Renal Tumors in Young Adults: Is Preoperative Computer Tomography Imaging Suggestive for the Nature of the Tumors?
Abstract
1. Introduction
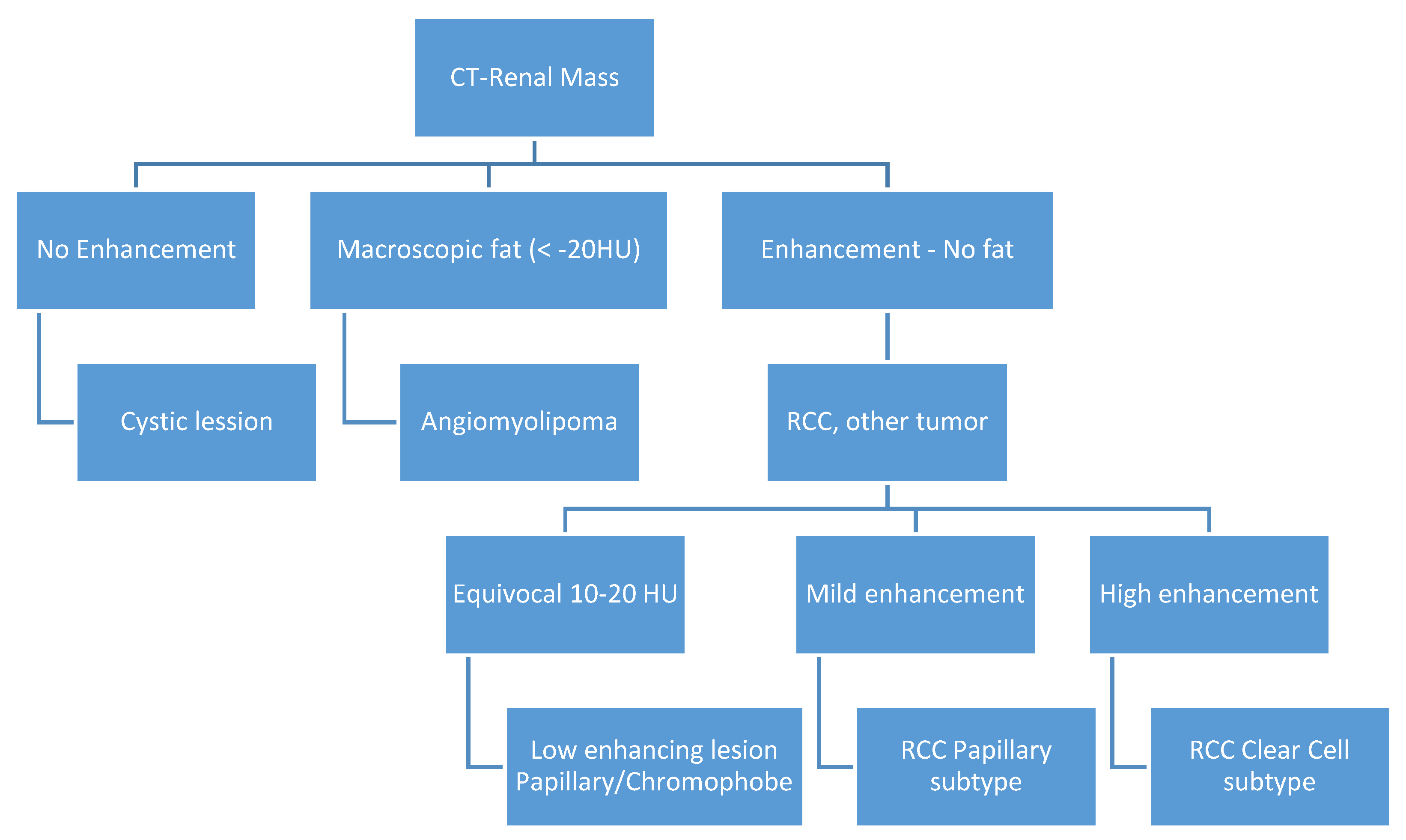
2. Materials and Methods
Data Analysis
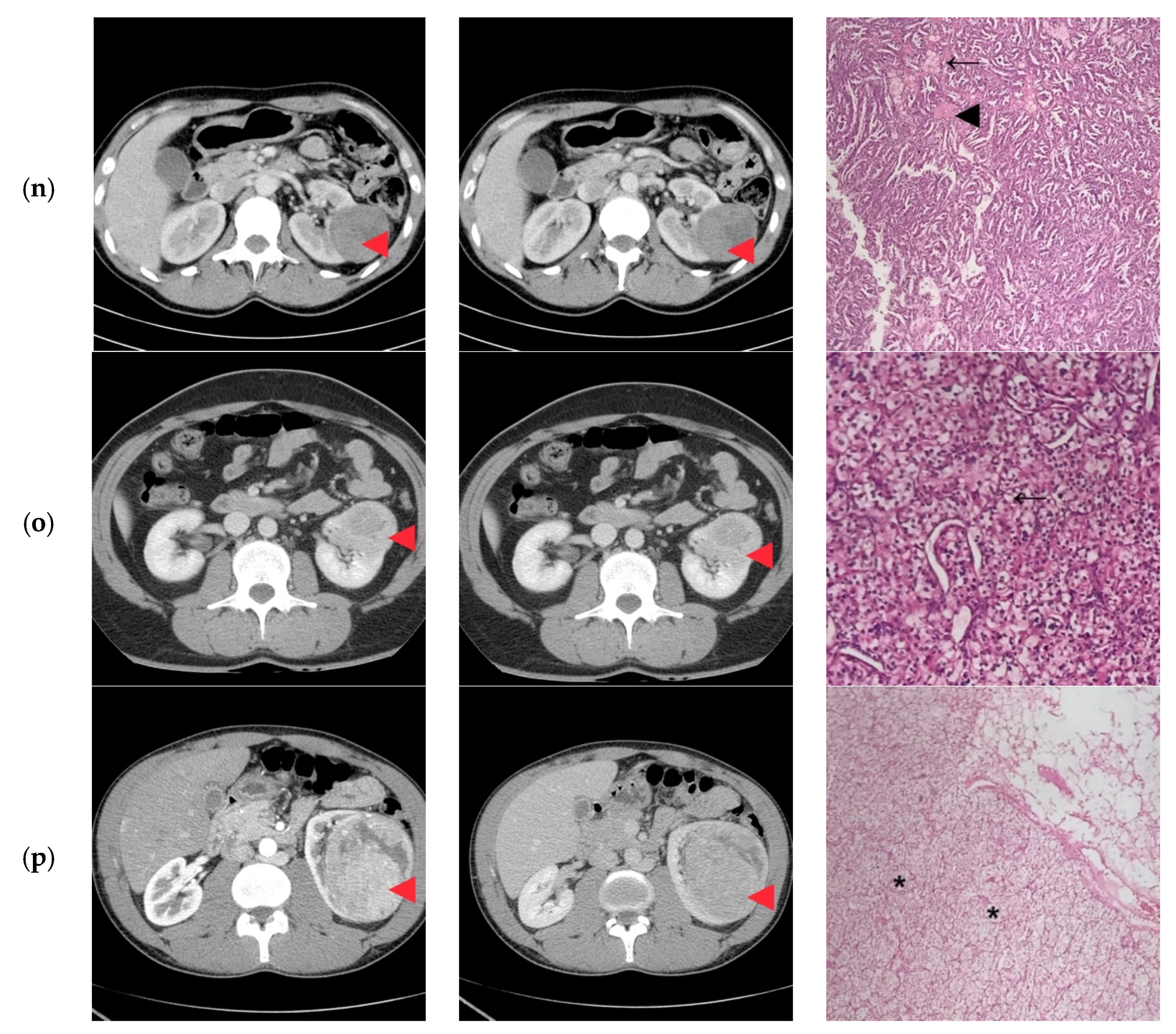
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html (accessed on 11 April 2020).
- Naitoh, Y.; Ishida, Y.; Nishida, M.; Soh, J.; Nakamura, M.; Yoneda, K.; Uchida, M.; Watanabe, H. A Case of Renal Cell Carcinoma in a Young Adult. Hinyokika Kiyo 1995, 41, 537–539. [Google Scholar] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018, 23, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.S.; Aron, M.; Gervais, D.A.; Jewett, M.A.S. Small Renal Mass. N. Engl. J. Med. 2010, 362, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G. Stumbling onto Cancer: Avoiding Overdiagnosis of Renal Cell Carcinoma. Am. Fam. Physician 2019, 99, 145–147. [Google Scholar]
- Sand, K.E.; Hjelle, K.M.; Rogde, A.J.; Gudbrandsdottir, G.; Bostad, L.; Beisland, C. Incidentally detected renal cell carcinomas are highly associated with comorbidity and mortality unrelated to renal cellcarcinoma. Scand. J. Urol. 2013, 47, 462–471. [Google Scholar] [CrossRef]
- Hellsten, S.; Berge, T.; Wehlin, L. Unrecognized renal cell carcinoma. Clinical and diagnostic aspects. Scand. J. Urol. Nephrol. 1981, 15, 269–272. [Google Scholar] [CrossRef]
- Sinner, D.G.; Colvin, R.B.; Vermillion, C.D.; Pfister, R.C.; Leadbetter, W.F. Diagnosis and management of renal cell carcinoma. A clinical andpathologic study of 309 cases. Cancer 1971, 28, 1165–1177. [Google Scholar] [CrossRef]
- Beisland, C.; Medby, P.C.; Beisland, H.O. Renal cell carcinoma: Gender difference in incidental detection and cancer-specific survival. Scand. J. Urol Nephrol. 2002, 36, 414–418. [Google Scholar] [CrossRef]
- Reinhard, R.; van der Zon-Conijn, M.; Smithuis, R. Kidney—Solid Masses. Available online: http://www.radiologyassistant.nl/en/p571eea20ec282/kidney-solid-masses.html (accessed on 14 January 2020).
- Silverman, S.G.; Israel, G.M.; Trinh, Q.D. Incompletely characterized incidental renal masses: Emerging data support conservative management. Radiology 2015, 275, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.; Grenier, N. Multiparametric MR imaging of solid renal tumors: A practical algorithm. Semin. Ultrasound CT MRI 2017, 38, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 99, 179–184. [Google Scholar] [PubMed]
- Cao, Y.; Paner, G.P.; Perry, K.T.; Flanigan, R.C.; Campbell, S.C.; Picken, M.M. Renal neoplasms in younger adults: Analysis of 112 tumors from a single institution according to the new 2004 World Health Organization classification and 2002 American Joint Committee on Cancer Staging System. Arch. Pathol. Lab. Med. 2005, 129, 487–491. [Google Scholar] [PubMed]
- Gillett, M.D.; Cheville, J.C.; Karnes, R.J.; Lohse, C.M.; Kwon, E.D.; Leibovich, B.C.; Zincke, H.; Blute, M.L. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J. Urol. 2005, 173, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, M.A.; Desai, M.; Mansukhani, M.; Goluboff, E.T.; Katz, A.E.; Sawczuk, I.S.; Benson, M.C.; Olsson, C.A.; McKiernan, J.M. Natural history and clinical outcome of sporadic renal cortical tumors diagnosed in theyoung adult. Urology 2004, 63, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.; Bratslavsky, G. Renal cell carcinoma in young patients: A review of recent literature. Curr. Urol. Rep. 2015, 16, 1. [Google Scholar] [CrossRef]
- King, S.C.; Pollack, L.A.; Li, J.; King, J.B.; Master, V.A. Continued Increase in Incidence of Renal Cell Carcinoma, Especially in Young Patients and High Grade Disease: United States 2001 to 2010. J. Urol. 2014, 191, 1665–1670. [Google Scholar] [CrossRef]
- Aslan, R.; Taken, K.; Eryılmaz, R. Clinicopathological Features and Survival Data of Localized Renal Masses in Young Adults. Asian Pac. J. Cancer Prev. 2018, 19, 3233–3236. [Google Scholar] [CrossRef]
- Abou El Fettouh, H.I.; Cherullo, E.E.; El-Jack, M.; Al Maslamani, Y.; Novick, A.C. Sporadic renal cell carcinoma in young adults: Presen-tation, treatment, and outcome. Urology 2002, 60, 806–810. [Google Scholar] [CrossRef]
- Sanchez-Ortiz, R.F.; Rosser, C.J.; Madsen, L.T.; Swanson, D.A.; Wood, C.G. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J. Urol. 2004, 171, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Simhan, J.; Smaldone, M.C.; Tsai, K.J.; Canter, D.J.; Li, T.; Kutikov, A.; Viterbo, R.; Chen, D.Y.; Greenberg, R.E.; Uzzo, R.G. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur. Urol. 2011, 60, 724–730. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. WHO Classification of Tumours of the Urinary System and Male Genital Organs; International Agency for Research on Cancer: Lyon, France, 2016; ISBN 9789283224372. [Google Scholar]
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef]
- Eggener, S.E.; Rubenstein, J.R.; Smith, N.D.; Nadler, R.B.; Kontak, J.; Flanigan, R.C.; Waters, W.B.; Picken, M.; Campbell, S.C. Renal tumors in young adults. J. Urol. 2004, 171, 106–110. [Google Scholar] [CrossRef]
- Xavier, T.; Valeri, A.; Descotes, J.-L.; Morin, V.; Stindel, E.; Doucet, L.; Joulin, V.; Bocqueraz, F.; Coulange, C.; Rambeaud, J.-J.; et al. The Oncology Committee of the Association Française d’Urologie. Renal Cell Carcinoma in Adults 40 Years Old or Less: Young Age is an Independent Prognostic Factor for Cancer-Specific Survival. Eur Urol. 2007, 51, 980–987. [Google Scholar]
- Sánchez-Ortiz, R.F.; Rosser, C.J.; Madsen, L.T.; Swanson, D.A.; Wood, C.G. Solid Renal Tumors: An Analysis of Pathological Features Related to Tumor Size. J. Urol. 2003, 170, 2217–2220. [Google Scholar]
| Renal Masses | What? | Additional Imaging? |
|---|---|---|
| Incomplete characterized incidental renal masses at unenhanced CT | ||
| Probably benign cyst | Probably, not necessarily |
| Possible malignant | Maybe warranted * |
| Incomplete characterized incidental renal masses at contrast-enhanced CT | ||
| Probably benign cyst | Probably, not necessarily |
| Possible malignant | Maybe warranted * |
| RENAL | 1 pt | 2 pts | 3 pts |
|---|---|---|---|
| Radius diameter (cm) | ≤4 | >4 but <7 | ≥7 |
| Exophytic/Endophytic | ≥50% Exophytic | <50% Endophytic | Entirely Endophytic |
| Nearest sinus or collecting system (mm) | ≥7 | >4 but <7 | ≤4 |
| Anterior/Posterior | Nonnumerical suffix a, p, x, h | ||
| Location, Polar lines | Entirely above or below the polar lines | The lesion crosses the polar lines | >50% of the mass crosses the polar line, or the mass is located entirely between the polar lines |
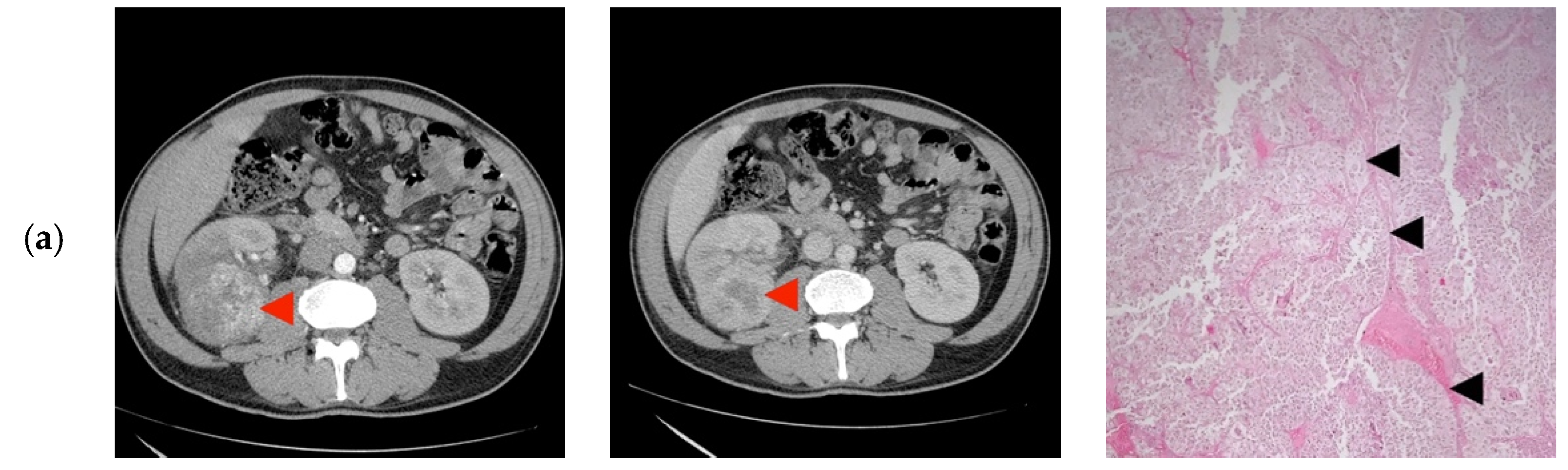
| ID | Age (years)/Gender | TNM | Tumor Diameter (mm) | R.E.N.A.L. Nephrometry Score | Preoperative CT Imaging Diagnosis |
|---|---|---|---|---|---|
| 1 | 45/M | cT2N0 | 73 | 9 | Clear Cell RCC |
| 2 | 27/F | cT1bN0 | 60 | 8 | Clear Cell RCC |
| 3 | 38/M | cT1bN0 | 47 | 10 | Papillary/Clear Cell RCC Oncocytoma |
| 4 | 24/M | cT2N0 | 85 | 10 | Clear Cell RCC |
| 5 | 31/F | cT1aN0 | 35 | 8 | Papillary Clear Cell RCC |
| 6 | 41/M | cT2N0 | 86 | 10 | Clear Cell RCC |
| 7 | 29/M | cT1bN0 | 40 | 8 | Oncocytoma |
| 8 | 30/F | cT1bN0 | 44 | 10 | Papillary/Clear Cell RCC |
| 9 | 32/F | cT1bN0 | 52 | 11 | Clear Cell RCC |
| 10 | 25/M | cT1bN0 | 45 | 11 | Papillary/Clear Cell RCC Complicated cyst Oncocytoma/Fat poor AML |
| 11 | 43/F | cT1bN0 | 50 | 9 | Papillary RCC/Oncocytoma Fat poop AML |
| 12 | 41/F | cT2N0 | 73 | 10 | Oncocytoma |
| 13 | 43/M | cT3aN0 | 33 | 7 | Clear Cell RCC |
| 14 | 38/F | cT1bN0 | 50 | 8 | Bleeding Clear Cell RCC |
| 15 | 32/M | cT3aN0 | 55 | 10 | Clear Cell RCC |
| 16 | 37/M | cT2N0 | 93 | 11 | Clear Cell RCC |
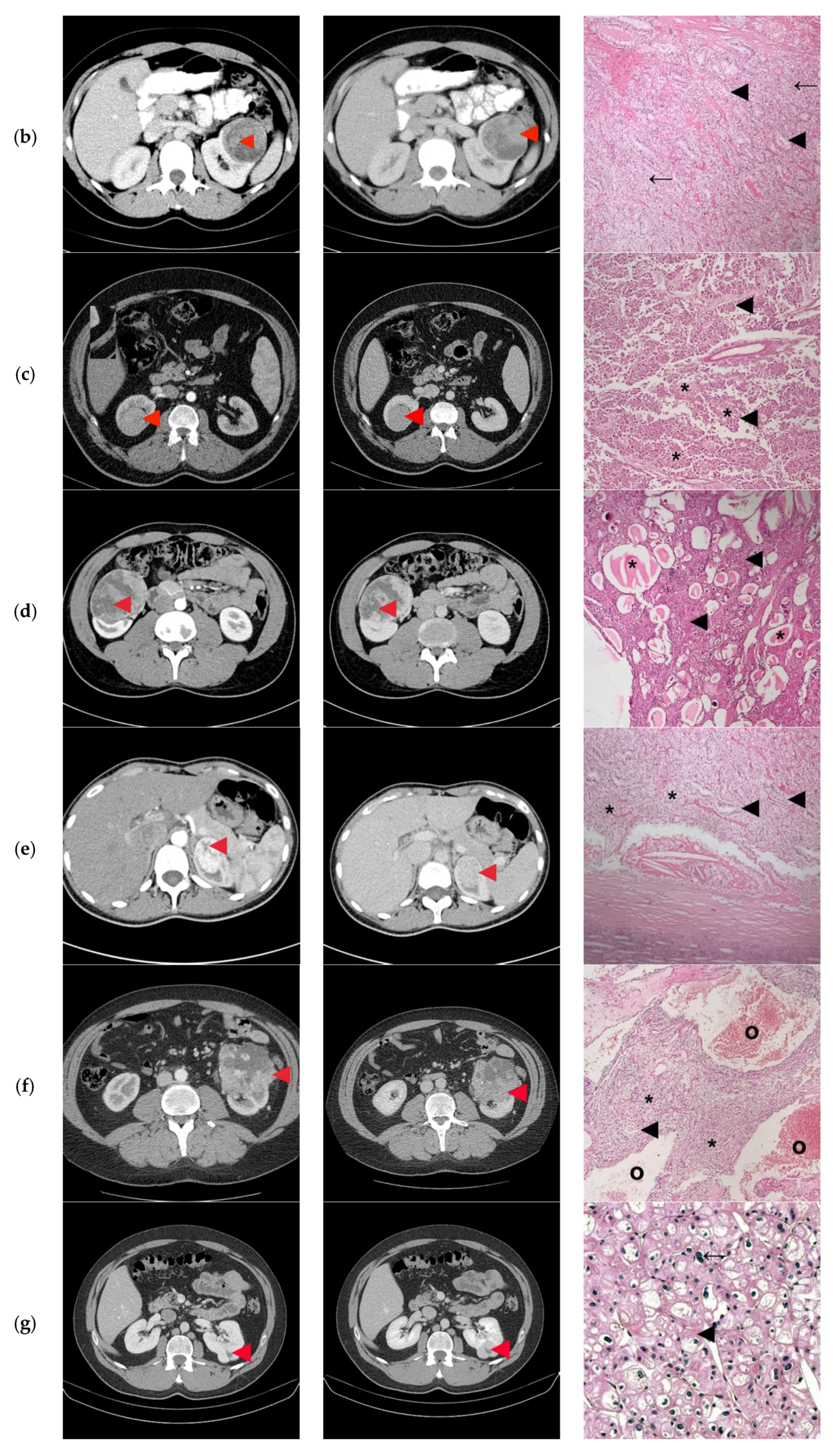
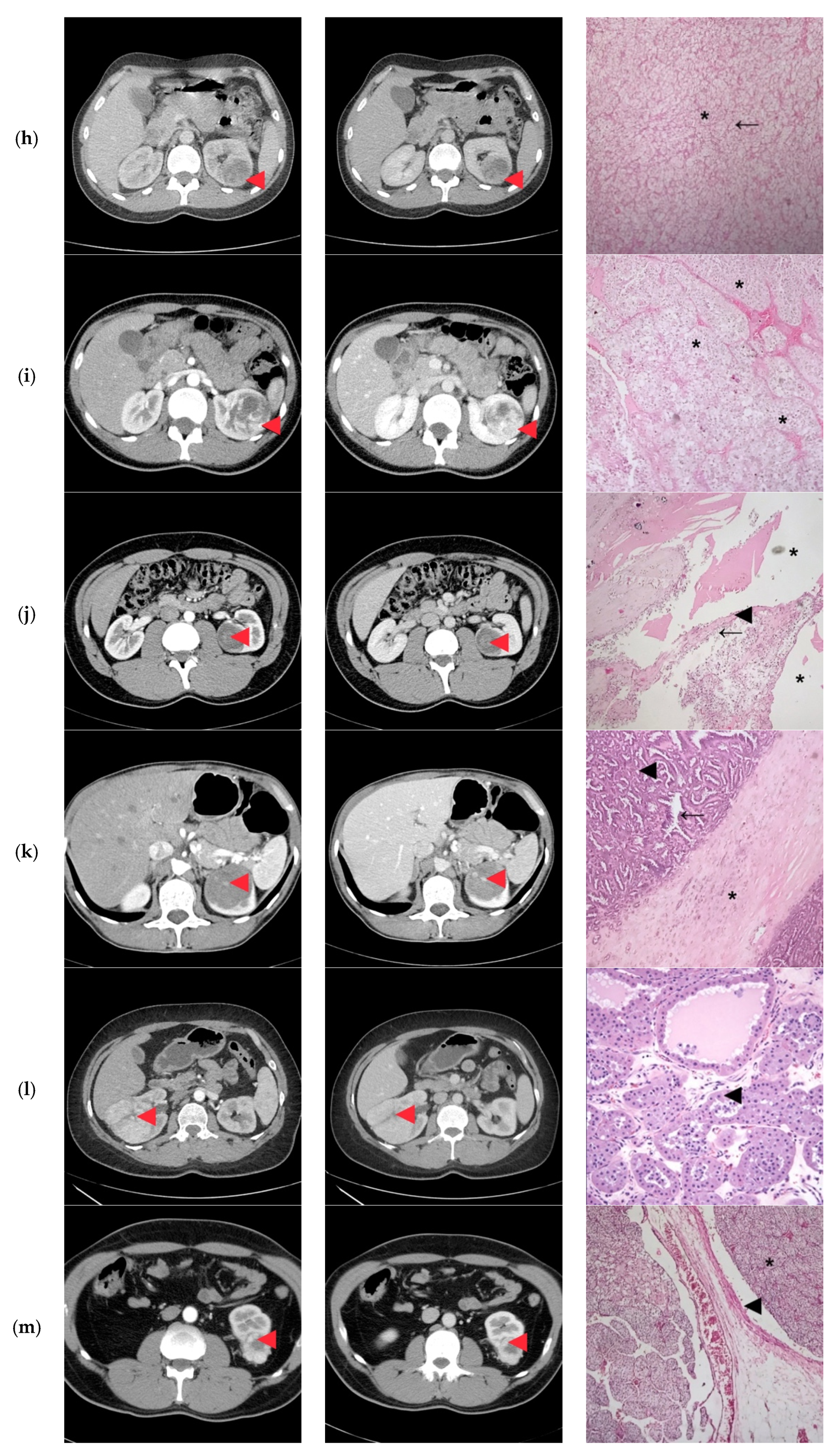
| ID | Surgical Approach | Histological Stage | Histological Exam | Concordance Histological-CT Diagnosis |
|---|---|---|---|---|
| 1 | Retroperitoneoscopic radical nephrectomy | pT2N0 | Clear Cell RCC Furhman 2 | Yes |
| 2 | Open radical nephrectomy | pT1bN0 | Clear Cell RCC Furhman 1 | Yes |
| 3 | Retroperitoneoscopic radical nephrectomy | pT1bN0 | Papillary-RCC, type II | Yes/No |
| 4 | Open Radical nephrectomy | pT2N0 | Cystic Nephroma | No |
| 5 | Retroperitoneoscopic radical nephrectomy | pT1aN0 | Clear Cell RCC Furhman 1 | Yes/No |
| 6 | Open radical nephrectomy | pT2N0 | Clear Cell RCCFurhman 2 | Yes |
| 7 | Open partial nephrectomy with local renal hypothermia and hilar clamping | PT1bN0 | Multicentric Chromophobe-RCC | No |
| 8 | Open partial nephrectomy with local renal hypothermia and hilar clamping | pT1bN0 | Clear Cell RCC Furhman 1 | Yes/No |
| 9 | Retroperitoneoscopic radical nephrectomy | pT1bN0 | Clear Cell RCC Furhman 2 | Yes |
| 10 | Open partial nephrectomy with local renal hypothermia and hilar clamping | pT1bN0 | Multiloculated cystic Clear Cell RCC Furhman 1 | No |
| 11 | Open partial nephrectomy with local renal hypothermia and hilar clamping | pT1bN0 | Metanephric adenofibroma | No |
| 12 | Open radical nephrectomy | pT2N0 | Oncocytoma | Yes |
| 13 | Open partial nephrectomy–segmentary renal vein invasion–radical nephrectomy | pT3aN0 | Clear Cell RCC Furhman 1 | Yes |
| 14 | Open partial nephrectomy with local renal hypothermia and hilar clamping | pT1bN0 | Papillary-RCC, type I | No |
| 15 | Open radical nephrectomy | pT3aN0 | Clear Cell RCC Furhman 1 | Yes |
| 16 | Retroperitoneoscopic radical nephrectomy | pT2N0 | Clear Cell RCC Furhman 1 | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharie, A.; Bolboacă, S.D.; Moisoiu, T.; Burghelea, D.; Iacob, G.; Ghervan, L.; Elec, F.I. Renal Tumors in Young Adults: Is Preoperative Computer Tomography Imaging Suggestive for the Nature of the Tumors? Diagnostics 2020, 10, 380. https://doi.org/10.3390/diagnostics10060380
Zaharie A, Bolboacă SD, Moisoiu T, Burghelea D, Iacob G, Ghervan L, Elec FI. Renal Tumors in Young Adults: Is Preoperative Computer Tomography Imaging Suggestive for the Nature of the Tumors? Diagnostics. 2020; 10(6):380. https://doi.org/10.3390/diagnostics10060380
Chicago/Turabian StyleZaharie, Andreea, Sorana D. Bolboacă, Tudor Moisoiu, Dan Burghelea, Gheorghita Iacob, Liviu Ghervan, and Florin Ioan Elec. 2020. "Renal Tumors in Young Adults: Is Preoperative Computer Tomography Imaging Suggestive for the Nature of the Tumors?" Diagnostics 10, no. 6: 380. https://doi.org/10.3390/diagnostics10060380
APA StyleZaharie, A., Bolboacă, S. D., Moisoiu, T., Burghelea, D., Iacob, G., Ghervan, L., & Elec, F. I. (2020). Renal Tumors in Young Adults: Is Preoperative Computer Tomography Imaging Suggestive for the Nature of the Tumors? Diagnostics, 10(6), 380. https://doi.org/10.3390/diagnostics10060380






