Determinants of the Cardiovascular Capacity of Amateur Long-Distance Skiers during the Transition Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric Measurements
2.3. Measurement of Aerobic Capacity (VO2 max Test)
2.4. Venous Blood Sampling and Analysis
2.5. Statistics
3. Results
3.1. Hematological Parameters of Participants
3.2. Correlations for Independent Variables
3.3. Regression Model
4. Discussion
4.1. Predictors for VO2 max
4.1.1. Monocytes
4.1.2. Sodium
4.1.3. Calcium
5. Conclusions
Funding
Conflicts of Interest
References
- Sandbakk, Ø.; Holmberg, H.C. Physiological Capacity and Training Routines of Elite Cross-Country Skiers: Approaching the Upper Limits of Human Endurance. Int. J. Sports Physiol. Perform. 2017, 12, 1003–1011. [Google Scholar] [CrossRef]
- Carlsson, M.; Assarsson, H.; Carlsson, T. The influence of sex, age, and race experience on pacing profiles during the 90 km Vasaloppet ski race. J. Sports Med. 2016, 7, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Skattebo, Ø.; Losnegard, T.; Stadheim, H.K. Double-Poling Physiology and Kinematics of Elite Cross-Country Skiers: Specialized Long-Distance Versus All-Round Skiers. Int. J. Sports Physiol. Perform. 2019, 14, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, N. Cardiovascular Adaptations to Four Months Training in Middle-Aged Amateur Long-Distance Skiers. Diagnostics 2020, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic, A.; Stankovic, D.; Ilic, D.; Joksimovic, I.; Jerkan, M. Hematological Profile of Serbian Youth National Soccer Teams. J. Hum. Kinet. 2009, 22, 51–60. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Protosygellou, M.; Petridou, A.; Tsalis, G.; Tsigilis, N.; Mougios, V. Hematologic and biochemical profile of juvenile and adult athletes of both sexes: Implications for clinical evaluation. Int. J. Sports Med. 2003, 24, 506–511. [Google Scholar]
- Manna, I.; Khanna, G.; Chandra Dhara, P. Effect of training on physiological and biochemical variables of soccer players of different age groups. Asian J. Sports Med. 2010, 1, 5–22. [Google Scholar] [CrossRef]
- Baffour-Awuah, B.; Addai-Mensah, O.; Moses, M.; Mensah, W.; Ibekwe, B.; Essaw, E.; Acheampong, I. Differences in Haematological and Biochemical Parameters of Athletes and Non-Athletes. JAMMR 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Gallagher, D.; Heymsfield, S.; Heo, M.; Jebb, S.; Murgatroyd, P.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef]
- Grzebisz, N.; Piejko, L.; Sulich, A. Determinants of cardiorespiratory fitness in amateur male cross-country skiers. Russ. J. Cardiol. 2019, 12, 109–113. [Google Scholar] [CrossRef]
- Undebakke, V.; Berg, J.; Tjønna, A.E.; Sandbakk, Ø. Comparison of Physiological and Perceptual Responses to Upper-, Lower-, and Whole-Body Exercise in Elite Cross-Country Skiers. J. Strength Cond. Res. 2019, 33, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.S.; Sampaio, N.L.F.; Cruz, C.J.G.; Vianna, B.; Pires, F.O. Haematological Responses on Amateur Cycling Stages Race. In Proceedings of the ICCSS 2018: International Conference on Cycle and Sports Science, Sydney, Australia, 29–30 January 2018. [Google Scholar]
- Diaz, E.; Ruiz, F.; Hoyos, I.; Zubero, J.; Gravina, L.; Gil, J.; Irazusta, J.; Gil, S.M. Cell damage, antioxidant status, and cortisol levels related to nutrition in ski mountaineering during a two-day race. J. Sports Sci. Med. 2010, 9, 338–346. [Google Scholar]
- Tayebi, S.; Ghanbari-Niaki, A. Effects of a low intensity circuit resistance exercise session on some hematological parameters of male collage students. Ann. Appl. Sport Sci. 2013, 1, 6–11. [Google Scholar]
- Kurowski, M.; Kowalski, M. Wpływ wysiłku fizycznego na odpowiedź immunologiczną–wybrane zagadnienia. Alerg. Astma Immunol. 2014, 19, 144–149. [Google Scholar]
- Gleeson, M.; Bishop, N.; Oliveira, M.; Tauler, P. Influence of training load on upper respiratory tract infection incidence and antigen-stimulated cytokine production. Scand. J. Med. Sci. Sports 2013, 23, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.; Roitt, I.M. Encyclopedia of Immunology, 2nd ed.; Academic Press: Michigan University, Ann Arbor, MI, USA, 1998; pp. 1793–1802. [Google Scholar]
- Geleijnse, J.; Kok, F.; Grobbee, D. Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. J. Hum. Hypertens. 2003, 17, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Chlíbková, D.; Nikolaidis, P.; Rosemann, T.; Knechtle, B.; Bednář, J. Fluid Metabolism in Athletes Running Seven Marathons in Seven Consecutive Days. Front. Physiol. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Baker, L. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, A.; Cox, G.R.; Costa, R. Sodium Intake Beliefs, Information Sources, and Intended Practices of Endurance Athletes Before and During Exercise. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Wentz, L.; Liu, P.; Ilich, J.; Haymes, E. Dietary and training predictors of stress fractures in female runners. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Moe, S. Disorders involving calcium, phosphorus, and magnesium. Prim. Care 2008, 35, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Ulimoen, S.; Enger, S.; Pripp, A.; Abdelnoor, M.; Arnesen, H.; Gjesdal, K.; Tveit, A. Calcium channel blockers improve exercise capacity and reduce N-terminal Pro-B-type natriuretic peptide levels compared with beta-blockers in patients with permanent atrial fibrillation. Eur. Heart J. 2014, 35, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.; Cohen, A. Review of WADA Prohibited Substances: Limited Evidence for Performance-Enhancing Effects. Sports Med. 2019, 49, 525–539. [Google Scholar] [CrossRef]
- Boisseau, N. Fat mass reduction and weight loss: Strategies and potential risk in Olympic athletes. In Proceedings of the Sport Nutrition Conference, INSEP, Paris, France, 7 May 2011. [Google Scholar]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing 2017, 14, 7. [Google Scholar] [CrossRef]

| Coefficient (r) | Interpretation |
|---|---|
| no correlation | |
| weak correlation | |
| average correlation | |
| strong correlation | |
| very strong correlation |
| Variable | Arithmetic Average (N = 16) Means ± SD (Difference Δ—Delta) |
|---|---|
| Age (years) | 38.69 ± 7.95 (28.00–56.00) |
| Body height (cm) | 181.44 ± 6.53 (169.00–197.00) |
| Body mass (kg) | 78.52 ± 6.18 (68.10–91.50) |
| Fat mass (kg) | 12.22 ± 2.53 (7.90–16.00) |
| Fat mass (%) | 15.51 ± 2.59 (10.00–19.30) |
| BMI (kg/m2) | 23.84 ± 1.35 (21.00–25.70) |
| VO2 max (mL/kg/min) | 48.37 ± 5.06 (38.54–55.81) |
| Morphology | Mean | Standard Deviation | Min | Max |
|---|---|---|---|---|
| Leukocytes (thou/µL) | 5.4 | 0.88 | 4.2 | 9.1 |
| Erythrocytes(M/µL) | 5.06 | 0.29 | 4.2 | 6 |
| Hemoglobin (g/dL) | 15.19 | 0.5 | 14 | 18 |
| Hematocrit % | 43.86 | 1.53 | 40 | 51 |
| Mean corpuscular value (MCV) (fL) | 88.03 | 3.41 | 80 | 99 |
| Mean corpuscular hemoglobin (MCH) (pg) | 30.49 | 1.01 | 27 | 35 |
| Mean corpuscular hemoglobin concentration (MCHC) (g/dL) | 34.66 | 0.93 | 32 | 37 |
| Platelets (thou/µL) | 214.06 | 35.74 | 140 | 440 |
| Red blood cell distribution width-standard deviation (RDW-SD) (fL) | 40.77 | 2.97 | 35.1 | 43.9 |
| Red blood cell distribution width-coefficient of variation (RDW-CV) % | 12.98 | 0.79 | 11.6 | 14.4 |
| Platelet distribution width (PDW) (fL) | 13.45 | 2.14 | 9.8 | 16.1 |
| Mean platelet volume (MPV) (fL) | 10.64 | 1.06 | 9 | 13 |
| Platelet-large cell ratio (P-LCR) % | 31.83 | 8.69 | 13 | 43 |
| Procalcitonin (PCT) % | 0.21 | 0.04 | 0.2 | 0.4 |
| Neutrophils (thou/µL) | 2.7 | 0.55 | 2 | 7 |
| Lymphocytes (thou/µL) | 2.04 | 0.56 | 1 | 3.5 |
| Monocytes (thou/µL) | 0.45 | 0.09 | 0.2 | 1 |
| Eosinophils (thou/µL) | 0.25 | 0.19 | 0.1 | 0.5 |
| Basophils (thou/µL) | 0.03 | 0.03 | 0 | 0.1 |
| Neutrophils % | 49.69 | 7.91 | 40 | 70 |
| Lymphocytes % | 38.26 | 7.43 | 20 | 45 |
| Eosinophils % | 4.46 | 2.83 | 1 | 6 |
| Basophils % | 0.55 | 0.37 | 0 | 2 |
| Erythrocyte sedimentation rate (ESR) (mm/h) | 5.06 | 3.73 | 2 | 12 |
| Urea (mg/dL) | 33.94 | 6.43 | 10 | 50 |
| Estimated glomerular filtration rate (eGFR) (mL/min/1.73m2) | 73.03 | 13.4 | - | - |
| Uric acid (mg/dL) | 5.6 | 1.45 | 3.4 | 7 |
| Glucose (mg/dL) | 85.25 | 17.52 | 70 | 99 |
| Total cholesterol (mg/dL) | 179.1 | 32.58 | 115 | 190 |
| Cholesterol high-density lipoproteins (HDL) (mg/dL) | 58.21 | 12.17 | ≥45 | - |
| Cholesterol non-HDL (mg/dL) | 119.84 | 37.31 | - | - |
| Cholesterol low-density lipoproteins (LDL) (mg/dL) | 105.46 | 31.23 | 0 | <115 |
| Triglycerides (mg/dL) | 81.36 | 34.51 | 0 | 150 |
| Aspartate transaminase (AST) (U/L) | 28.81 | 22.87 | 0 | 40 |
| Alanine aminotransferase (ALT) (U/L) | 22.22 | 7.59 | 0 | 41 |
| Alkaline phosphatase (U/L) | 59.22 | 10.44 | 40 | 129 |
| Gamma-glutamyl transferase (GGTP) (U/L) | 19.89 | 10.59 | 8 | 61 |
| Serum amylase (U/L) | 63.58 | 21.38 | 28 | 100 |
| Sodium (mmol/L) | 141.46 | 2.27 | 136 | 145 |
| Potassium (mmol/L) | 4.53 | 0.37 | 3.5 | 5.1 |
| Total calcium (mmol/L) | 2.42 | 0.11 | 2.15 | 2.5 |
| Magnesium (mmol/L) | 0.86 | 0.06 | 0.66 | 1.07 |
| Iron (µg/dL) | 11.05 | 50.3 | 33 | 193 |
| C-reactive protein (CRP) (mg/dL) | 0.71 | 0.97 | 0 | 5 |
| Thyroid-stimulating hormone (µIU/mL) | 1.71 | 0.71 | 0.27 | 4.2 |
| Testosterone (ng/dL) | 591.94 | 210.72 | 239 | 836 |
| Cortisol (µg/dL) 7–10 AM | 14.24 | 4.32 | 6.2 | 19.4 |
| Variable | p-Value | Correlation |
|---|---|---|
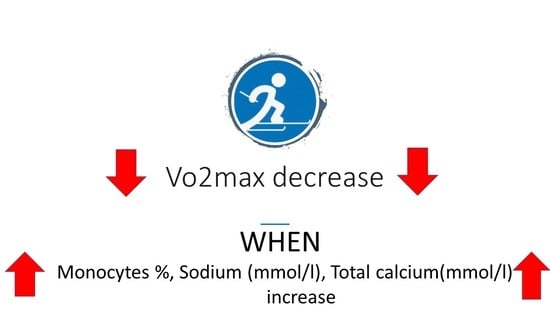
| Monocytes (thou/μL) | 0.001 | −0.750 |
| Eosinophils (thou/μL) | 0.026 | 0.613 |
| Monocytes % | <0.001 | −0.797 |
| Eosinophils % | 0.027 | 0.610 |
| Erythrocyte sedimentation rate (ESR) (mm/h) | 0.010 | −0.620 |
| Estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) | 0.041 | 0.531 |
| Sodium (mmol/L) | 0.004 | −0.680 |
| Total calcium (mmol/L) | 0.035 | −0.530 |
| Variable | Regression Coefficient | Statistical Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 237.147 | 37.655 | 6.30 | 0.000 |
| Monocytes % | −0.902 | 0.354 | −2.55 | 0.031 |
| Sodium (mmol/L) | −0.980 | 0.261 | −3.76 | 0.004 |
| Total Calcium (mmol/L) | −18.074 | 4.387 | −4.12 | 0.03 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzebisz, N. Determinants of the Cardiovascular Capacity of Amateur Long-Distance Skiers during the Transition Period. Diagnostics 2020, 10, 675. https://doi.org/10.3390/diagnostics10090675
Grzebisz N. Determinants of the Cardiovascular Capacity of Amateur Long-Distance Skiers during the Transition Period. Diagnostics. 2020; 10(9):675. https://doi.org/10.3390/diagnostics10090675
Chicago/Turabian StyleGrzebisz, Natalia. 2020. "Determinants of the Cardiovascular Capacity of Amateur Long-Distance Skiers during the Transition Period" Diagnostics 10, no. 9: 675. https://doi.org/10.3390/diagnostics10090675
APA StyleGrzebisz, N. (2020). Determinants of the Cardiovascular Capacity of Amateur Long-Distance Skiers during the Transition Period. Diagnostics, 10(9), 675. https://doi.org/10.3390/diagnostics10090675