Predicting Therapeutic Efficacy of Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. CEUS Imaging
2.3. CECT Imaging
2.4. DEB-TACE Procedure
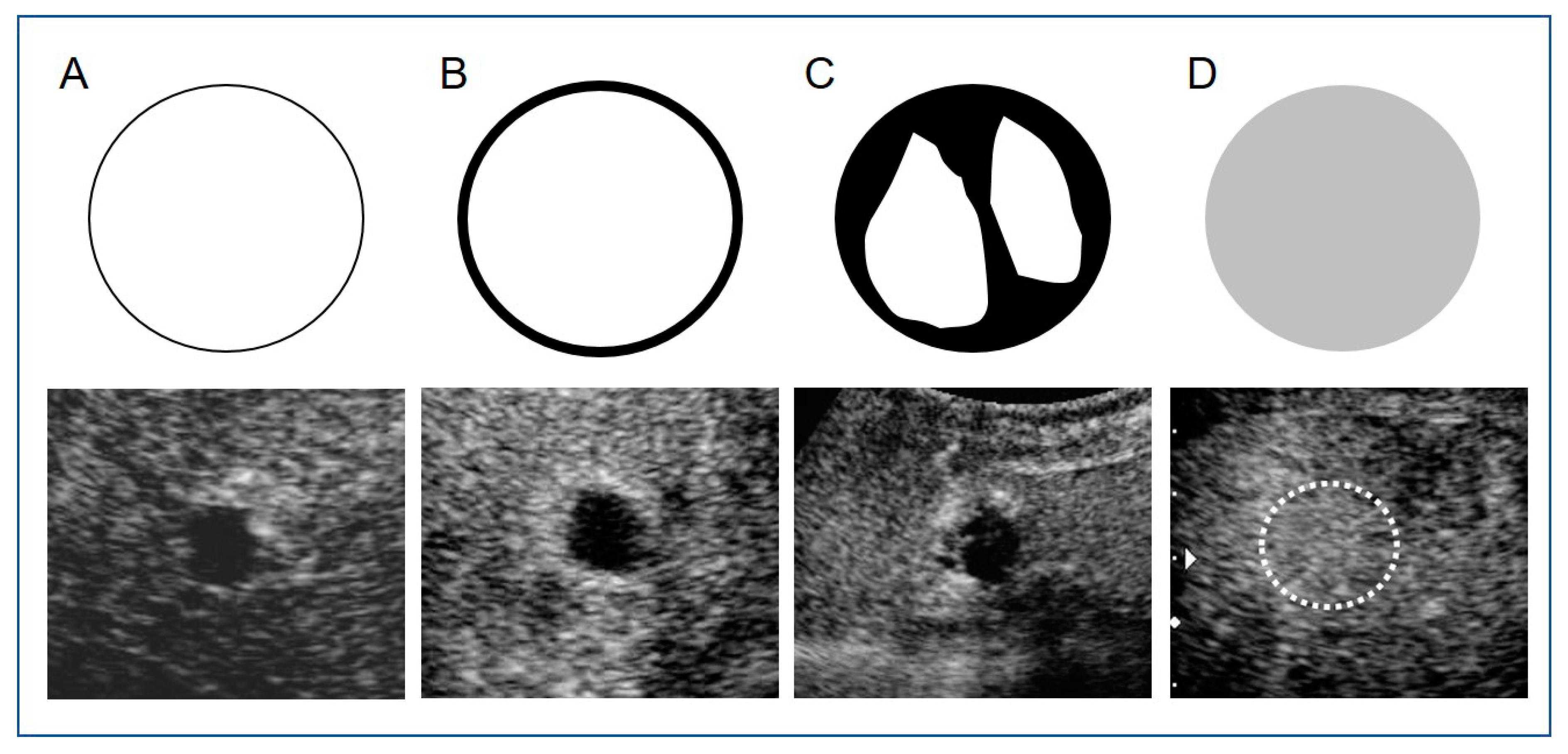
2.5. Image Analysis
2.6. Statistical Analysis
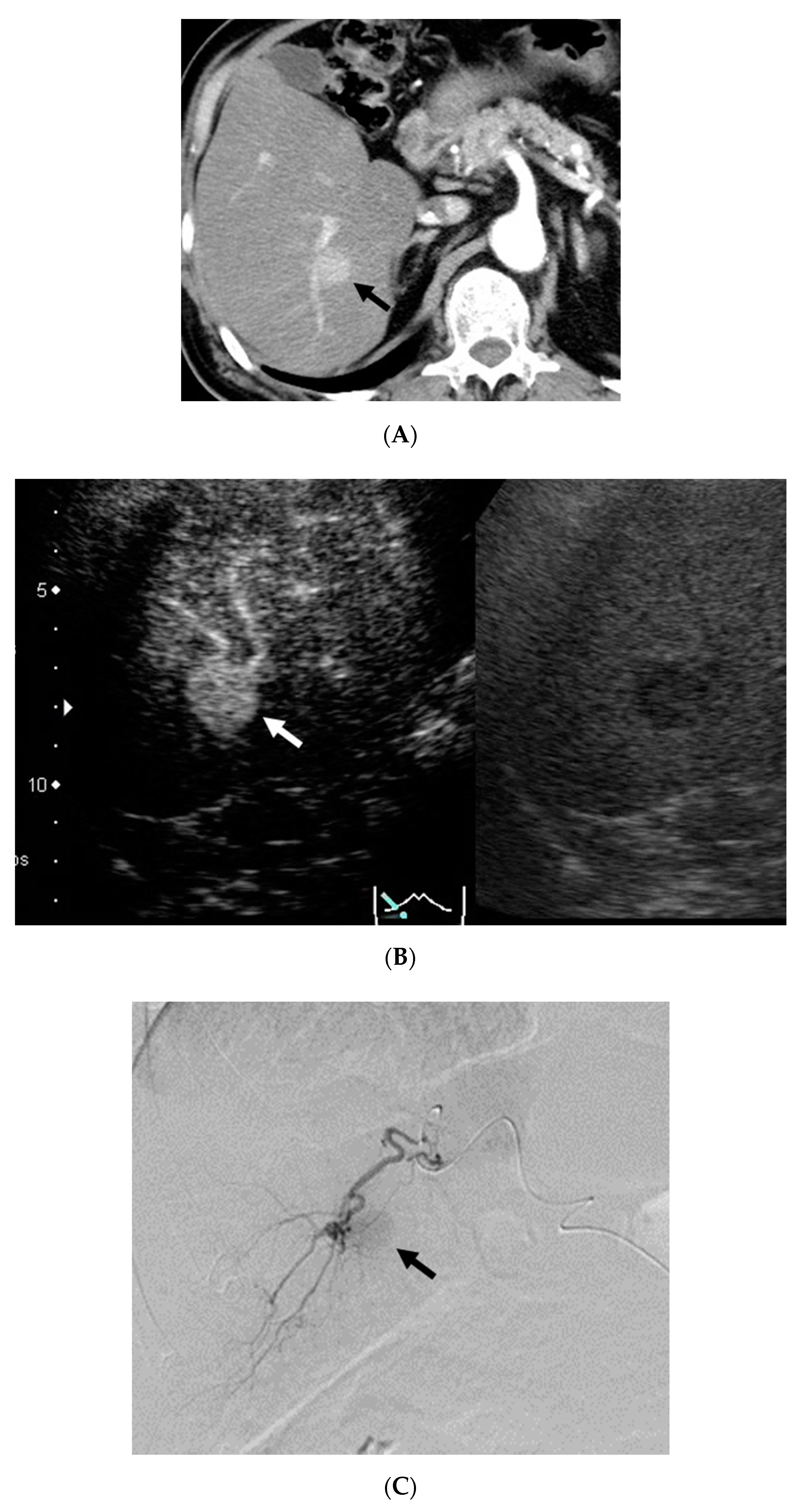
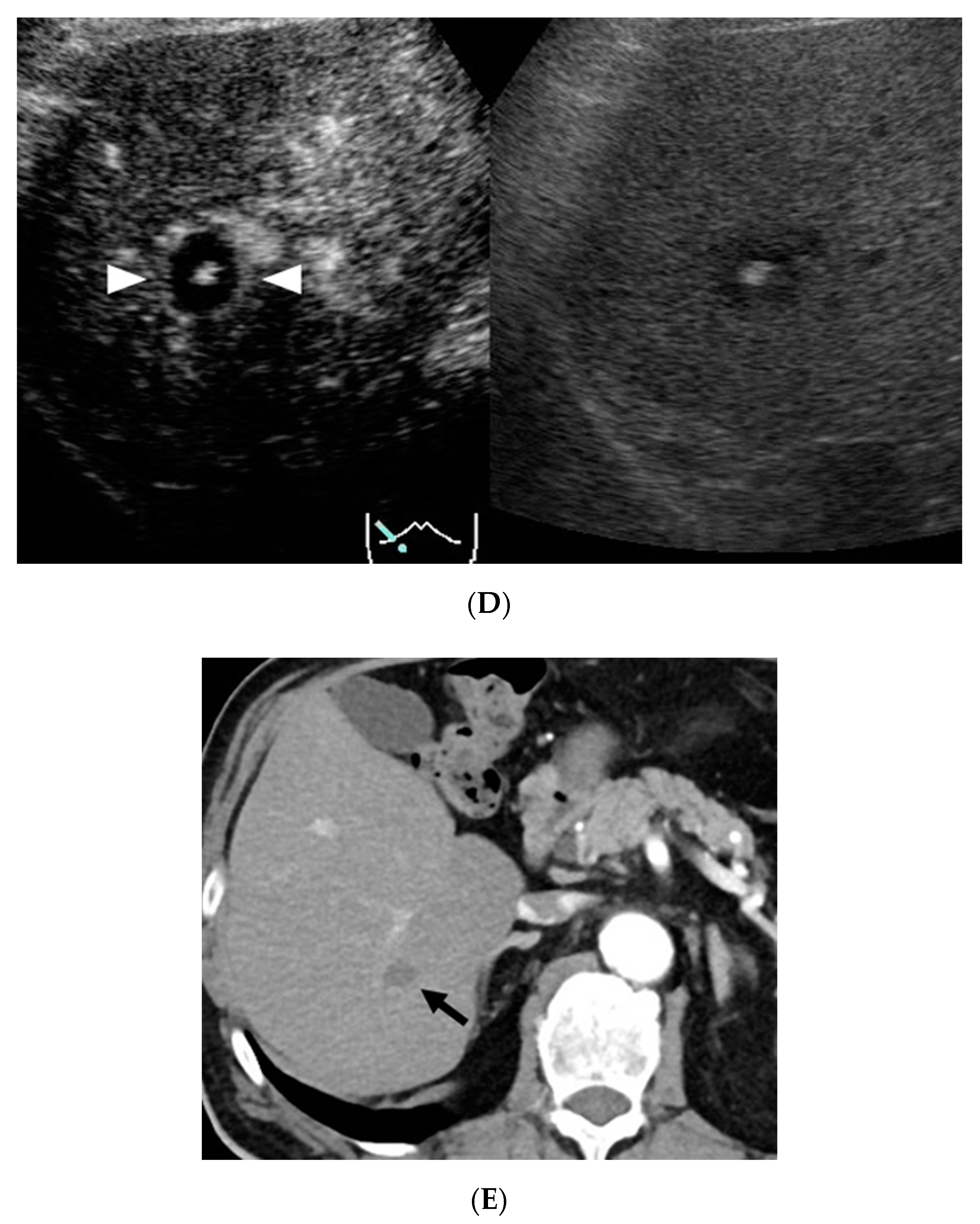
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kakeda, S.; Korogi, Y.; Ohnari, N.; Moriya, J.; Oda, N.; Nishino, K.; Miyamoto, W. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J. Vasc. Interv. Radiol. 2007, 18, 1508–1516. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Ikehara, T.; Shimizu, R.; Shinohara, M.; Igarashi, Y.; Sumino, Y. Evaluation of sorafenib for advanced hepatocellular carcinoma with low α-fetoprotein in arrival time parametric imaging using contrast-enhanced ultrasonography. J. Med. Ultrason. 2017, 44, 101–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiozawa, K.; Watanabe, M.; Ikehara, T.; Kobayashi, K.; Ochi, Y.; Suzuki, Y.; Fuchinoue, K.; Yoneda, M.; Kenmochi, T.; Okubo, Y.; et al. Evaluation of contrast-enhanced ultrasonography for hepatocellular carcinoma prior to and following stereotactic body radiation therapy using the CyberKnife® system: A preliminary report. Oncol. Lett. 2016, 11, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef]
- Lewis, A.L.; Gonzalez, M.V.; Leppard, S.W.; Brown, J.E.; Stratford, P.W.; Phillips, G.J.; Lloyd, A.W. Doxorubicin eluting beads-1: Effects of drug loading on bead characteristics and drug distribution. J. Mater. Sci. Mater. Med. 2007, 18, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Maluccio, M.; Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012, 62, 394–399. [Google Scholar] [CrossRef]
- Cazejust, J.; Bessoud, B.; Colignon, N.; Garcia-Alba, C.; Planché, O.; Menu, Y. Hepatocellular carcinoma vascularization: From the most common to the lesser known arteries. Diagn. Interv. Imaging 2014, 95, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Park, C.H.; Kim, J.D.; Kim, H.Y.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chun, H.J.; Choi, B.G.; Lee, H.G. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: A case-control study of Asian patients. Eur. J. Gastroenterol. Hepatol. 2011, 23, 521–527. [Google Scholar] [CrossRef]
- Kalva, S.P.; Iqbal, S.I.; Yeddula, K.; Blaszkowsky, L.S.; Akbar, A.; Wicky, S.; Zhu, A.X. Transarterial chemoemboliza tion with Doxorubicin-eluting microspheres for inoperable hepatocellular carcinoma. Gastrointest. Cancer Res. 2011, 4, 2–8. [Google Scholar]
- Chung, W.S.; Lee, K.H.; Park, M.S.; Lee, Y.J.; Kwon, J.; Baek, S.E.; Kim, M.J. Enhancement patterns of hepatocellular carcinoma after transarterial chemoembolization using drug-eluting beads on arterial phase CT images: A pilot retrospective study. Am. J. Roentgenol. 2012, 199, 349–359. [Google Scholar] [CrossRef]
- Shaw, C.M.; Eisenbrey, J.R.; Lyshchik, A.; O’Kane, P.L.; Merton, D.A.; Machado, P.; Pino, L.; Brown, D.B.; Forsberg, F. Contrast-enhanced ultrasound evaluation of residual blood flow to hepatocellular carcinoma after treatment with transarterial chemoembolization using drug-eluting beads: A prospective study. J. Ultrasound Med. 2015, 34, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carci noma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Kokudo, N.; Hasegawa, K.; Akahane, M.; Igaki, H.; Izumi, N.; Ichida, T.; Uemoto, S.; Kaneko, S.; Kawasaki, S.; Ku, Y.; et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol. Res. 2015, 45, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Kudo, M.; Hatanaka, K.; Maekawa, K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology 2010, 78 (Suppl. S1), 40–45. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Ikehara, T.; Yamamoto, S.; Matsui, T.; Saigusa, Y.; Igarashi, Y.; Maetani, I. Efficacy of intra-arterial contrast-enhanced ultrasonography during transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. World J. Hepatol. 2018, 10, 95–104. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Hammerstingl, R.; Huppertz, A.; Breuer, J.; Balzer, T.; Blakeborough, A.; Carter, R.; Fusté, L.C.; Heinz-Peer, G.; Judmaier, W.; Laniado, M.; et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: Comparison with intraoperative and histopathologic findings in focal liver lesions. Eur. Radiol. 2008, 18, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Akai, H.; Kiryu, S.; Matsuda, I.; Satou, J.; Takao, H.; Tajima, T.; Watanabe, Y.; Imamura, H.; Kokudo, N.; Akahane, M.; et al. Detection of hepatocellular carcinoma by Gd-EOB-DTPA-enhanced liver MRI: Comparison with triple phase 64 detector row helical CT. Eur. J. Radiol. 2011, 80, 310–315. [Google Scholar] [CrossRef]
- Mannelli, L.; Kim, S.; Hajdu, C.H.; Babb, J.S.; Clark, T.W.; Taouli, B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: Diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. Am. J. Roentgenol. 2009, 193, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Mannelli, L.; Hajdu, C.H.; Babb, J.S.; Clark, T.W.; Hecht, E.M.; Taouli, B. Hepatocellular carcinoma: Assessment of response to transarterial chemoembolization with image subtraction. J. Magn. Reson. Imaging 2010, 31, 348–355. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Grassi, C.J.; Cardella, J.F.; Charboneau, J.W.; Dodd, G.D., 3rd; Dupuy, D.E.; Gervais, D.; Gillams, A.R.; Kane, R.A.; Lee, F.T., Jr.; et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology 2005, 235, 728–739. [Google Scholar] [CrossRef]
| Characteristics | All (n = 32) |
|---|---|
| Age (years)(median) | 72 (range 44–89) |
| Gender Male/female | 28/4 |
| Etiology | |
| Alcohol/HBV/HCV/NASH | 14/4/12/2 |
| Child-Pugh classification A/B | 23/9 |
| Previous treatment y/n | 18/14 |
| Tumor number | 39 |
| Tumor size(mm) (median) | 21 (range 8–50) |
| DC Bead (100–300 μm, mL) (median) | 0.6 (range 0.2–1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiozawa, K.; Matsui, T.; Murakami, T.; Watanabe, M.; Maetani, I. Predicting Therapeutic Efficacy of Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound. Diagnostics 2021, 11, 291. https://doi.org/10.3390/diagnostics11020291
Shiozawa K, Matsui T, Murakami T, Watanabe M, Maetani I. Predicting Therapeutic Efficacy of Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound. Diagnostics. 2021; 11(2):291. https://doi.org/10.3390/diagnostics11020291
Chicago/Turabian StyleShiozawa, Kazue, Takashi Matsui, Takahiro Murakami, Manabu Watanabe, and Iruru Maetani. 2021. "Predicting Therapeutic Efficacy of Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound" Diagnostics 11, no. 2: 291. https://doi.org/10.3390/diagnostics11020291
APA StyleShiozawa, K., Matsui, T., Murakami, T., Watanabe, M., & Maetani, I. (2021). Predicting Therapeutic Efficacy of Transarterial Chemoembolization with Drug-Eluting Beads for Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound. Diagnostics, 11(2), 291. https://doi.org/10.3390/diagnostics11020291






