Exploring Association of Breast Pain, Pregnancy, and Body Mass Index with Breast Tissue Elasticity in Healthy Women: Glandular and Fat Differences
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Elastography and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Pandya, S.; Moore, R.G. Breast Development and Anatomy. Clin. Obstet. Gynecol. 2011, 54, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Arendt, L.M.; Kuperwasser, C. Form and function: How estrogen and progesterone regulate the mammary epithelial hierarchy. J. Mammary Gland. Biol. Neoplasia 2015, 20, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, S.A.; Sindelar, W.F. Risk factors in breast cancer. Prog. Clin. Cancer 1975, 6, 99–114. [Google Scholar] [PubMed]
- Gefen, A.; Dilmoney, B. Mechanics of the normal woman’s breast. Technol. Health Care 2007, 15, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Nightingale, K.R. What challenges must be overcome before ultrasound elasticity imaging is ready for the clinic? Imaging Med. 2011, 3, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Athanasiou, A.; Deffieux, T.; Gennisson, J.-L.; Montaldo, G.; Muller, M.; Tardivon, A.; Fink, M. Quantitative Assessment of Breast Lesion Viscoelasticity: Initial Clinical Results Using Supersonic Shear Imaging. Ultrasound Med. Biol. 2008, 34, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Real-Time Ultrasound Elasticity of the Breast. Ultrasound Q. 2010, 26, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Destounis, S.; Lackey, L.B.; Svensson, W.E.; Balleyguier, C.; Smith, C. Evaluation of breast lesions using sonographic elasticity imaging: A multicenter trial. J. Ultrasound Med. 2012, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Cosgrove, D.; Doré, C.J.; Schäfer, F.K.W.; Svensson, W.E.; Hooley, R.J.; Ohlinger, R.; Mendelson, E.B.; Balu-Maestro, C.; Locatelli, M.; et al. Shear-wave Elastography Improves the Specificity of Breast US: The BE1 Multinational Study of 939 Masses. Radiology 2012, 262, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Bojanic, K.; Katavic, N.; Smolic, M.; Peric, M.; Kralik, K.; Sikora, M.; Vidačić, K.; Pacovski, M.; Štimac, D.; Ivanac, G. Implementation of Elastography Score and Strain Ratio in Combination with B-Mode Ultrasound Avoids Unnecessary Biopsies of Breast Lesions. Ultrasound Med. Biol. 2017, 43, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Dominković, M.D.; Ivanac, G.; Kelava, T.; Brkljacic, B. Elastographic features of triple negative breast cancers. Eur. Radiol. 2015, 26, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Skórzewska, A.; Skibińska-Zielińska, M.; Opala, T. Factors influencing breast elasticity measured by the ultrasound Shear Wave elastography–preliminary results. Arch. Med Sci. 2011, 7, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Tardivon, A.; Tanter, M.; Sigal-Zafrani, B.; Bercoff, J.; Deffieux, T.; Gennisson, J.-L.; Fink, M.; Neuenschwander, S. Breast Lesions: Quantitative Elastography with Supersonic Shear Imaging—Preliminary Results. Radiology 2010, 256, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Tešić, V.; Kolarić, B.; Znaor, A.; Kuna, S.K.; Brkljacic, B. Mammographic Density and Estimation of Breast Cancer Risk in Intermediate Risk Population. Breast J. 2012, 19, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Skórzewska, A.; Opala, T. Changes in ultrasound shear wave elastography properties of normal breast during menstrual cycle. Clin. Exp. Obstet. Gynecol. 2011, 38, 137–142. [Google Scholar] [PubMed]
- Rzymski, P.; Wysocki, P.; Kycler, W.; Opala, T. Correlation between insulin resistance and breast elasticity heterogeneity measured by shear wave elastography in premenopausal women—A pilot study. Arch. Med Sci. 2011, 7, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Zhang, Z. Effects of precompression on elasticity imaging of the breast: Development of a clinically useful semiquantitative method of precompression assessment. J. Ultrasound Med. 2012, 31, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Krouskop, T.A.; Wheeler, T.M.; Kallel, F.; Garra, B.S.; Hall, T.J. Elastic moduli of breast and prostate tissues under compression. Ultrason. Imaging 1998, 20, 260–2744. [Google Scholar] [CrossRef] [PubMed]
- Sinkus, R.; Tanter, M.; Xydeas, T.; Catheline, S.; Bercoff, J.; Fink, M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn. Reson. Imaging 2005, 23, 159–165. [Google Scholar] [CrossRef] [PubMed]
- McKnight, A.L.; Kugel, J.L.; Rossman, P.J.; Manduca, A.; Hartmann, L.C.; Ehman, R.L. MR Elastography of Breast Cancer: Preliminary Results. Am. J. Roentgenol. 2002, 178, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, D.L.; Ellsworth, R.E.; Love, B.; Deyarmin, B.; Lubert, S.M.; Mittal, V.; Hooke, J.A.; Shriver, C.D. Outer Breast Quadrants Demonstrate Increased Levels of Genomic Instability. Ann. Surg. Oncol. 2004, 11, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Sarp, S.; Fioretta, G.; Verkooijen, H.M.; Vlastos, G.; Rapiti, E.; Schubert, H.; Sappino, A.-P.; Bouchardy, C. Tumor Location of the Lower-Inner Quadrant Is Associated with an Impaired Survival for Women With Early-Stage Breast Cancer. Ann. Surg. Oncol. 2006, 14, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, Ö.; Johansson, O. Sleep on the right side—Get cancer on the left? Pathophysiology 2010, 17, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, J.L.; Meier, P.P.; Jegier, B.; Motykowski, J.E.; Zuleger, J.L. Comparison of Milk Output from the Right and Left Breasts During Simultaneous Pumping in Mothers of Very Low Birthweight Infants. Breastfeed. Med. 2007, 2, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Amarosa, A.R.; McKellop, J.; Leite, A.P.K.; Moccaldi, M.; Clendenen, T.V.; Babb, J.S.; Zeleniuch-Jacquotte, A.; Moy, L.; Kim, S. Evaluation of the kinetic properties of background parenchymal enhancement throughout the phases of the menstrual cycle. Radiology 2013, 268, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, W.P.; Chan, S.; Yeh, D.C.; Su, M.Y.; McLaren, C.E. Correlation of endogenous hormonal levels, fibroglandular tissue volume and percent density measured using 3D MRI during one menstrual cycle. Ann. Oncol. 2013, 24, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, G.; Chow, L.; Schlosser, A.; Yaffe, M.J.; Boyd, N.; Martin, L.J. Differences in Measured Mammographic Density in the Menstrual Cycle. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1993–1999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buist, D.S.; Bowles, E.J.A.; Miglioretti, D.L.; White, E. Mammographic Breast Density, Dense Area, and Breast Area Differences by Phase in the Menstrual Cycle. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2303–2306. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Su, M.-Y.L.; Lei, F.-J.; Wu, J.-P.; Lin, M.; Nalcioglu, O.; Feig, S.A.; Chen, J.H. Menstrual cycle-related fluctuations in breast density measured by using three-dimensional MR imaging. Radiology 2011, 261, 744–751. [Google Scholar] [CrossRef]
- Wojcinski, S.; Cassel, M.; Farrokh, A.; Soliman, A.A.; Hille, U.; Schmidt, W.; Degenhardt, F.; Hillemanns, P. Variations in the Elasticity of Breast Tissue During the Menstrual Cycle Determined by Real-time Sonoelastography. J. Ultrasound Med. 2012, 31, 63–72. [Google Scholar] [CrossRef] [PubMed]
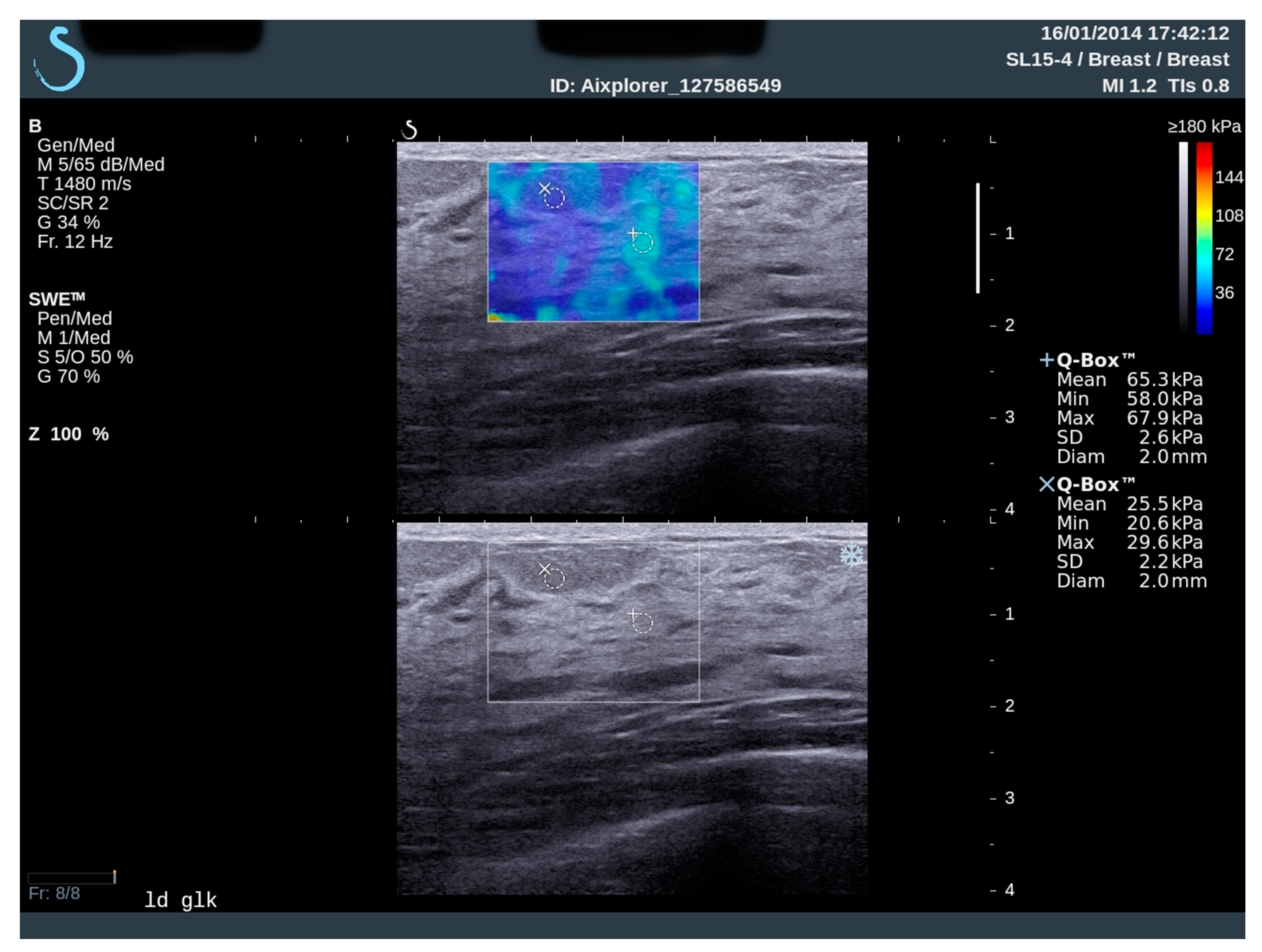
| Subjects Characteristics | Total Number | % | |
|---|---|---|---|
| Family history of breast cancer | Negative | 166 | 83% |
| Positive | 34 | 17% | |
| Exogenous hormones | No | 146 | 73% |
| Yes | 54 | 27% | |
| Smoking | No | 131 | 65.5% |
| Yes | 69 | 34.5% | |
| Alcohol consumption | No | 135 | 67.5% |
| Yes | 65 | 32.5% | |
| Breast pain | No | 112 | 56% |
| Yes | 88 | 44% | |
| Subjects Characteristics | |
|---|---|
| Age (years) * | 37 (29–52) |
| Age of menarche * | 13 (12–14) |
| Age of menopause * | 50 (45–52) |
| Day of menstrual cycle at the time of the examination * | 15 (8–20) |
| Women ever being pregnant [n (%)] | 110 (55) |
| Age of first pregnancy * | 26 (21–28) |
| Duration of lactation (months) * | 8 (4–22) |
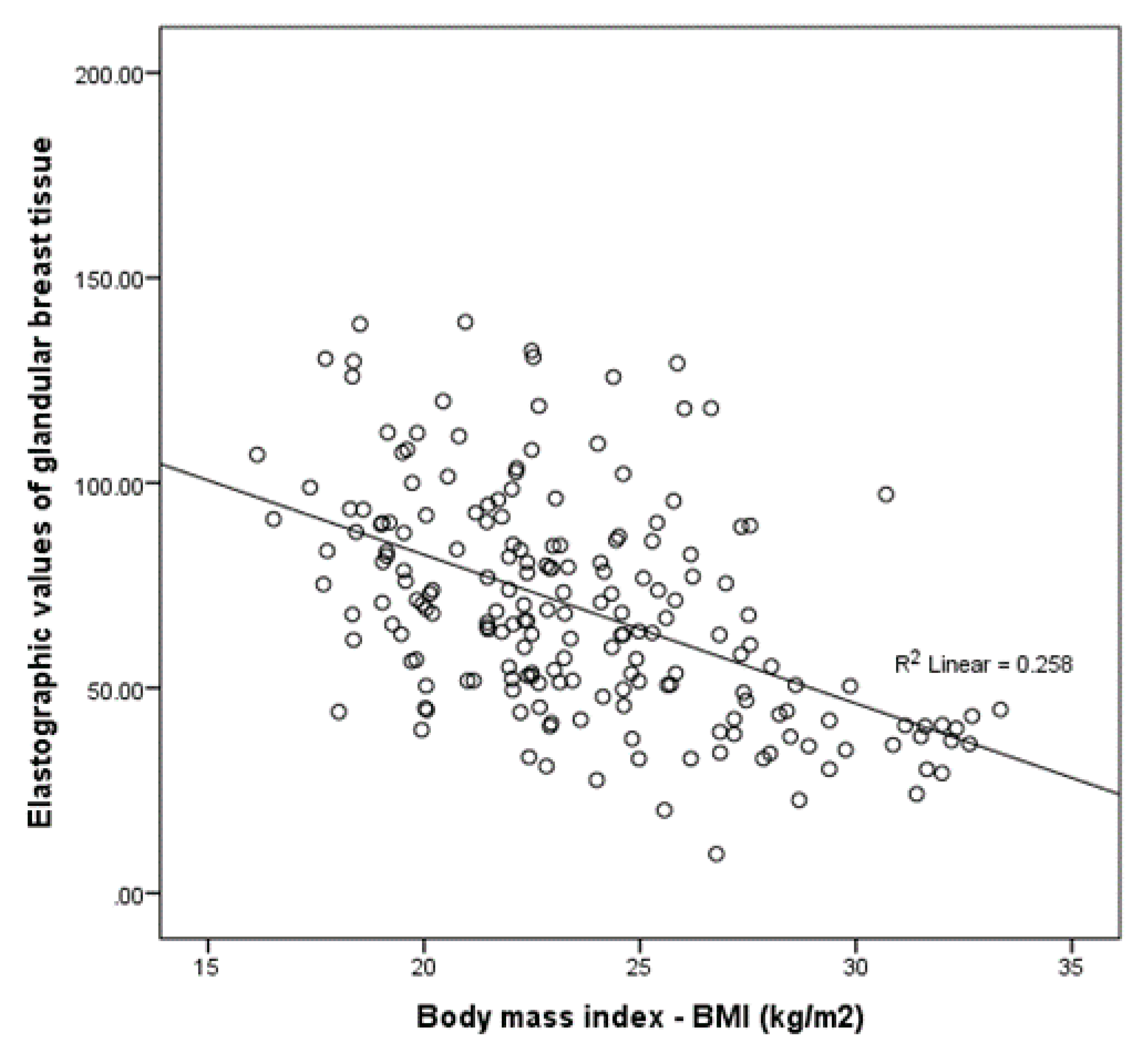
| Body mass index (kg/m2) * | 23 (21–26.2) |
| Total number of breast lesions * | 2 (1–3) |
| Number of malignant lesions [n (%)] | 8 (4) |
| Number of benign lesions [n (%)] | 94 (47) |
| Breast Quadrants | Elastographic Values of Glandular Breast Tissue | Elastographic Values of Fat Tissue in Breast | p§ | ||
|---|---|---|---|---|---|
| Median (IQR) | p * | Median (IQR) | p * | ||
| UOQ | 69.6 (50.4–93.9) | <0.001 † | 26.5 (20.1–36.7) | <0.001 † | <0.001 |
| LOQ | 60.3 (39.8–75.9) | 21.18 (16.5–28.4) | <0.001 | ||
| UIQ | 69.5 (47.3–97.4) | 28.5 (21.2–37.6) | <0.001 | ||
| LIQ | 65.5 (43.9–94.1) | 25.28 (19.1–34.3) | <0.001 | ||
| Breast | Elastographic Values of Glandular Breast Tissue | Elastographic Values of Fat Tissue in Breast | p† | ||
|---|---|---|---|---|---|
| Median (IQR) | p * | Median (IQR) | p * | ||
| Left | 68.9 (51.2–95.4) | 0.005 | 27.1 (26–28.4) | <0.001 | <0.001 |
| Right | 63.3 (47.7–85.3) | 24.3 (18.4–31.7) | <0.001 | ||
| Medical History Data | Elastographic Values of Glandular Breast Tissue | Elastographic Values of Fat Tissue in Breast | |||
|---|---|---|---|---|---|
| Median (IQR) | p * | Median (IQR) | p * | ||
| Family history of BC | no | 65.2 (49.5–83.6) | 0.25 | 25.9 (20.6–32.3) | 0.13 |
| yes | 73.8 (45.1–91.7) | 27.7 (23–35.7) | |||
| Exogenous hormones | no | 68.2 (45.7–89.6) | 0.35 | 26.1 (21.3–33.4) | 0.66 |
| yes | 61.9 (49.6–82.1) | 25.8 (21–30.7) | |||
| Smoking | no | 67 (49.6–85) | 0.72 | 26.6 (21.7–34) | 0.12 |
| yes | 65.5 (45.7–86.9) | 24.1 (20.6–31) | |||
| Alcohol | no | 63.8 (43.5–84.7) | 0.11 | 26.3 (21.4–33.4) | 0.77 |
| yes | 69.3 (52.8–91.7) | 26 (21–31.6) | |||
| Breast pain | no | 61.7 (42.2–83.5) | 0.02 | 25.6 (21.3–32.3) | 0.78 |
| yes | 72.2 (518–90.8) | 26.3 (21–33) | |||
| Ever being pregnant | no | 72.2 (52.8–93.6) | 0.001 | 26.8 (22.03–35.1) | 0.07 |
| yes | 61.7 (42.2–80.03) | 25.1 (20.9–31.2) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzoic Dominkovic, M.; Ivanac, G.; Bojanic, K.; Kralik, K.; Smolic, M.; Divjak, E.; Smolic, R.; Brkljacic, B. Exploring Association of Breast Pain, Pregnancy, and Body Mass Index with Breast Tissue Elasticity in Healthy Women: Glandular and Fat Differences. Diagnostics 2020, 10, 393. https://doi.org/10.3390/diagnostics10060393
Dzoic Dominkovic M, Ivanac G, Bojanic K, Kralik K, Smolic M, Divjak E, Smolic R, Brkljacic B. Exploring Association of Breast Pain, Pregnancy, and Body Mass Index with Breast Tissue Elasticity in Healthy Women: Glandular and Fat Differences. Diagnostics. 2020; 10(6):393. https://doi.org/10.3390/diagnostics10060393
Chicago/Turabian StyleDzoic Dominkovic, Martina, Gordana Ivanac, Kristina Bojanic, Kristina Kralik, Martina Smolic, Eugen Divjak, Robert Smolic, and Boris Brkljacic. 2020. "Exploring Association of Breast Pain, Pregnancy, and Body Mass Index with Breast Tissue Elasticity in Healthy Women: Glandular and Fat Differences" Diagnostics 10, no. 6: 393. https://doi.org/10.3390/diagnostics10060393
APA StyleDzoic Dominkovic, M., Ivanac, G., Bojanic, K., Kralik, K., Smolic, M., Divjak, E., Smolic, R., & Brkljacic, B. (2020). Exploring Association of Breast Pain, Pregnancy, and Body Mass Index with Breast Tissue Elasticity in Healthy Women: Glandular and Fat Differences. Diagnostics, 10(6), 393. https://doi.org/10.3390/diagnostics10060393





