A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography
Abstract
1. Introduction
2. Method
2.1. Subjects
2.2. Tests
2.3. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Diet
3.3. Performance
3.4. Echocardiographic Findings
4. Discussion
5. Conclusions
- A vegan diet does not result in impaired performance in amateur runners.
- Following a plant-based diet may influence both morphological and functional heart remodelling.
- The vegan diet may be associated with certain, most likely positive, characteristics in echocardiography (lower RWT, better LV systolic and diastolic function).
Author Contributions
Funding
Conflicts of Interest
References
- Parker, J. The Year of the Vegan. Econ. 2019. Available online: https://worldin2019.economist.com/theyearofthevegan?utm_source=412&utm_medium=COM (accessed on 5 June 2020).
- Forgrieve, J. The Growing Acceptance of Veganism. Forbes 2018. Available online: https://www.forbes.com/sites/janetforgrieve/2018/11/02/picturing-a-kindler-gentler-world-vegan-month/#5bdba7b02f2b (accessed on 5 June 2020).
- Pluim, B.M.; Zwinderman, A.H.; Van Der Laarse, A.; E Van Der Wall, E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Dores, H.; Mendes, L.; Dinis, P.; Cardim, N.; Monge, J.C.; Santos, J.F. Myocardial deformation and volume of exercise: A new overlap between pathology and athlete’s heart? Int. J. Cardiovasc. Imaging 2018, 34, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Simsek, Z.; Tas, M.H.; Degirmenci, H.; Yazıcı, A.G.; Duman, H.; Gundogdu, F.; Karakelleoglu, S.; Senocak, H. PP-059 Speckle tracking echocardiographic analysis of left ventricular SYSTOLIC and diastolic functions of young elite athletes with eccentric and concentric type of cardiac remodelling. Echocardiography 2013, 30, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Montesanti, D.; Autore, C.; Di Paolo, F.M.; Pisicchio, C.; Squeo, M.R.; Musumeci, B.; Spataro, A.; Pandian, N.G.; Pelliccia, A.; et al. Patterns of Left Ventricular Longitudinal Strain and Strain Rate in Olympic Athletes. J. Am. Soc. Echocardiogr. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Kerley, C.P. A Review of Plant-based Diets to Prevent and Treat Heart Failure. Card. Fail. Rev. 2018, 4, 54–61. [Google Scholar] [CrossRef]
- Tuso, P.; Stoll, S.; Li, W.W. A Plant-Based Diet, Atherogenesis, and Coronary Artery Disease Prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef]
- Parol, D. Ocena wpływu długoterminowego stosowania diety wegańskiej na wydolność fizyczną u osób amatorsko uprawiających biegi długodystansowe. Ph.D. Thesis, Medical University of Warsaw, Warsaw, Poland, 2018. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Mihl, C.; Dassen, W.R.M.; Kuipers, H. Cardiac remodelling: Concentric versus eccentric hypertrophy in strength and endurance athletes. Neth. Hear. J. 2008, 16, 129–133. [Google Scholar] [CrossRef]
- Nassenstein, K.; Breuckmann, F.; Lehmann, N.; Schmermund, A.; Hunold, P.; Broecker-Preuss, M.; Sandner, T.A.; Halle, M.; Mann, K.; Jockel, K.-H.; et al. Left ventricular volumes and mass in marathon runners and their association with cardiovascular risk factors. Int. J. Cardiovasc. Imaging 2009, 25, 71–79. [Google Scholar] [CrossRef]
- Neves, P.O.; Andrade, J.; Monção, H. Coronary artery calcium score: Current status. Radiol. Bras. 2017, 50, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Möhlenkamp, P.D.M.S.; Lehmann, N.; Breuckmann, F.; Bröcker-Preuss, M.; Nassenstein, K.; Halle, M.; Budde, T.; Mann, K.; Barkhausen, J.; Heusch, G.; et al. Running: The risk of coronary events: Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur. Heart J. 2008, 29, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Radford, N.; Defina, L.F.; Leonard, D.; E Barlow, C.; Willis, B.L.; Gibbons, L.W.; Gilchrist, S.C.; Khera, A.; Levine, B.D. Cardiorespiratory Fitness, Coronary Artery Calcium, and Cardiovascular Disease Events in a Cohort of Generally Healthy Middle-Age Men. Circulation 2018, 137, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.V.; Lavie, C.J.; Mehra, M.R.; Ventura, H.O.; Kurtz, J.D.; Messerli, F.H. Left Ventricular Geometry and Survival in Patients With Normal Left Ventricular Ejection Fraction. Am. J. Cardiol. 2006, 97, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; Stoicescu, C.; Magda, S.; Petcu, I.; Radu, M.; Palombo, C.; Cinteza, M.; Lichiardopol, R.; Vinereanu, D. “Supranormal” Cardiac Function in Athletes Related to Better Arterial and Endothelial Function. Echocardiography 2010, 27, 659–667. [Google Scholar] [CrossRef]
- Hejazi, K.; Hosseini, S.R.A. Influence of Selected Exercise on Serum Immunoglobulin, Testosterone and Cortisol in Semi-Endurance Elite Runners. Asian J. Sports Med. 2012, 3, 185–192. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23012638 (accessed on 26 July 2017).
- Nadimi, H.; Nejad, A.Y.; Djazayery, A.; Hosseini, M.; Hosseini, S. Association of vegan diet with RMR, body composition and oxidative stress. Acta Sci. Pol. Technol. Aliment. 2013, 12, 311–318. [Google Scholar]
- Carbone, S.; Billingsley, H.E.; Canada, J.M.; Kadariya, D.; De Chazal, H.M.; Rotelli, B.; Potere, N.; Paudel, B.; Markley, R.; Dixon, D.L.; et al. Unsaturated Fatty Acids to Improve Cardiorespiratory Fitness in Patients With Obesity and HFpEF: The UFA-Preserved Pilot Study. JACC Basic Transl. Sci. 2019, 4, 563–565. [Google Scholar]
- Dores, H.; Freitas, A.; Malhotra, A.; Mendes, M.; Sharma, S. The hearts of competitive athletes: An up-to-date overview of exercise-induced cardiac adaptations. Rev. Port. Cardiol. 2015, 34, 51–64. [Google Scholar] [CrossRef]
- Dawes, T.J.; Corden, B.; Cotter, S.; De Marvao, A.; Walsh, R.; Ware, J.S.; Cook, S.A.; O Regan, D.P. Moderate Physical Activity in Healthy Adults Is Associated With Cardiac Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004712. [Google Scholar] [CrossRef]
- Baggish, A.L.; Yared, K.; Wang, F.; Weiner, R.B.; Hutter, A.M.; Picard, M.H.; Wood, M.J. The impact of endurance exercise training on left ventricular systolic mechanics. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1109–H1116. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, A.; Grace, F.; Richards, J.; Hough, J.; Oxborough, D.; Sculthorpe, N. Left Ventricular Speckle Tracking-Derived Cardiac Strain and Cardiac Twist Mechanics in Athletes: A Systematic Review and Meta-Analysis of Controlled Studies. Sports Med. 2017, 47, 1145–1170. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Caselli, S.; Solari, M.; Pelliccia, A.; Cameli, M.; Focardi, M.; Padeletti, M.; Corrado, D.; Bonifazi, M.; Mondillo, S. Novel echocardiographic techniques for the evaluation of athletes’ heart: A focus on speckle-tracking echocardiography. Eur. J. Prev. Cardiol. Engl. 2016, 23, 437–446. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal Ranges of Left Ventricular Strain: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, P.G.; Jensen, M.T.; Biering-Sørensen, T.; Møgelvang, R.; Galatius, S.; Fritz-Hansen, T.; Rossing, P.; Vilsbøll, T.; Jensen, J.S. Cholesterol remnants and triglycerides are associated with decreased myocardial function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 137. [Google Scholar] [CrossRef]
- Król, W.; Jędrzejewska, I.; Konopka, M.; Burkhard-Jagodzińska, K.; Klusiewicz, A.; Pokrywka, A.; Chwalbińska, J.; Sitkowski, D.; Dłużniewski, M.; Mamcarz, A.; et al. Left Atrial Enlargement in Young High-Level Endurance Athletes—Another Sign of Athlete’s Heart? J. Hum. Kinet. 2016, 53, 81–90. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Focardi, M.; Mondillo, S. Atrial Enlargement in the Athlete’s Heart: Assessment of Atrial Function May Help Distinguish Adaptive from Pathologic Remodeling. J. Am. Soc. Echocardiogr. 2018, 31, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Nørregaard, L.; Thorsøe, F. The Effect of Carbohydrate Diet on Intermittent Exercise Performance. Int. J. Sports Med. 1992, 13, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.M.; Wharton, C.M.; Johnston, C.S. Cardiorespiratory Fitness and Peak Torque Differences between Vegetarian and Omnivore Endurance Athletes: A Cross-Sectional Study. Nutrients 2016, 8, 726. [Google Scholar] [CrossRef] [PubMed]
| Group | V | C |
|---|---|---|
| Age (years) | 32 ± 5 | 30 ± 5 |
| Height (cm) | 178.5 ± 7 | 180.5 ± 7 |
| Weight (kg) | 68.6 ± 7 * | 75.1 ± 6 * |
| BSA (m2) | 1.75 ± 0.1 * | 1.83 ± 0.1 * |
| BMI (kg/m2) | 21.6 ± 2.1 * | 23 ± 1.3 * |
| Weekly practice time (h) | 5.5 ± 4 | 4.9 ± 2 |
| Weekly distance (km) | 48.7 ± 3 | 48.5 ± 21 |
| Training experience (years) | 4.9 ± 4 | 3.9 ± 3 |
| Group | V | C |
|---|---|---|
| VO2max (L/min) | 3.70 ± 0.5 | 3.75 ± 0.6 |
| VO2 AT (L/min) | 2.24 ± 0.7 | 2.29 ± 0.7 |
| VO2max (mL/kg/min) | 54.0 ± 7.0 * | 50.1 ± 7.2 * |
| Power max (W) | 309 ± 36 | 324.2 ± 40.3 |
| Power AT (W) | 197 ± 48 | 216 ± 49 |
| HR max | 167 ± 29 | 168 ± 27 |
| HR AT | 144 ± 24 | 142 ± 19 |
| SBP max (mmHg) | 158 ± 30 | 157 ± 26 |
| DBP max (mmHg) | 86.8 ± 10 | 83 ± 21 |
| Group | V | C | p-Value |
|---|---|---|---|
| LVIDd (cm) | 5.12 ± 0.2 | 5.11 ± 0.2 | NS |
| LVIDd/BSA (cm/m2) | 2.93 ± 0.3 | 2.81 ± 0.2 | 0.04 |
| IVSd (cm) | 1.00 ± 0.10 | 1.08 ± 0.1 | 0.01 |
| IVSd/BSA (cm/m2) | 0.58 ± 0.1 | 0.59 ± 0.1 | NS |
| RVOT | 2.92 ± 0.2 | 2.89 ± 0.3 | NS |
| RVOT/BSA (cm/m2) | 2.3 ± 0.2 | 2.1 ± 0.2 | 0.003 |
| LVM (g) | 190 ± 34 | 210 ± 31 | 0.01 |
| LVMI (g/m2) | 108 ± 17 | 115 ± 14 | NS |
| RWT | 0.39 ± 0.07 | 0.42 ± 0.06 | 0.03 |
| LAV (mL) | 66.5 ± 19 | 74.6 ± 16 | NS |
| LAV/BSA (mL/m2) | 38 ± 10 | 40.3 ± 10 | NS |
| RAA/BSA (cm2/m2) | 11.9 ± 2.7 | 11.1 ± 2.2 | NS |
| Group | V | C | p-Value |
|---|---|---|---|
| E (m/s) | 0.87 ± 0.1 | 0.79 ± 0.1 | 0.02 |
| A (m/s) | 0.44 ± 1.1 | 0.44 ± 1.4 | NS |
| e′ ivs (cm/s) | 14.2 ± 2.7 | 14.3 ± 2.5 | NS |
| e′ lat (cm/s) | 19.6 ± 3.5 | 19.1 ± 3.1 | NS |
| E/e′ | 6.3 ± 1.3 | 5.60 ± 1 | 0.03 |
| TVs′ | 16.1 ± 2.8 | 15.4 ± 2.8 | NS |
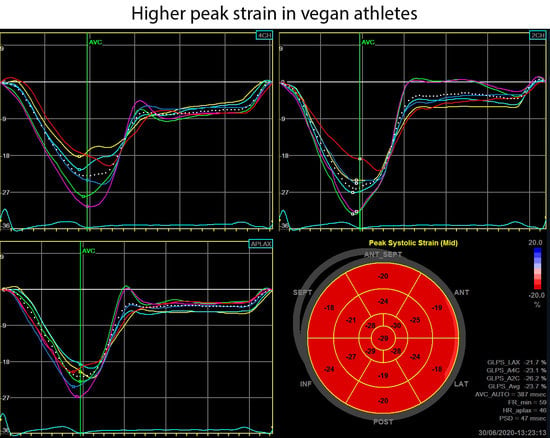
| Peak GLS (%) | 20.5 ± 2.2 | 19.6 ± 1.5 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, W.; Price, S.; Śliż, D.; Parol, D.; Konopka, M.; Mamcarz, A.; Wełnicki, M.; Braksator, W. A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography. Diagnostics 2020, 10, 477. https://doi.org/10.3390/diagnostics10070477
Król W, Price S, Śliż D, Parol D, Konopka M, Mamcarz A, Wełnicki M, Braksator W. A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography. Diagnostics. 2020; 10(7):477. https://doi.org/10.3390/diagnostics10070477
Chicago/Turabian StyleKról, Wojciech, Szymon Price, Daniel Śliż, Damian Parol, Marcin Konopka, Artur Mamcarz, Marcin Wełnicki, and Wojciech Braksator. 2020. "A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography" Diagnostics 10, no. 7: 477. https://doi.org/10.3390/diagnostics10070477
APA StyleKról, W., Price, S., Śliż, D., Parol, D., Konopka, M., Mamcarz, A., Wełnicki, M., & Braksator, W. (2020). A Vegan Athlete’s Heart—Is It Different? Morphology and Function in Echocardiography. Diagnostics, 10(7), 477. https://doi.org/10.3390/diagnostics10070477