The Additional Value of 18F-FDG PET and MRI in Patients with Glioma: A Review of the Literature from 2015 to 2020
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Selection
3. Results
3.1. Diagnosis and Differential Diagnosis
3.2. Grading
3.3. Prognosis
3.4. Assessment of Recurrence
3.5. Treatment Planning and Evaluation of Response to Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daumas-Duport, C.; Scheithauer, B.; O’Fallon, J.; Kelly, P. Grading of astrocytomas. A simple and reproducible method. Cancer 1988, 62, 2152–2165. [Google Scholar] [CrossRef]
- Gupta, A.; Dwivedi, T. A simplified overview of world health organization classification update of central nervous system tumors 2016. J. Neurosci. Rural Pract. 2017, 8, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N.; Asselin, M.C. The validation path of hypoxia pet imaging: A focus on brain tumours. Curr. Med. Chem. 2017, 25, 3074–3095. [Google Scholar] [CrossRef] [PubMed]
- Chiavazza, C.; Pellerino, A.; Ferrio, F.; Cistaro, A.; Soffietti, R.; Ruda, R. Primary cns lymphomas: Challenges in diagnosis and monitoring. BioMed Res. Int. 2018, 2018, 3606970. [Google Scholar] [CrossRef] [PubMed]
- Cistaro, A.; Caobelli, F.; Quartuccio, N.; Fania, P.; Pagani, M. Uncommon 18f-fdg-pet/ct findings in patients affected by limbic encephalitis: Hyper-hypometabolic pattern with double antibody positivity and migrating foci of hypermetabolism. Clin. Imaging 2015, 39, 329–333. [Google Scholar] [CrossRef]
- Gokden, M. If it is not a glioblastoma, then what is it? A differential diagnostic review. Adv. Anat. Pathol. 2017, 24, 379–391. [Google Scholar] [CrossRef]
- Mellai, M.; Piazzi, A.; Casalone, C.; Grifoni, S.; Melcarne, A.; Annovazzi, L.; Cassoni, P.; Denysenko, T.; Valentini, M.C.; Cistaro, A.; et al. Astroblastoma: Beside being a tumor entity, an occasional phenotype of astrocytic gliomas? OncoTargets Ther. 2015, 8, 451–460. [Google Scholar] [CrossRef][Green Version]
- Mangiola, A.; de Bonis, P.; Maira, G.; Balducci, M.; Sica, G.; Lama, G.; Lauriola, L.; Anile, C. Invasive tumor cells and prognosis in a selected population of patients with glioblastoma multiforme. Cancer 2008, 113, 841–846. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neurooncol. 2017, 134, 495–504. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: Imaging challenges for the evaluation of treated gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N.; Laudicella, R.; Mapelli, P.; Guglielmo, P.; Pizzuto, D.A.; Boero, M.; Arnone, G.; Picchio, M.; Young, A.W.G. Hypoxia pet imaging beyond 18f-fmiso in patients with high-grade glioma: 18f-faza and other hypoxia radiotracers. Clin. Transl. Imaging 2020, 8, 11–20. [Google Scholar] [CrossRef]
- Riccardo, L.; Natale, Q.; Pierpaolo, A.; Domenico, A.; Maria, G.; Rexhep, D.; Francesco, B.; Sergio, B.; On the behalf of Young AIMN Working Group. 18f-fmiso pet imaging: Insights over MRI in patients with glioma. Clin. Transl. Imaging 2020, 8, 3–10. [Google Scholar] [CrossRef]
- Singh, H.; Maurya, V.; Gill, S.S. Computerised tomography features in gliomas. Med. J. Armed Forces India 2002, 58, 221–225. [Google Scholar] [CrossRef]
- Abd-Elghany, A.A.; Naji, A.A.; Alonazi, B.; Aldosary, H.; Alsufayan, M.A.; Alnasser, M.; Mohammad, E.A.; Mahmoud, M.Z. Radiological characteristics of glioblastoma multiforme using CT and MRI examination. J. Radiat. Res. Appl. Sci. 2019, 12, 289–293. [Google Scholar] [CrossRef]
- Bruzzone, M.G.; D’Incerti, L.; Farina, L.L.; Cuccarini, V.; Finocchiaro, G. CT and MRI of brain tumors. Q. J. Nucl. Med. Mol. Imaging. 2012, 56, 112–137. [Google Scholar] [PubMed]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality brain tumor imaging: MR imaging, PET, and PET/MR imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef]
- Shukla, G.; Alexander, G.S.; Bakas, S.; Nikam, R.; Talekar, K.; Palmer, J.D.; Shi, W. Advanced magnetic resonance imaging in glioblastoma: A review. Chin. Clin. Oncol. 2017, 6, 7. [Google Scholar] [CrossRef]
- Thust, S.C.; Heiland, S.; Falini, A.; Jäger, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef]
- Valentini, M.C.; Mellai, M.; Annovazzi, L.; Melcarne, A.; Denysenko, T.; Cassoni, P.; Casalone, C.; Maurella, C.; Grifoni, S.; Fania, P.; et al. Comparison among conventional and advanced MRI, (18)f-fdg PET/CT, phenotype and genotype in glioblastoma. Oncotarget 2017, 8, 91636–91653. [Google Scholar] [CrossRef]
- Thomas, D.G.; Beaney, R.P.; Brooks, D.J. Positron emission tomography in the study of cerebral tumours. Neurosurg. Rev. 1984, 7, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Valentini, C.; Melcarne, A.; Mellai, M.; Prodi, E.; Carrara, G.; Denysenko, T.; Junemann, C.; Casalone, C.; Corona, C. Spatial relationships of MR imaging and positron emission tomography with phenotype, genotype and tumor stem cell generation in glioblastoma multiforme. In Tumors of the Central Nervous System—Primary and Secondary; INTECH: London, UK, 2014; pp. 63–93. [Google Scholar]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougere, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-oncology 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: A systematic review of published meta-analyses. Int. J. Mol. Sci 2019, 20, 4669. [Google Scholar] [CrossRef] [PubMed]
- DeLaPaz, R.L.; Patronas, N.J.; Brooks, R.A.; Smith, B.H.; Kornblith, P.L.; Milam, H.; Di Chiro, G. Positron emission tomographic study of suppression of gray-matter glucose utilization by brain tumors. AJNR. Am. J. Neuroradiol. 1983, 4, 826–829. [Google Scholar] [PubMed]
- Li, L.; Mu, W.; Wang, Y.; Liu, Z.; Liu, Z.; Wang, Y.; Ma, W.; Kong, Z.; Wang, S.; Zhou, X.; et al. A non-invasive radiomic method using (18)f-fdg pet predicts isocitrate dehydrogenase genotype and prognosis in patients with glioma. Fron. Oncol. 2019, 9, 1183. [Google Scholar] [CrossRef]
- Arora, G.; Sharma, P.; Sharma, A.; Mishra, A.K.; Hazari, P.P.; Biswas, A.; Garg, A.; Aheer, D.; Kumar, R. 99mtc-methionine hybrid SPECT/CT for detection of recurrent glioma: Comparison with 18f-fdg PET/CT and contrast-enhanced MRI. Clin. Nucl. Med. 2018, 43, e132–e138. [Google Scholar] [CrossRef]
- Back, M.; LeMottee, M.; Crasta, C.; Bailey, D.; Wheeler, H.; Guo, L.; Eade, T. Reducing radiation dose to normal brain through a risk adapted dose reduction protocol for patients with favourable subtype anaplastic glioma. Radiat. Oncol. 2017, 12, 46. [Google Scholar] [CrossRef]
- Chiang, G.C.; Galla, N.; Ferraro, R.; Kovanlikaya, I. The added prognostic value of metabolic tumor size on FDG-PET at first suspected recurrence of glioblastoma multiforme. J. Neuroimaging 2017, 27, 243–247. [Google Scholar] [CrossRef]
- Hatzoglou, V.; Yang, T.J.; Omuro, A.; Gavrilovic, I.; Ulaner, G.; Rubel, J.; Schneider, T.; Woo, K.M.; Zhang, Z.; Peck, K.K.; et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 fdg PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro-oncology 2016, 18, 873–880. [Google Scholar] [CrossRef]
- Hirata, T.; Kinoshita, M.; Tamari, K.; Seo, Y.; Suzuki, O.; Wakai, N.; Achiha, T.; Umehara, T.; Arita, H.; Kagawa, N.; et al. 11c-methionine-18f-fdg dual-pet-tracer-based target delineation of malignant glioma: Evaluation of its geometrical and clinical features for planning radiation therapy. J. Neurosurg. 2019, 131, 676–686. [Google Scholar] [CrossRef]
- Hojjati, M.; Badve, C.; Garg, V.; Tatsuoka, C.; Rogers, L.; Sloan, A.; Faulhaber, P.; Ros, P.R.; Wolansky, L.J. Role of fdg-Pet/Mri, fdg-Pet/Ct, and dynamic susceptibility contrast perfusion mri in differentiating radiation necrosis from tumor recurrence in glioblastomas. J. Neuroimaging 2018, 28, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Iagaru, A.; Mosci, C.; Mittra, E.; Zaharchuk, G.; Fischbein, N.; Harsh, G.; Li, G.; Nagpal, S.; Recht, L.; Gambhir, S.S. Glioblastoma multiforme recurrence: An exploratory study of (18)f fpprgd2 pet/ct. Radiology 2015, 277, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, M.; Nishizaki, T.; Ikeda, N.; Okamura, T.; Tanaka, Y.; Fujii, N.; Ohno, M.; Shimabukuro, T.; Kimura, T.; Ikeda, E.; et al. A surgical strategy using a fusion image constructed from 11c-methionine pet, 18f-fdg-PET and MRI for glioma with no or minimum contrast enhancement. J. Neurooncol 2018, 138, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Taneja, S.; Jha, A.; Damesha, N.K.; Negi, P.; Jadhav, G.K.; Verma, S.M.; Sogani, S.K. Multiparametric evaluation in differentiating glioma recurrence from treatment-induced necrosis using simultaneous (18)f-fdg-PET/MRI: A single-institution retrospective study. AJNR. Am. J. Neuroradiol. 2017, 38, 899–907. [Google Scholar] [CrossRef]
- Leiva-Salinas, C.; Schiff, D.; Flors, L.; Patrie, J.T.; Rehm, P.K. Fdg PET/MR imaging coregistration helps predict survival in patients with glioblastoma and radiologic progression after standard of care treatment. Radiology 2017, 283, 508–514. [Google Scholar] [CrossRef]
- Lundemann, M.; Munck Af Rosenschold, P.; Muhic, A.; Larsen, V.A.; Poulsen, H.S.; Engelholm, S.A.; Andersen, F.L.; Kjaer, A.; Larsson, H.B.W.; Law, I.; et al. Feasibility of multi-parametric pet and mri for prediction of tumour recurrence in patients with glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 603–613. [Google Scholar] [CrossRef]
- Nakajima, S.; Okada, T.; Yamamoto, A.; Kanagaki, M.; Fushimi, Y.; Okada, T.; Arakawa, Y.; Takagi, Y.; Miyamoto, S.; Togashi, K. Primary central nervous system lymphoma and glioblastoma: Differentiation using dynamic susceptibility-contrast perfusion-weighted imaging, diffusion-weighted imaging, and (18)f-fluorodeoxyglucose positron emission tomography. Clin. Imaging 2015, 39, 390–395. [Google Scholar] [CrossRef]
- O’Neill, A.F.; Qin, L.; Wen, P.Y.; de Groot, J.F.; Van den Abbeele, A.D.; Yap, J.T. Demonstration of DCE-MRI as an early pharmacodynamic biomarker of response to vegf trap in glioblastoma. J. Neurooncol. 2016, 130, 495–503. [Google Scholar] [CrossRef]
- Sacconi, B.; Raad, R.A.; Lee, J.; Fine, H.; Kondziolka, D.; Golfinos, J.G.; Babb, J.S.; Jain, R. Concurrent functional and metabolic assessment of brain tumors using hybrid PET/MR imaging. J. Neurooncol. 2016, 127, 287–293. [Google Scholar] [CrossRef]
- Sakata, A.; Okada, T.; Yamamoto, Y.; Fushimi, Y.; Dodo, T.; Arakawa, Y.; Mineharu, Y.; Schmitt, B.; Miyamoto, S.; Togashi, K. Addition of amide proton transfer imaging to fdg-PET/CT improves diagnostic accuracy in glioma grading: A preliminary study using the continuous net reclassification analysis. AJNR Am. J. Neuroradiol. 2018, 39, 265–272. [Google Scholar] [CrossRef]
- Seligman, L.; Kovanlikaya, I.; Pisapia, D.J.; Naeger, D.M.; Magge, R.; Fine, H.A.; Chiang, G.C. Integrated PET-MRI for glioma surveillance: Perfusion-metabolism discordance rate and association with molecular profiling. AJR Am. J. Roentgenol. 2019, 212, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; D’Souza, M.; Jaimini, A.; Hazari, P.P.; Saw, S.; Pandey, S.; Singh, D.; Solanki, Y.; Kumar, N.; Mishra, A.K.; et al. A comparison study of (11)c-methionine and (18)f-fluorodeoxyglucose positron emission tomography-computed tomography scans in evaluation of patients with recurrent brain tumors. Indian J. Nucl. Med. 2016, 31, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.B.; Jeffree, R.L.; Thomas, P.; Goodman, S.; Debowski, M.; Lwin, Z.; Chua, B. Diagnostic performance of 18f-fluorodeoxyglucose positron emission tomography in the evaluation of glioma. J. Med. Imaging. Radiat. Oncol. 2019, 63, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Song, P.J.; Lu, Q.Y.; Li, M.Y.; Li, X.; Shen, F. Comparison of effects of 18f-fdg PET-CT and MRI in identifying and grading gliomas. J. Biol. Regul. Homeost. Agent. 2016, 30, 833–838. [Google Scholar]
- Takano, K.; Kinoshita, M.; Arita, H.; Okita, Y.; Chiba, Y.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Kanemura, Y.; Nonaka, M.; et al. Diagnostic and prognostic value of 11c-methionine pet for nonenhancing gliomas. AJNR Am. J. Neuroradiol. 2016, 37, 44–50. [Google Scholar] [CrossRef]
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Kitamura, Y.; Mizoguchi, M.; Yoshimoto, K.; Kuga, D.; Suzuki, S.O.; Baba, S.; et al. Diagnostic utility of intravoxel incoherent motion MR imaging in differentiating primary central nervous system lymphoma from glioblastoma multiforme. J. Magn. Reson. Imaging JMRI 2016, 44, 1256–1261. [Google Scholar] [CrossRef]
- Makino, K.; Hirai, T.; Nakamura, H.; Murakami, R.; Kitajima, M.; Shigematsu, Y.; Nakashima, R.; Shiraishi, S.; Uetani, H.; Iwashita, K.; et al. Does adding fdg-pet to MRI improve the differentiation between primary cerebral lymphoma and glioblastoma? Observer performance study. Ann. Nucl. Med. 2011, 25, 432–438. [Google Scholar] [CrossRef]
- Zou, Y.; Tong, J.; Leng, H.; Jiang, J.; Pan, M.; Chen, Z. Diagnostic value of using 18f-fdg PET and PET/CT in immunocompetent patients with primary central nervous system lymphoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 41518–41528. [Google Scholar] [CrossRef]
- Herholz, K. Brain tumors: An update on clinical pet research in gliomas. Semin. Nucl. Med. 2017, 47, 5–17. [Google Scholar] [CrossRef]
- Manabe, O.; Hattori, N.; Yamaguchi, S.; Hirata, K.; Kobayashi, K.; Terasaka, S.; Kobayashi, H.; Motegi, H.; Shiga, T.; Magota, K.; et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur. J. Nucl Med. Mol. Imaging 2015, 42, 896–904. [Google Scholar] [CrossRef]
- Tsiouris, S.; Bougias, C.; Fotopoulos, A. Principles and current trends in the correlative evaluation of glioma with advanced MRI techniques and PET. Hell. J. Nucl. Med. 2019, 22, 206–219. [Google Scholar] [PubMed]
- Zukotynski, K.A.; Fahey, F.H.; Vajapeyam, S.; Ng, S.S.; Kocak, M.; Gururangan, S.; Kun, L.E.; Poussaint, T.Y. Exploratory evaluation of MR permeability with 18f-fdg PET mapping in pediatric brain tumors: A report from the pediatric brain tumor consortium. J. Nucl. Med. 2013, 54, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Amide proton transfer imaging of the human brain. Methods Mol. Biol. 2011, 711, 227–237. [Google Scholar] [PubMed]
- Enslow, M.S.; Zollinger, L.V.; Morton, K.A.; Butterfield, R.I.; Kadrmas, D.J.; Christian, P.E.; Boucher, K.M.; Heilbrun, M.E.; Jensen, R.L.; Hoffman, J.M. Comparison of 18f-fluorodeoxyglucose and 18f-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin. Nucl. Med. 2012, 37, 854–861. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Xie, P.; Li, W.; Li, X.; Ma, L. Comparison of magnetic resonance spectroscopy and positron emission tomography in detection of tumor recurrence in posttreatment of glioma: A diagnostic meta-analysis. Asia Pac. J. Clin. Oncol. 2015, 11, 97–105. [Google Scholar] [CrossRef]
- Kinoshita, M.; Arita, H.; Goto, T.; Okita, Y.; Isohashi, K.; Watabe, T.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Shimosegawa, E.; et al. A novel pet index, 18f-fdg-11c-methionine uptake decoupling score, reflects glioma cell infiltration. J. Nucl. Med. 2012, 53, 1701–1708. [Google Scholar] [CrossRef]
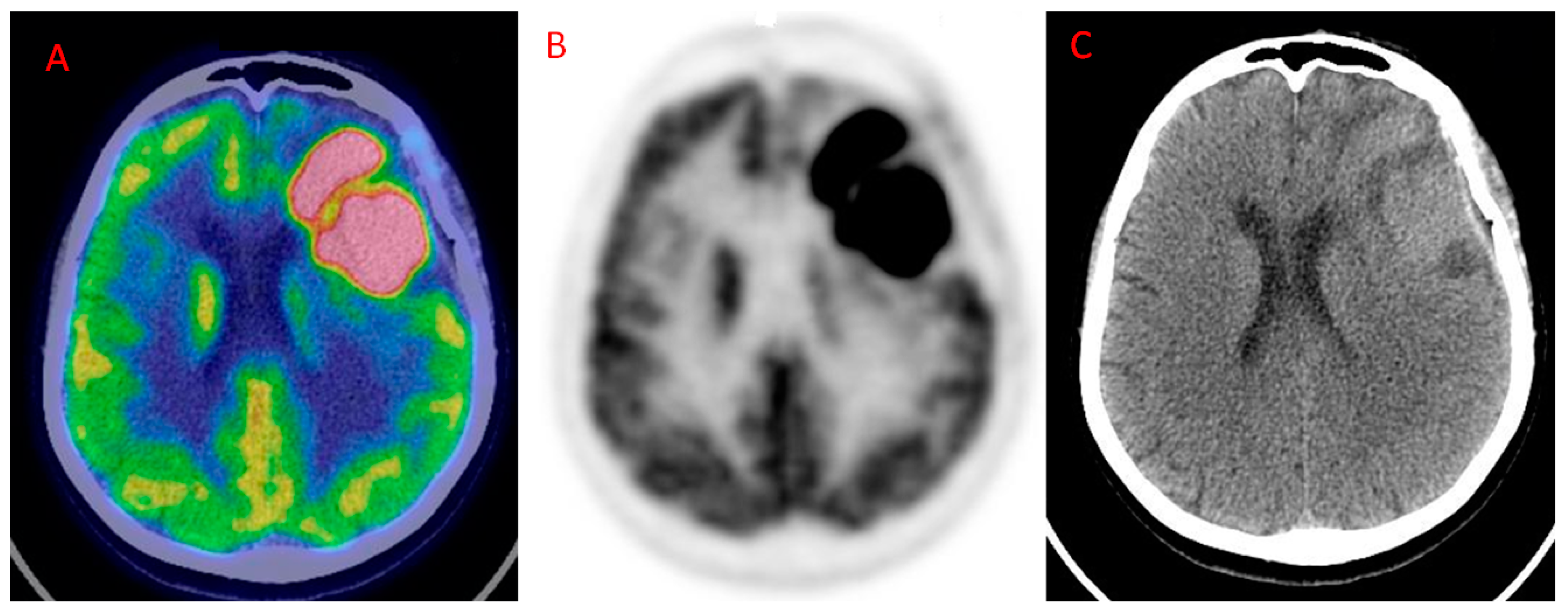
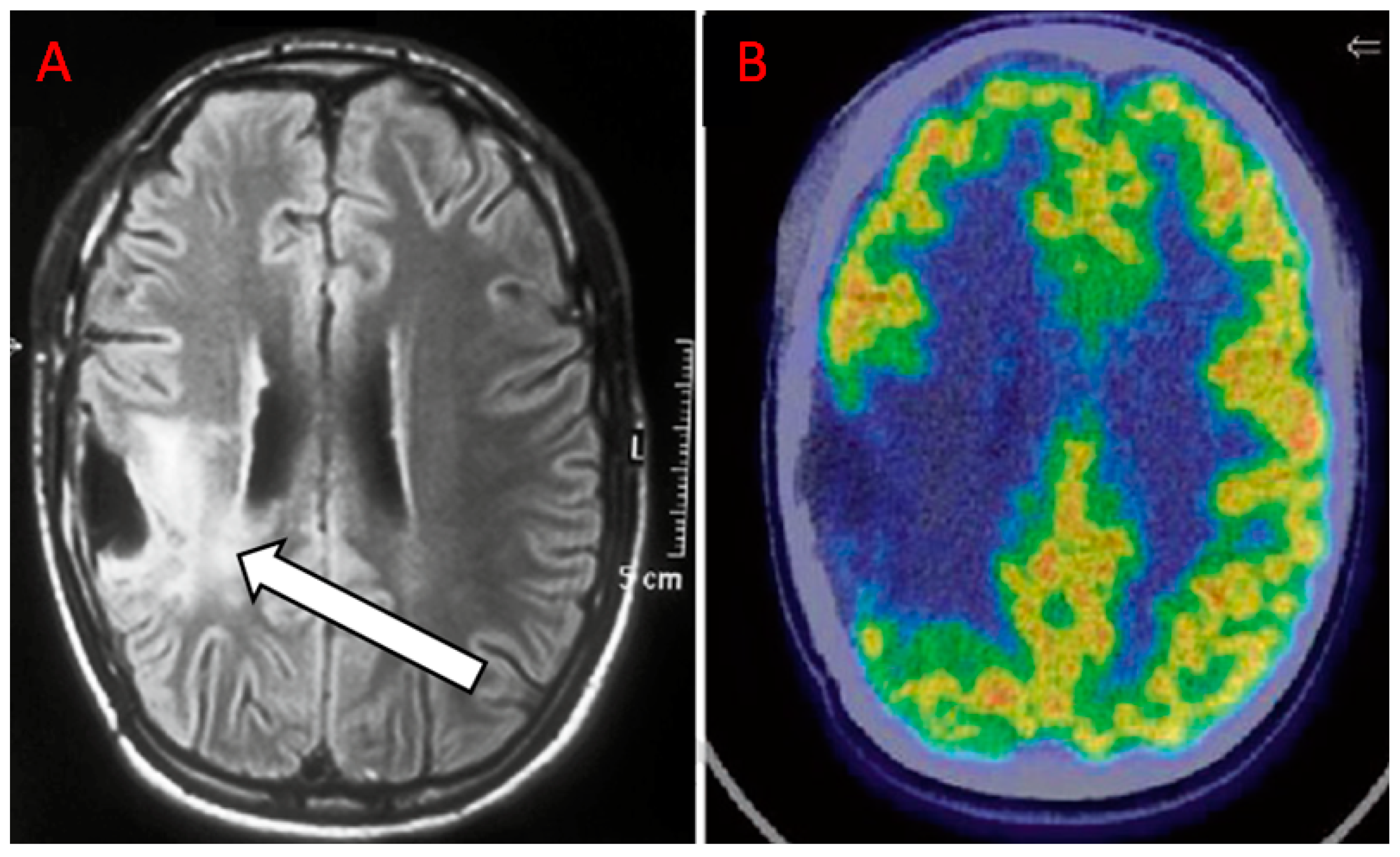
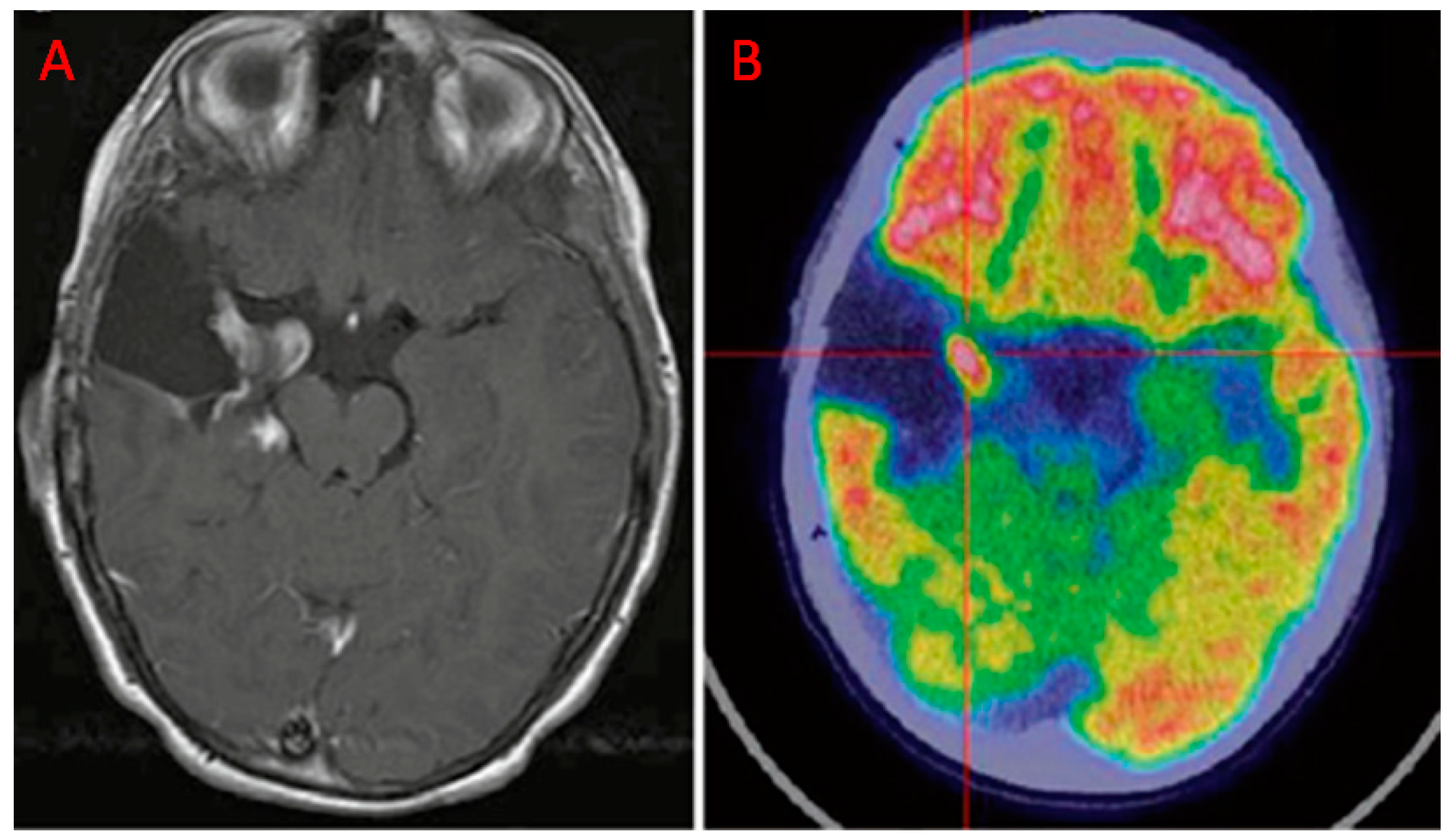
| Authors | Year | Study Design | Number of Patients | Tumor Histotype/ Glioma Grade | PET Scanner Type | MRI Technique | Main Findings |
|---|---|---|---|---|---|---|---|
| Diagnosis and Differential Diagnosis | |||||||
| Valentini et al. [20] | 2017 | R | 12 (48 biopsy specimens) | GBM | PET/CT | DWI, DTI, DSC-PWI, MRSI | Highest values of rCBV, Cho/Cr, Cho/NAA, proportional decrease of SUVmax with increasing distance from the CE region. At histological examination, the CE region showed maximum tumor histological malignancy and presented the maximum values of rCBV, Cho/Cr, Cho/NAA, LL and SUVmax. |
| Yamashita et al. [47] | 2016 | R | 50 | GBM = 33 PCNSL = 17 | PET/CT | DWI, IVIM | Significantly higher fmax (p < 0.001) and Dmin (p < 0.0001) and significantly lower SUVmax (p < 0.0005) in GBM than in PCNSL. |
| Nakajima et al. [38] | 2015 | R | 34 | GBM = 23 PCNSL = 11 | PET/CT | DWI, DSC-PWI | High SS (100%) and SP (73.9%) of 18F-FDG PET in differentiating GBM from PCNSL. Good accuracy of ADC5% and uncorr |
| Grading | |||||||
| Shaw et al. [44] | 2019 | R | 33 | 36 histology samples: II = 11 III = 17 IV = 4 metastases = 1 benign = 3 | PET/CT | Gd MRI | Combination of PET and MRI imaging enhances AC in identifying high-grade regions of glioma. PET: SS = 59%, SP = 79%, PPV = 89%, NPV = 55%. MRI: SS = 77%, SP = 86%, PPV = 89%, NPV = 71%. Combined PET and MRI: SS = 79%, SP = 100%, PPV = 100%, NPV = 75%. |
| Sakata et al. [41] | 2018 | R | 49 | II = 15 III-IV = 34 | PET/CT | DWI, APT | Comparable AC of T/N and ADCmin and amide proton transfer in the discrimination of HGGs from LGGs. A larger increase for the diagnosis of HGGs with the combination APT + T/N compared to ADCmin + T/N. |
| Takano et al. [46] | 2016 | R | 35 | II = 23 III = 12 | PET/CT | DTI, DWI | No satisfactory performance for average fractional anisotropy, and maximum fractional anisotropy, minimum ADC, T/Nmax and T/Nave in discriminating III from II grade. |
| Song et al. [45] | 2016 | R | 70 | LGG and HGG | PET/CT | Gd MRI | 18F-FDG PET/CT performs better (in terms of SS, SP and AC) than MRI (p < 0.05) for identifying different grades of glioma. |
| Sacconi et al. [40] | 2016 | R | 20 | II = 6 III = 3 IV = 6 metastases = 2 meningioma = 2 lymphoma = 1 | PET/MR | PWI | Utility of rCBVmean and SUVmean in discriminating HGGs from LGGS. rCBVmean (optimal cut-off value = 1.74): SS = 100%, SP = 74%. SUVmean, (optimal cut-off value = 4.0): SS = 50%, SP = 79.5%. |
| Prognosis | |||||||
| Lundemann et al. [37] | 2019 | P | 16 | GBM | PET/CT (18F-FET) PET/MR (18F-FDG) | DWI, DCE | 18F-FDG and 18F-FET uptake demonstrate the highest mutual correlation in CELs and NELs, with 18F-FET being the most important to predict recurrence. Fractional anisotropy resulted in the second most important parameter for recurrence probability in apparently healthy tissue. |
| Chiang et al. [29] | 2017 | R | 44 | GBM | PET/CT | ADC | Metabolic tumor volume and tumor cross products on 18F-FDG PET and on MRI may serve as prognostic variables. Combining the cross products of both PET and MRI, the AC in predicting poor survival increased to 74% from 58% using MRI alone. |
| Leiva-Salinas et al. [36] | 2017 | R | 56 | GBM | PET/CT | Gd MRI | SUVr may be a useful imaging marker to identify patients’ decreased survival after standard therapy. SUVr was not influenced by tumor size and location on MRI images at diagnosis. |
| Assessment of Recurrence | |||||||
| Seligman et al. [42] | 2019 | R | 41 | III = 21 IV = 20 | PET/MRI | DCE | 18F-FDG PET and DCE-MRI hold comparable AC (80% vs. 83%) in identifying tumor recurrence. |
| Hojjati et al. [32] | 2018 | R | 24 (28 lesions) | GBM | PET/MRI PET/CT | DCE, DSC-PWI, DWI | The authors documented an AUC of 1.0 in a joint predictive model including r-mean ≥ 1.31 and a CBV ≥ 3.32. By contrast, a model encompassing only CBV ≥ 3.32 demonstrated a lower AUC (0.94). |
| Arora et al. [27] | 2018 | P | 29 | LGG = 15, HGG = 14 | PET/CT | Gd MRI | On per-patient analysis, no significance difference was found between the performance of 18F-FDG PET/CT and MRI (AC = 82.8% vs. 76.6%) in detecting recurrence. MRI did not detect significantly more lesions than 18F-FDG PET/CT (p = 0.14). |
| Jena et al. [35] | 2017 | R | 35 | II = 9 III = 13 IV = 19 | PET/MR | DWI, PWI, MRS | PET provides complementary information to MRI. The AUC obtained combining MRI metrics (rCBV, mean ADC, Cho/Cr) and the PET parameter (mean T/N) was higher (0.935 ± 0.046) than the curve that resulted only from the three MRI parameters (0.913 ± 0.053). |
| Hatzoglou et al. [30] | 2016 | P | 29 | II = 7 III = 8 IV = 18 | PET/CT | DCE | The combination of a plasma volume ratio ≥ 2.1 and a SUVratio ≥ 1.2 improve the performance in distinguishing progression from radiation injury compared to individual PET and DCE metrics. |
| Sharma et al. [43] | 2016 | R | 64 | Low-grade astrocytoma = 22 High-grade astrocytoma = 16 Medulloblastoma = 10 Other miscellaneous brain tumors = 6 | PET/CT | NR | Good performance of PET and MRI in detecting recurrence in oligodendroglioma. In low-grade astrocytomas, a high rate of false positive cases (10/22 patients) were documented for PET. Nevertheless, PET was helpful in all cases reported as equivocal (n = 5) by MRI. |
| Iagaru et al. [33] | 2015 | P | 17 | GBM | PET/CT | Gd MRI | Similar diagnostic performance of the two modalities for recurrent GBM (13/15 detected recurrences for PET vs. 14/15 MR). |
| Treatment Planning and Evaluation of Response to Therapy | |||||||
| Idegushi et al. [34] | 2018 | P | 16 | II = 8 III = 8 | PET/CT | Gd MRI, T2-w, FLAIR | 18F-FDG PET may also help in planning surgical resection. Only partial overlap between 18F-FDG uptake and the contrast-enhancement area. Tissue extracted from the 18F-FDG and Gd MRI positive areas presented anaplastic features. Tissue extracted from 18F-FDG and Gd MRI negative areas resulted in grade II glioma at pathological examination. |
| Hirata et al. [31] | 2019 | P | 25 | III = 10 IV = 15 | PET | Gd MRI, T2-w | Tumor delineation is underestimated by Gd MRI. High overlap of DS and T1-Gd positively influenced survival. |
| Back et al. [28] | 2017 | P | 10 | III | PET/CT | T1-w, Gd MRI, T2-w | 18F-FDG PET guided integrated boost intensity-modulated RT (b-IMRT) that may result in a reduced dose to the normal brain when compared to standard IMRT (s-IMRT). |
| O’Neill et al. [39] | 2016 | P | 12 | III | PET/CT | DCE, DWI | The MRI-derived metrics (ADCmean, Ktrans, Ve) demonstrated significant variation in the patients (median difference of Ktrans = −41.8%, p < 0.02, median difference of Ve = −42.6%, p < 0.04), possibly reflecting the early effects of VEGF trap on tumour vasculature. No systematic changes were observed for SUVmax (median difference = −7.8%, p > 0.67). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, N.; Laudicella, R.; Vento, A.; Pignata, S.; Mattoli, M.V.; Filice, R.; Comis, A.D.; Arnone, A.; Baldari, S.; Cabria, M.; et al. The Additional Value of 18F-FDG PET and MRI in Patients with Glioma: A Review of the Literature from 2015 to 2020. Diagnostics 2020, 10, 357. https://doi.org/10.3390/diagnostics10060357
Quartuccio N, Laudicella R, Vento A, Pignata S, Mattoli MV, Filice R, Comis AD, Arnone A, Baldari S, Cabria M, et al. The Additional Value of 18F-FDG PET and MRI in Patients with Glioma: A Review of the Literature from 2015 to 2020. Diagnostics. 2020; 10(6):357. https://doi.org/10.3390/diagnostics10060357
Chicago/Turabian StyleQuartuccio, Natale, Riccardo Laudicella, Antonio Vento, Salvatore Pignata, Maria Vittoria Mattoli, Rossella Filice, Alessio Danilo Comis, Annachiara Arnone, Sergio Baldari, Manlio Cabria, and et al. 2020. "The Additional Value of 18F-FDG PET and MRI in Patients with Glioma: A Review of the Literature from 2015 to 2020" Diagnostics 10, no. 6: 357. https://doi.org/10.3390/diagnostics10060357
APA StyleQuartuccio, N., Laudicella, R., Vento, A., Pignata, S., Mattoli, M. V., Filice, R., Comis, A. D., Arnone, A., Baldari, S., Cabria, M., & Cistaro, A. (2020). The Additional Value of 18F-FDG PET and MRI in Patients with Glioma: A Review of the Literature from 2015 to 2020. Diagnostics, 10(6), 357. https://doi.org/10.3390/diagnostics10060357







