Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD?
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Population
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Ultrasound Determinations
2.6. Anthropometric Measures and Clinical Data
2.7. Metabolic Assessment
2.8. Laboratory Testing
2.9. Statistics
3. Results
3.1. Prevalence
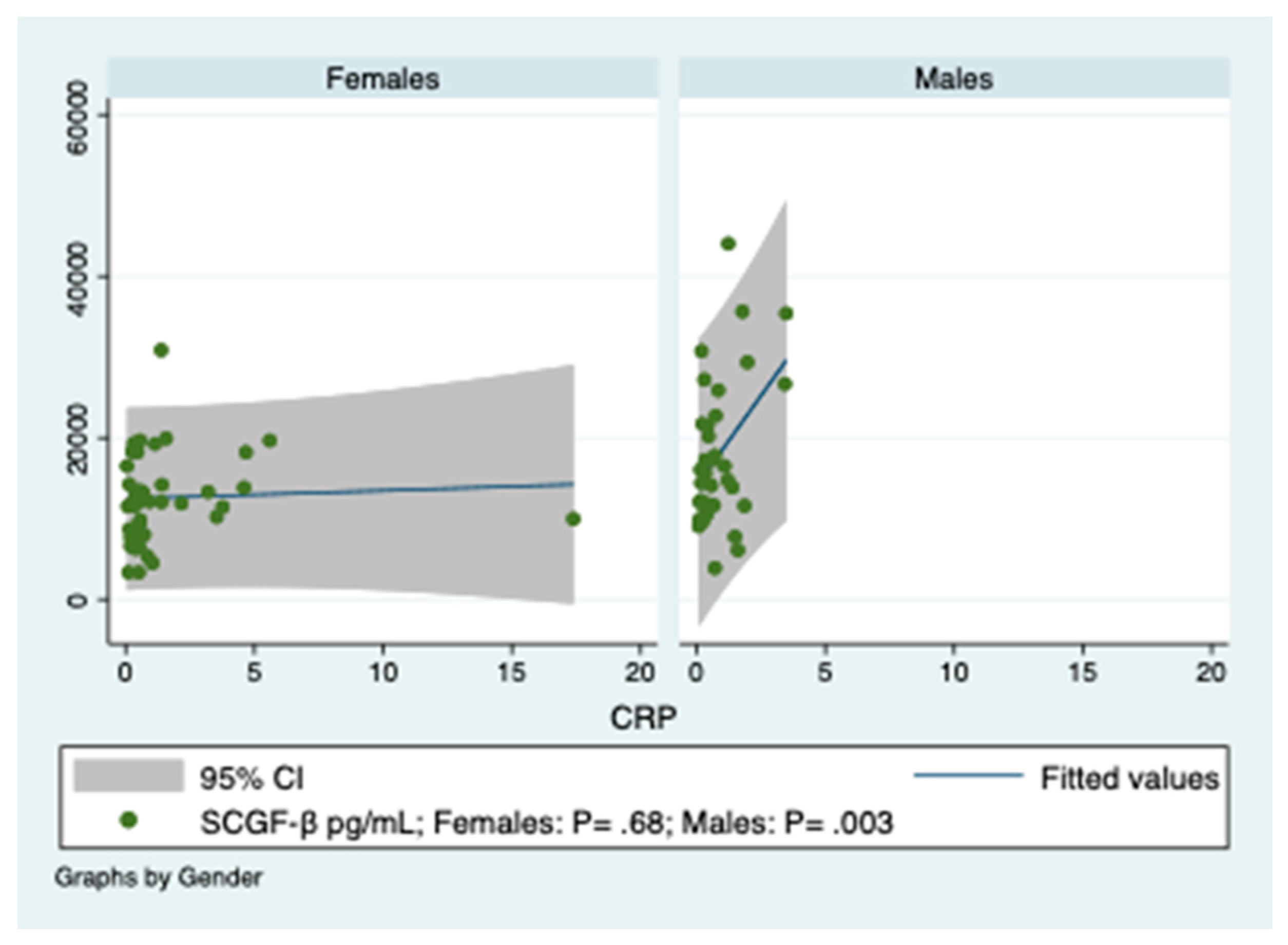
3.2. Predictions of SCGF-β Levels by Indices of Inflammation in Obese Patients
3.3. Predictions of SCGF-β Levels by Colony-Stimulating Factors
3.4. Prediction of HOMA by SCGF-β, M-CSF, TNF-β, IL-12p40, IL-6 and IL-10
3.5. Prediction of SCGF-β Levels by the Four Surrogate Markers of Insulin Resistance
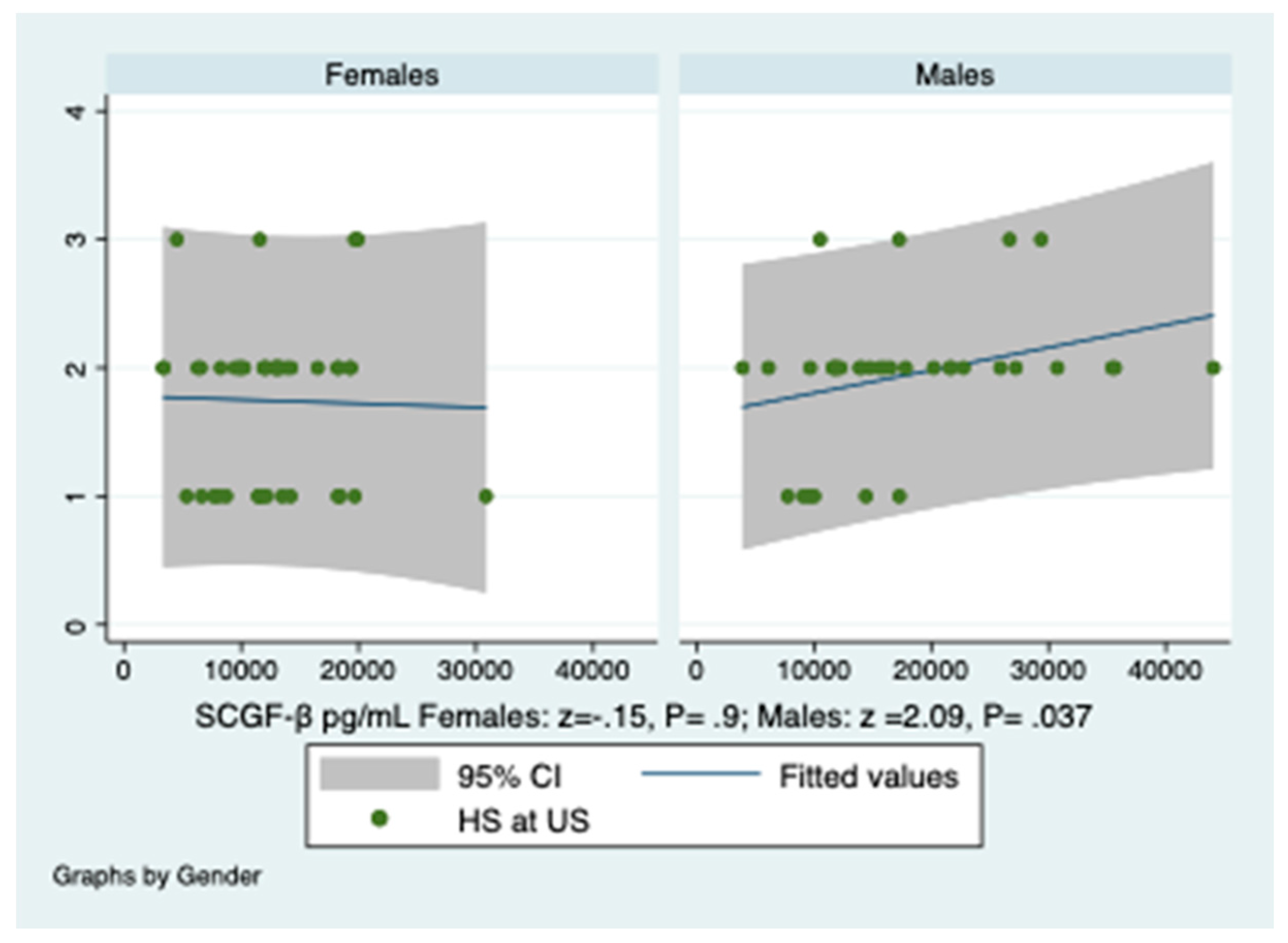
3.6. Prediction of Hepatic Steatosis by SCGF-β Levels and HOMA
3.7. Tackling the Problem of Unobserved Confounding Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modu, lation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Del Proposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008, 57, 3239–3246. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Milleck, J.; Korobko, V.G.; Shingarova, L.N.; Schmidt, H.E. Differences in the biological activity of TNF alpha and TNF beta correlate with their different abilities for binding to the target cells. Eur. Cytokine Netw. 1992, 3, 381–390. [Google Scholar] [PubMed]
- Cooper, A.M.; Khader, S.A. IL-12p40: An inherently agonistic cytokine. Trends Immunol. 2007, 8, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Tarantino, G.; Colicchio, P.; Conca, P.; Finelli, C.; Di Minno, M.N.; Tarantino, M.; Capone, D.; Pasanisi, F. Young adult obese subjects with and without insulin resistance: What is the role of chronic inflammation and how to weigh it non-invasively? J. Inflamm. 2009, 6, 6. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Utzschneider, M.K.; Kahn, S.E. The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002, 3, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Sugimura, A.; Seki, T.; Nagasawa, T.; Ohta, N.; Shimonishi, M.; Hagiya, M.; Shimizu, S. Cloning, expression, and characterization of a cDNA encoding a novel human growth factor for primitive hematopoietic progenitor cells. Proc. Natl. Acad. Sci. USA 1997, 94, 7577–7582. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.A.; Zhao, C.; Pavicic, P.G.; Datta, S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front. Immunol. 2014, 5, 554. [Google Scholar] [CrossRef]
- Boroumand, P.; Klip, A. Bone marrow adipose cells cellular interactions and changes with obesity. J. Cell Sci. 2020, 133, 5. [Google Scholar] [CrossRef]
- Tarantino, G.; Costantini, S.; Finelli, C.; Capone, F.; Guerriero, E.; La Sala, N.; Gioia, S.; Castello, G. Carotid intima-media thickness is predicted by combined eotaxin levels and severity of hepatic steatosis at ultrasonography in obese patients with nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e105610. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Miccoli, R.; Bianchi, C.; Odoguardi, L.; Penno, G.; Caricato, F.; Giovannitti, M.G.; Pucci, L.; Del Prato, S. Prevalence of the metabolic syndrome among Italian adults according to ATP III definition. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 250–254. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Paulmichl, K.; Hatunic, M.; Højlund, K.; Jotic, A.; Krebs, M.; Mitrakou, A.; Porcellati, F.; Tura, A.; Bergsten, P.; Forslund, A.; et al. Modification and validation of the triglyceride-to-HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: The single point insulin sensitivity estimator (SPISE). Clin. Chem. 2016, 62, 1211–1219. [Google Scholar] [CrossRef]
- Dubois, S.; McGovern, M.; Ehrhardt, V. Eisenstoffwechsel-diagnostik mit boehringer Mannheim/Hitachi-analysen systemen: Ferritin, transferrin und eisen. GIT Labor Medizin 1988, 9, 468–471. (In German) [Google Scholar]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage polarization and meta-inflammation. Transl. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Baron, R.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Khera, A.; McGuire, D.K.; de Lemos, J.A. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 2005, 46, 464–469. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Dasu, M.R.; Ciobanu, D.; Reusch, J.; Jialal, I. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Artherioscler. Thromb. Vas. Biol. 2006, 26, 469–475. [Google Scholar] [CrossRef]
- Hiraoka, A. Leukemia cell lines require self-stem cell growth factor (SCGF) for their proliferation. Leuk. Res. 2008, 32, 1623–1625. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Woo, S.L.; Kim, S.M.; Shende, V.R.; Neuendorff, N.; Guo, X.; Guo, T.; Qi, T.; Pei, Y.; et al. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 2014, 289, 16374–16388. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.; Chen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Hui-Yun, C.; Horng-Yuan, W.; Wen-Hsiung, C.; Shee-Chan, L.; Cheng-Hsin, C.; Tsang-En, W.; Chuan-Chuan, L.; Shou-Chuan, S. Nonalcoholic fatty liver disease: Prevalence, influence on age and sex, and relationship with metabolic syndrome and insulin resistance. Int. J. Gerontol. 2013, 7, 194–198. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]

| Age (years) | 46 (34–53) * | Gender M/F (n) | 36/44 |
| Obesity classes I/II/III (n) | 8/26/46 | MS (APT III) Yes/Not (n) | 51/29 |
| MS (IDF) Yes/Not (n) | 51/29 | ||
| T2DM (n) | 16 | OSAS/PCOS (n) | 0/0 |
| Hypertension (n) | 4 | ACV/CCV disease (n) | 0/0 |
| HOMA-B% | 37.11 (22.4–49.8) * | HOMA Total | 2.78 (1.85–4.18) * |
| HOMA (M) | 2.75 (1.9–4.7) * | HOMA (F) | 3–12 (1.55–4.18) * |
| SPISE | 6.6 ±1.7 ° | QUICKI | 0.32 (0.31–0.35) * |
| GM-CSF (pg/mL) | 2 (0.14–18.7) * | M-CSF | 17.3 (13.9–22) * |
| SCGF-β (pg/mL) | 13,113 (9976–18,299) * | TNF-β (pg/mL) | 2.7 (0.21–6.2) * |
| CRP (M) (mg/L) | 0.59 (0.23–1.22) * | IL-12p40 (pg/mL) | 234 (130–317) * |
| CRP (F) (mg/L) | 0.55 (0.34–1.38) * | IL-6 (M) (pg/mL) | 4.3 (1.6–15.2) * |
| Ferritin (M) (ng/mL) | 167.5 (85–234.5) * | Il-6 (F)(pg/mL) | 6.4 (3–19.3) * |
| Ferritin (F) (ng/mL) | 41.5 (20–69) * | IL-10 (pg/mL) | 11.6 (2.4–33.1) * |
| IR > 2 (M) (n) | 26 | IR > 2 (F) (n) | 29 |
| IR > 2.76 (M) (n) | 19 | IR > 2.76 (F) (n) | 23 |
| HS at US grades 1/2/3 | 22/50/8 | SLD cm | 11.0 (10.2–12.4) * |
| ALT (U/I) | 28 (21.5–39) * | Gamma-GT U/I | 25 (16.5–42.5) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarantino, G.; Citro, V.; Balsano, C.; Capone, D. Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics 2020, 10, 395. https://doi.org/10.3390/diagnostics10060395
Tarantino G, Citro V, Balsano C, Capone D. Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics. 2020; 10(6):395. https://doi.org/10.3390/diagnostics10060395
Chicago/Turabian StyleTarantino, Giovanni, Vincenzo Citro, Clara Balsano, and Domenico Capone. 2020. "Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD?" Diagnostics 10, no. 6: 395. https://doi.org/10.3390/diagnostics10060395
APA StyleTarantino, G., Citro, V., Balsano, C., & Capone, D. (2020). Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics, 10(6), 395. https://doi.org/10.3390/diagnostics10060395







