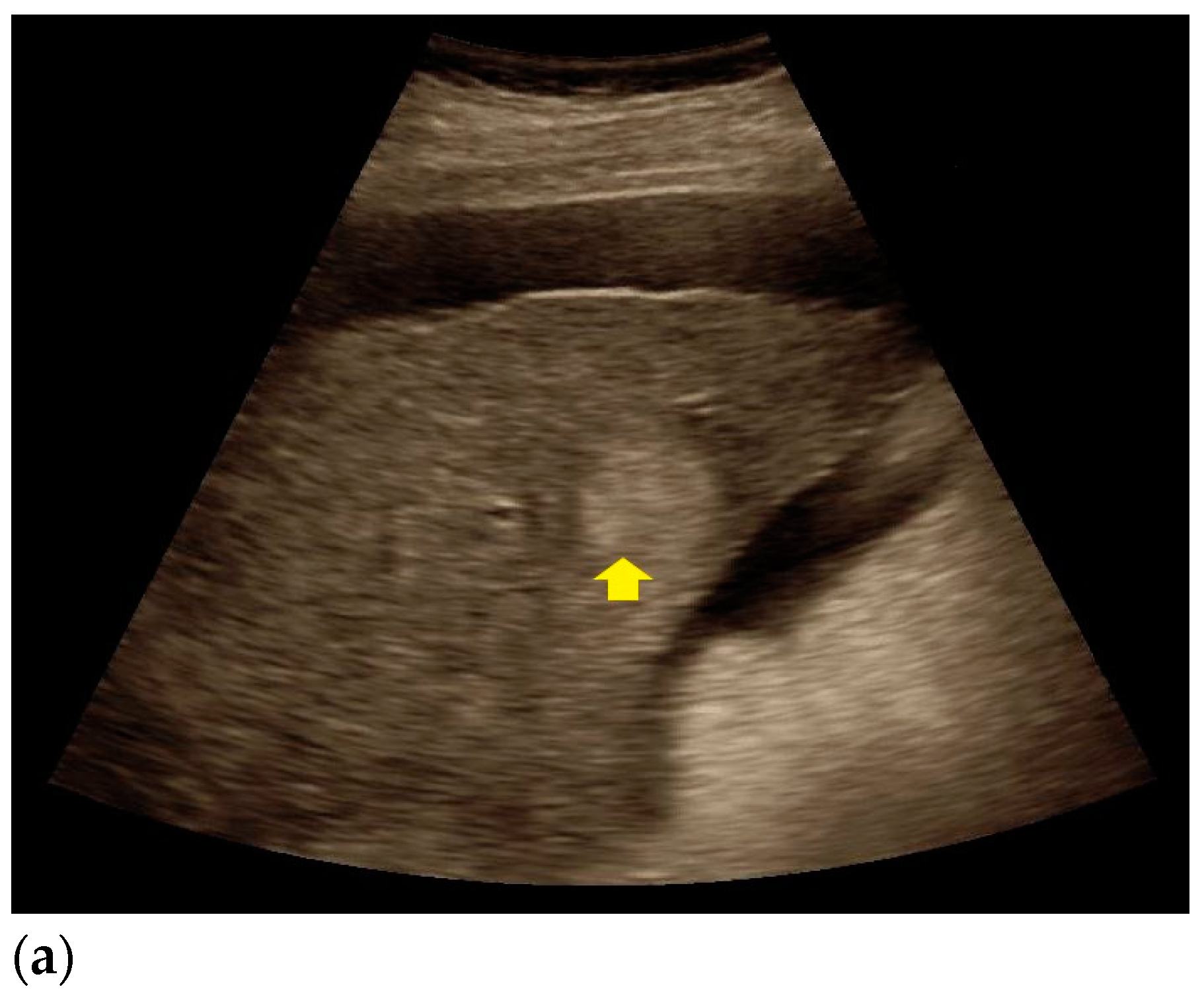
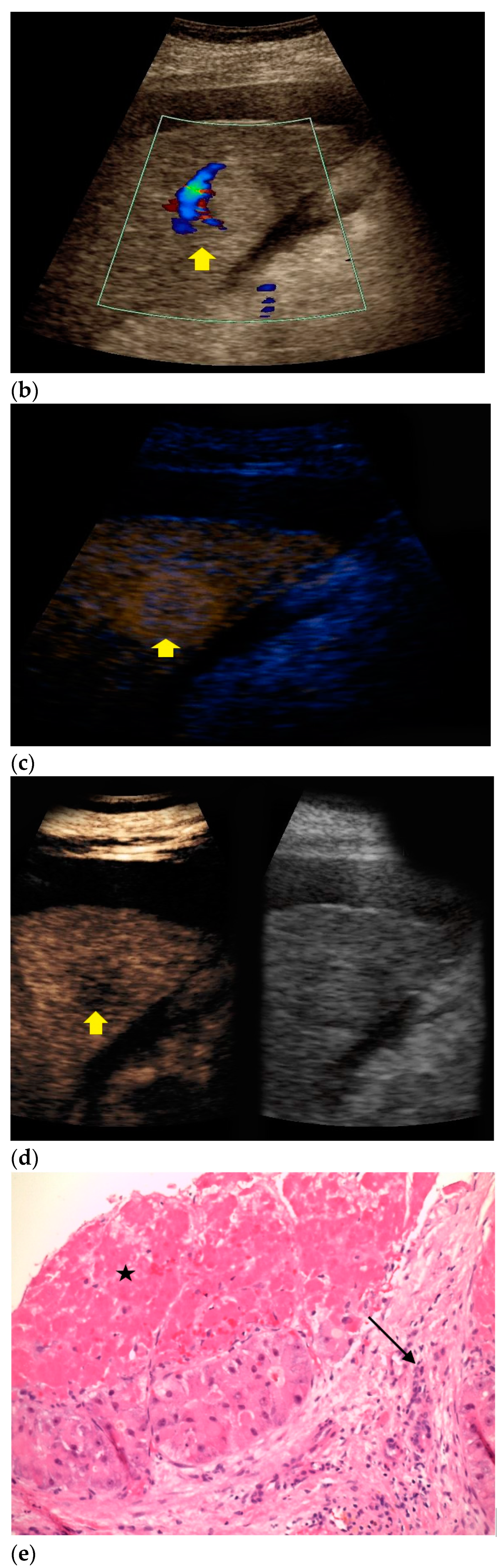
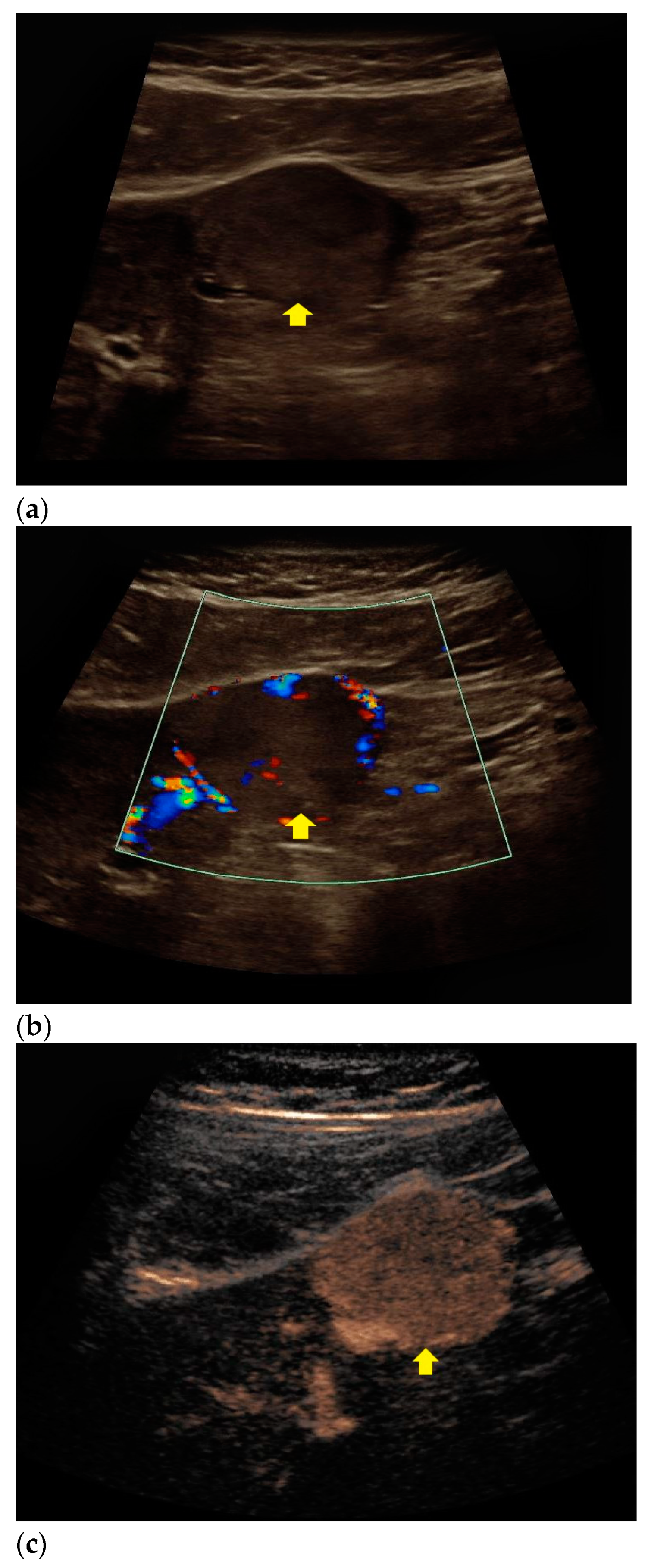
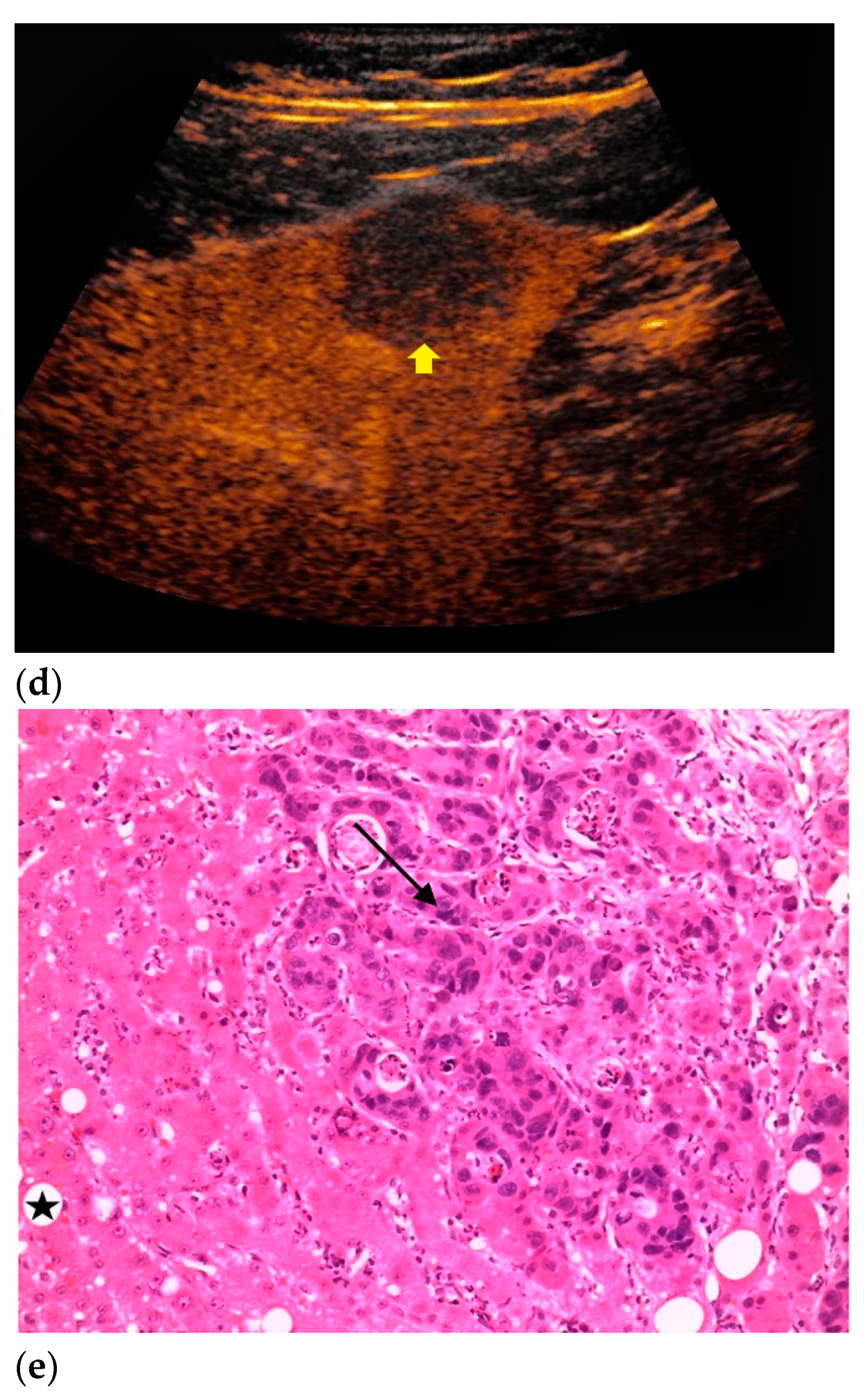
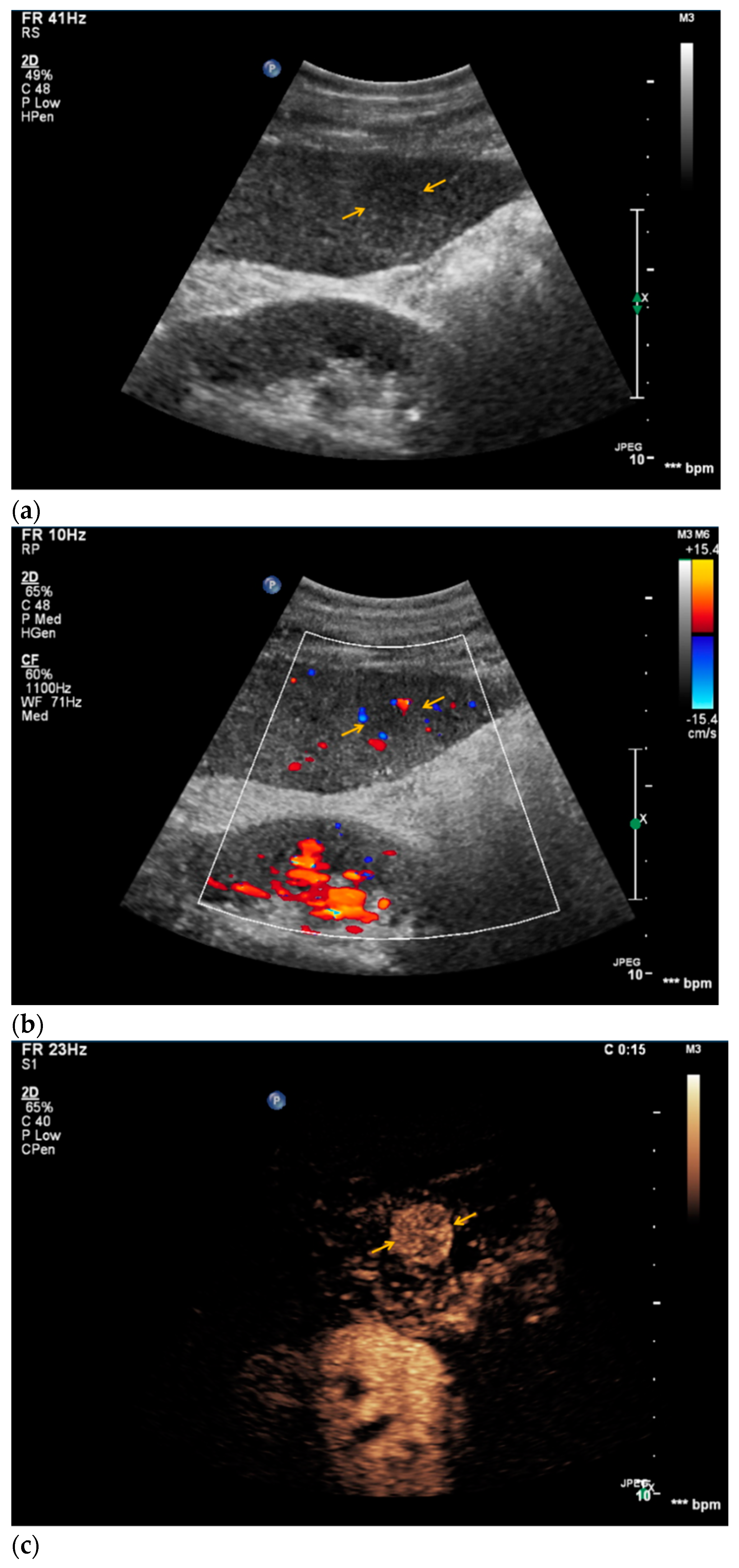
| Histopathology | Frequency |
|---|---|
| HCC | 38 |
| CCC | 4 |
| Metastasis | 59 |
| Lymphoma | 4 |
| HCC/CCC mixed type | 4 |
| Hemangioma | 3 |
| Cyst | 2 |
| Abscess | 3 |
| Hepatitis | 4 |
| Fibrosis | 10 |
| Cirrhosis | 9 |
| Candida infection | 1 |
| FNH | 2 |
| Hemangioma/FNH mixed type | 1 |
| Echinococcus cyst | 1 |
| Focal hepatic steatosis | 3 |
| Other reactive changes | 3 |
| Inconspicuous | 13 |
| Total (n = 160) | CEUS Positive | CEUS Negative | ||
|---|---|---|---|---|
| Sensitivity | ||||
| Malignant | n = 109 | 103 | 6 | 94.5% |
| Specificity | ||||
| Benign | n = 51 | 15 | 36 | 70.6% |
| TPR/ Concordance | TNR/ 1-Discordance | |||
| 87.3% | 85.7% |
| Misclassification | Histopathology |
|---|---|
| False positive | Fibrotic lesions: n = 7 |
| Cirrhotic lesions: n = 3 | |
| No pathologic changes: n = 2 | |
| Adenoma: n = 1 | |
| Reactive changes: n = 1 | |
| Abscess: n = 1 | |
| False negative | HCC: n = 4 |
| Neuroendocrine tumor (Metastasis): n = 1 | |
| Adenoma carcinoma (Metastasis): n = 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geyer, T.; Clevert, D.-A.; Schwarz, S.; Reidler, P.; Gassenmaier, S.; Knösel, T.; Rübenthaler, J.; Schwarze, V.; Armbruster, M. Diagnostic Value of CEUS Prompting Liver Biopsy: Histopathological Correlation of Hepatic Lesions with Ambiguous Imaging Characteristics. Diagnostics 2021, 11, 35. https://doi.org/10.3390/diagnostics11010035
Geyer T, Clevert D-A, Schwarz S, Reidler P, Gassenmaier S, Knösel T, Rübenthaler J, Schwarze V, Armbruster M. Diagnostic Value of CEUS Prompting Liver Biopsy: Histopathological Correlation of Hepatic Lesions with Ambiguous Imaging Characteristics. Diagnostics. 2021; 11(1):35. https://doi.org/10.3390/diagnostics11010035
Chicago/Turabian StyleGeyer, Thomas, Dirk-André Clevert, Sonja Schwarz, Paul Reidler, Sebastian Gassenmaier, Thomas Knösel, Johannes Rübenthaler, Vincent Schwarze, and Marco Armbruster. 2021. "Diagnostic Value of CEUS Prompting Liver Biopsy: Histopathological Correlation of Hepatic Lesions with Ambiguous Imaging Characteristics" Diagnostics 11, no. 1: 35. https://doi.org/10.3390/diagnostics11010035
APA StyleGeyer, T., Clevert, D.-A., Schwarz, S., Reidler, P., Gassenmaier, S., Knösel, T., Rübenthaler, J., Schwarze, V., & Armbruster, M. (2021). Diagnostic Value of CEUS Prompting Liver Biopsy: Histopathological Correlation of Hepatic Lesions with Ambiguous Imaging Characteristics. Diagnostics, 11(1), 35. https://doi.org/10.3390/diagnostics11010035






