Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ultrasound (US) Examination
2.3. Reference Standard
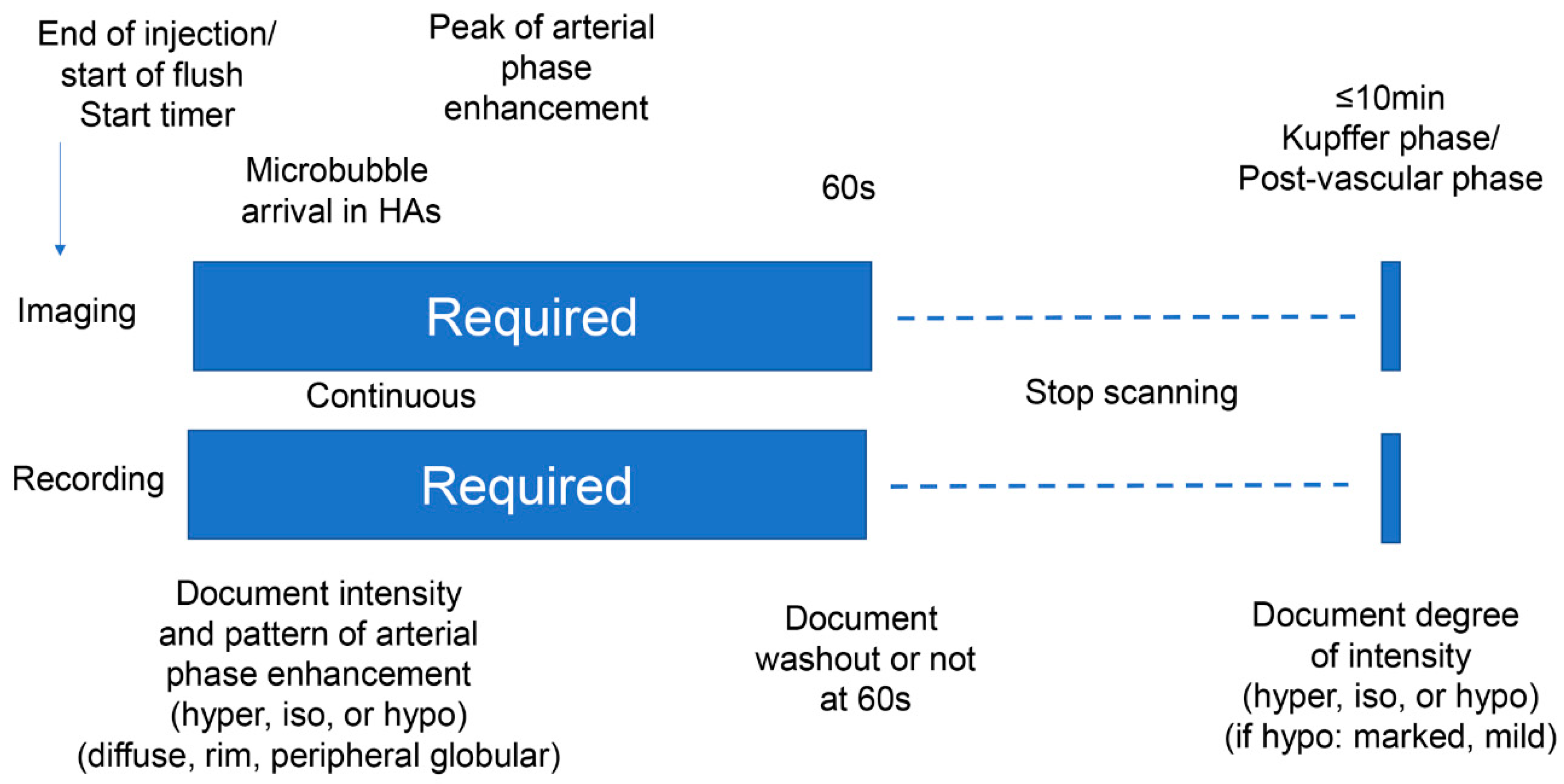
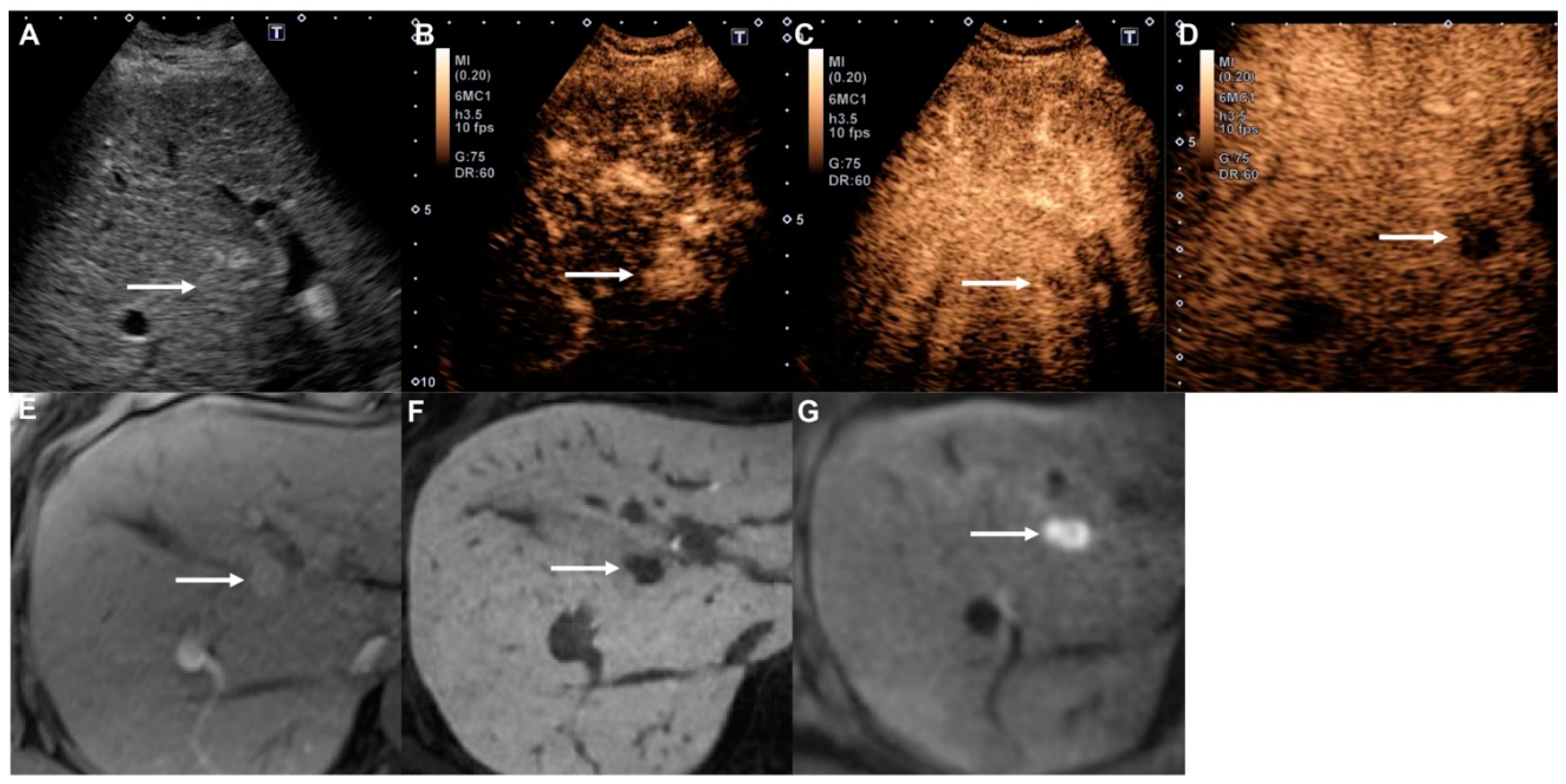
2.4. Contrast-Enhanced Ultrasound (CEUS) Image Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Distribution of Nodules in the Modified CEUS LI-RADS Categories
3.3. Imaging Characteristics of All Liver Nodules
3.4. Diagnostic Performance of the Modified CEUS LI-RADS LR-5 and LR-M Categories
3.5. Modified CEUS LI-RADS Categories and Degree of Histopathologic Differentiation of HCC
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [PubMed]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013, 34, 11–29. [Google Scholar] [PubMed] [Green Version]
- Italian Association for the Study of the Liver (AISF); AISF Expert Panel; AISF Coordinating Committee; Bolondi, L.; Cillo, U.; Colombo, M.; Craxì, A.; Farinati, F.; Giannini, E.G.; Golfieri, R.; et al. Position paper of the Italian Association for the Study of the Liver (AISF): The multidisciplinary clinical approach to hepatocellular carcinoma. Dig. Liver Dis. 2013, 45, 712–723. [Google Scholar]
- Omata, M.; Lesmana, L.A.; Tateishi, R.; Chen, P.J.; Lin, S.M.; Yoshida, H.; Kudo, M.; Lee, J.M.; Choi, B.I.; Poon, R.T.; et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010, 4, 439–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Vilana, R.; Forner, A.; Bianchi, L.; García-Criado, A.; Rimola, J.; de Lope, C.R.; Reig, M.; Ayuso, C.; Brú, C.; Bruix, J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010, 51, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Bernatik, T.; Blank, W.; Schuler, A.; Greis, C.; Dietrich, C.F.; Seitz, K. Diagnostic accuracy of CEUS in the differential diagnosis of small (≤20 mm) and subcentimetric (≤10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. 2011, 32, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Wang, W.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Xu, Z.F.; Chen, L.D.; Wang, Z.; Liang, J.Y.; Huang, Y.; et al. Contrast-enhanced ultrasound for the characterization of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Liver Cancer 2015, 4, 241–252. [Google Scholar] [CrossRef]
- Kono, Y.; Lyshchik, A.; Cosgrove, D.; Dietrich, C.F.; Jang, H.J.; Kim, T.K.; Piscaglia, F.; Willmann, J.K.; Wilson, S.R.; Santillan, C.; et al. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): The official version by the American College of Radiology (ACR). Ultraschall Med. 2017, 38, 85–86. [Google Scholar] [CrossRef] [Green Version]
- Terminology and Diagnostic Criteria Committee; Japan Society of Ultrasonics in Medicine. Ultrasound diagnostic criteria for hepatic tumors. J. Med. Ultrason. 2014, 41, 113–123. [Google Scholar] [CrossRef]
- Hatanaka, K.; Kudo, M.; Minami, Y.; Ueda, T.; Tatsumi, C.; Kitai, S.; Takahashi, S.; Inoue, T.; Hagiwara, S.; Chung, H.; et al. Differential diagnosis of hepatic tumors: Value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology 2008, 51 (Suppl. 1), 61–69. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, T.K.; Burns, P.N.; Wilson, S.R. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: Comparison with histologic differentiation. Radiology 2007, 244, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Numata, K.; Nakano, M.; Tanabe, M.; Chuma, M.; Nihonmatsu, H.; Nozaki, A.; Ogushi, K.; Luo, W.; Ruan, L.; et al. Diagnostic Value of Imaging Methods in the Histological Four Grading of Hepatocellular Carcinoma. Diagnostics 2020, 10, 321. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Li, C.; Wen, T.; Yan, L.; Yang, J. The impact of tumor differentiation on the prognosis of HBV-associated solitary hepatocellular carcinoma following hepatectomy: A propensity score matching analysis. Dig. Dis. Sci. 2018, 63, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Saito, K.; Yoshiara, H.; Imai, Y. Kupffer-phase findings of hepatic hemangiomas in contrast-enhanced ultrasound with Sonazoid. Ultrasound Med. Biol. 2014, 40, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.J.; Yu, J.S.; Cho, E.S.; Kim, J.H.; Chung, J.J. High-flow haemangiomas versus hypervascular hepatocellular carcinoma showing “pseudo-washout” on gadoxetic acid-enhanced hepatic MRI: Value of diffusion-weighted imaging in the differential diagnosis of small lesions. Clin. Radiol. 2017, 72, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Shunichi, S.; Hiroko, I.; Fuminori, M.; Waki, H. Definition of contrast enhancement phases of the liver using a perfluoro-based microbubble agent, perflubutane microbubbles. Ultrasound Med. Biol. 2009, 35, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Shiraishi, J.; Moriyasu, F.; Saito, K.; Doi, K. Improved detection of hepatic metastases with contrast-enhanced low mechanical-index pulse inversion ultrasonography during the liver-specific phase of Sonazoid: Observer performance study with JAFROC analysis. Acad. Radiol. 2009, 16, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Shiraishi, J.; Saito, K.; Taira, J.; Saguchi, T.; Imai, Y. Assessment of arterial hypervascularity of hepatocellular carcinoma: Comparison of contrast-enhanced US and gadoxetate disodium-enhanced MR imaging. Eur. Radiol. 2012, 22, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Kim, T.K.; Jang, H.J.; Burns, P.N. Enhancement patterns of focal liver masses: Discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am. J. Roentgenol. 2007, 189, W7–W12. [Google Scholar] [CrossRef] [PubMed]


| Rating Criteria with Nodule Size | Arterial Phase | Early Washout (<60 s) or Not | Kupffer Phase (≥10 min) |
|---|---|---|---|
| LR-5 | |||
| ≥1 cm | Hyperenhancement (not rim, not peripheral discontinuous globular hyperenhancement) | No | Hypoechoic |
| LR-M | |||
| Nodule size not considered | Rim hyperenhancement or not rim, not peripheral discontinuous globular hyperenhancement | Yes | Hypoechoic |
| LR-4 | |||
| ≥2 cm | No hyperenhancement | No | Hypoechoic |
| ≥1 cm | Not rim, not peripheral discontinuous globular hyperenhancement | No | Isoechoic or Hyperechoic |
| <1 cm | Not rim, not peripheral discontinuous globular hyperenhancement | No | Hypoechoic |
| LR-3 | |||
| <2 cm | No hyperenhancement | No | Any |
| ≥2 cm | No hyperenhancement | No | Isoechoic or Hyperechoic |
| <1 cm | Not rim, not peripheral discontinuous globular hyperenhancement | No | Isoechoic or Hyperechoic |
| LR-2 | |||
| <1 cm | Iso-enhancement | No | Isoechoic or Hyperechoic |
| LR-1 | |||
| Nodule size not considered | Definitely benign arterial phase pattern (cyst, hemangioma, focal fatty deposition/sparing, or other definitely benign finding) | No | N/A |
| Characteristic | Result |
|---|---|
| Median age (y) * | 70.0 (54.5–78.0) |
| Sex | |
| Male | 74 (71.2%) |
| Female | 30 (28.8%) |
| Median nodule size (mm) * | 17.9 (13.1–28.2) |
| Liver disease etiology | |
| HCV | 36 (34.6%) |
| HBV | 27 (26.0%) |
| Alcohol | 26 (25.0%) |
| NASH | 11 (10.6%) |
| HCV + HBV | 1 (1.0%) |
| AIH | 1 (1.0%) |
| Unknown | 2 (1.9%) |
| Presence of cirrhosis | 80 (87.9%) |
| Histopathologic analysis | |
| HCC | 64 (61.5%) |
| Well-differentiated | 28 |
| Moderately differentiated | 32 |
| Poorly differentiated | 4 |
| ICC | 6 (5.8%) |
| Metastasis | 9 (8.7%) |
| FNH | 5 (4.8%) |
| Dysplastic nodule | 3 (2.9%) |
| AML | 1 (1.0%) |
| Focal fatty change | 1 (1.0%) |
| Diffuse large B-cell lymphoma | 1 (1.0%) |
| Adrenal rest tumor | 1 (1.0%) |
| No histopathologic analysis | |
| Contrast-enhanced CT or MRI and follow-up | |
| Hemangioma | 10 (9.6%) |
| FNH | 2 (1.9%) |
| Focal spared lesion | 1 (1.0%) |
| Category | No. of Nodules (% of total) | Percentage HCC | Percentage Non-HCC Malignancy | Histopathologic Analysis | Contrast-Enhanced CT or MRI and Follow-Up |
|---|---|---|---|---|---|
| LR-1 | 7 (6.7) | 0 | 0 | 0 | 7 |
| LR-2 | 1 (1.0) | 0 | 0 | 1 | 0 |
| LR-3 | 10 (9.6) | 70.0 (7/10) | 0 | 9 | 1 |
| LR-4 | 16 (15.3) | 37.5 (6/16) | 0 | 13 | 3 |
| LR-5 | 48 (46.2) | 93.8 (45/48) | 0 | 46 | 2 |
| LR-M | 22 (21.2) | 27.3 (6/22) | 68.2 (15/22) | 22 | 0 |
| Total Nodules (104) n (%) | LR-1 7 (6.7) | LR-2 1 (1.0%) | LR-3 10 (9.6%) | LR-4 16 (15.3%) | LR-5 48 (46.2%) | LR-M 22 (21.2%) |
|---|---|---|---|---|---|---|
| HCC | 0 | 0 | 7 (70.0%) | 6 (37.5%) | 45 (93.8%) | 6 (27.3%) |
| Metastasis | 0 | 0 | 0 | 0 | 0 | 9 (40.9%) |
| ICC | 0 | 0 | 0 | 0 | 0 | 6 (27.3%) |
| Lymphoma | 0 | 0 | 0 | 0 | 0 | 1 (4.6%) |
| Hemangioma | 6 (85.7%) | 0 | 0 | 2 (12.5%) | 2 (4.2%) | 0 |
| FNH | 0 | 0 | 1 (10.0%) | 6 (37.5%) | 0 | 0 |
| DN | 0 | 0 | 2 (20.0%) | 1 (6.3%) | 0 | 0 |
| AML | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 |
| Adrenal rest tumor | 0 | 0 | 0 | 0 | 1 (2.1%) | 0 |
| Focal fatty area | 0 | 1 (100%) | 0 | 0 | 0 | 0 |
| Focal spread lesion | 1 (14.3%) | 0 | 0 | 0 | 0 | 0 |
| Imaging Features | Malignant Lesions | Benign Lesions | Total (n = 104) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC (n = 64) | Metastasis (n = 9) | ICC (n = 6) | Lymphoma (n = 1) | Hemangioma (n = 10) | FNH (n = 7) | DN (n = 3) | AML (n = 1) | Adrenal Rest Tumor (n = 1) | Focal Fatty Area (n = 1) | Focal Spared Lesion (n = 1) | ||
| Gray-scale echogenicity | ||||||||||||
| Hyperechoic | 13 | 3 | 1 | 0 | 2 | 3 | 1 | 0 | 1 | 1 | 0 | 25 |
| Isoechoic | 10 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 13 |
| Hypoechoic | 41 | 5 | 5 | 1 | 7 | 3 | 2 | 1 | 0 | 0 | 1 | 66 |
| Arterial phase | ||||||||||||
| Hyperenhancement | 56 | 2 | 5 | 1 | 7 | 7 | 1 | 1 | 1 | 0 | 0 | 81 |
| Diffuse | 56 | 2 | 4 | 1 | 4 | 7 | 1 | 1 | 1 | 0 | 0 | 77 |
| Rim | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Peripheral nodular | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Iso-enhancement | 7 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 14 |
| Hypo-enhancement | 1 | 3 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 9 |
| Kupffer phase | ||||||||||||
| Isoechoic | 11 | 0 | 0 | 0 | 7 | 5 | 3 | 1 | 0 | 1 | 1 | 29 |
| Hypoechoic | 53 | 9 | 6 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 73 |
| Marked | 16 | 9 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 |
| Mild | 37 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 41 |
| Hyperechoic | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Washout | ||||||||||||
| <60 s | 6 | 9 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| Criteria | Se (%) | 95% CI | Sp (%) | 95% CI | PPV (%) | 95% CI | NPV (%) | 95% CI | Accuracy (%) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| LR-5 | 70.3 | 57.6–81.1 | 92.5 | 79.6–98.4 | 93.8 | 82.8–98.7 | 66.1 | 52.2–78.2 | 78.7 | 69.7–86.2 |
| LR-M | 68.2 | 45.1–86.1 | 100 | 93.5–100 | 100 | 69.8–100 | 92.1 | 84.5–96.8 | 93.3 | 86.6–97.3 |
| CEUS LI-RADS | Well-Differentiated | Moderately Differentiated | Poorly Differentiated |
|---|---|---|---|
| LR-3 | 6 | 1 | 0 |
| LR-4 | 5 | 1 | 0 |
| LR-5 | 17 | 28 | 0 |
| LR-M | 0 | 2 | 4 |
| Total | 28 | 32 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, K.; Kakegawa, T.; Takahashi, H.; Tomita, Y.; Abe, M.; Yoshimasu, Y.; Takeuchi, H.; Kasai, Y.; Itoi, T. Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid. Diagnostics 2020, 10, 828. https://doi.org/10.3390/diagnostics10100828
Sugimoto K, Kakegawa T, Takahashi H, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Kasai Y, Itoi T. Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid. Diagnostics. 2020; 10(10):828. https://doi.org/10.3390/diagnostics10100828
Chicago/Turabian StyleSugimoto, Katsutoshi, Tatsuya Kakegawa, Hiroshi Takahashi, Yusuke Tomita, Masakazu Abe, Yu Yoshimasu, Hirohito Takeuchi, Yoshitaka Kasai, and Takao Itoi. 2020. "Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid" Diagnostics 10, no. 10: 828. https://doi.org/10.3390/diagnostics10100828
APA StyleSugimoto, K., Kakegawa, T., Takahashi, H., Tomita, Y., Abe, M., Yoshimasu, Y., Takeuchi, H., Kasai, Y., & Itoi, T. (2020). Usefulness of Modified CEUS LI-RADS for the Diagnosis of Hepatocellular Carcinoma Using Sonazoid. Diagnostics, 10(10), 828. https://doi.org/10.3390/diagnostics10100828





