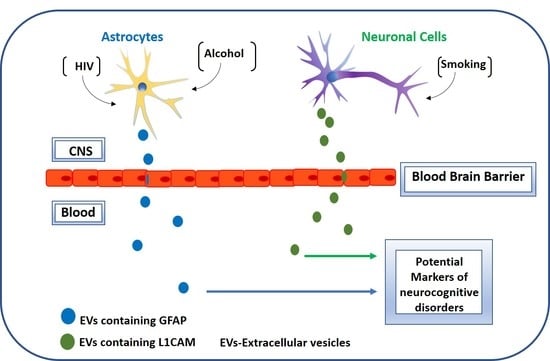
Circulatory Astrocyte and Neuronal EVs as Potential Biomarkers of Neurological Dysfunction in HIV-Infected Subjects and Alcohol/Tobacco Users
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
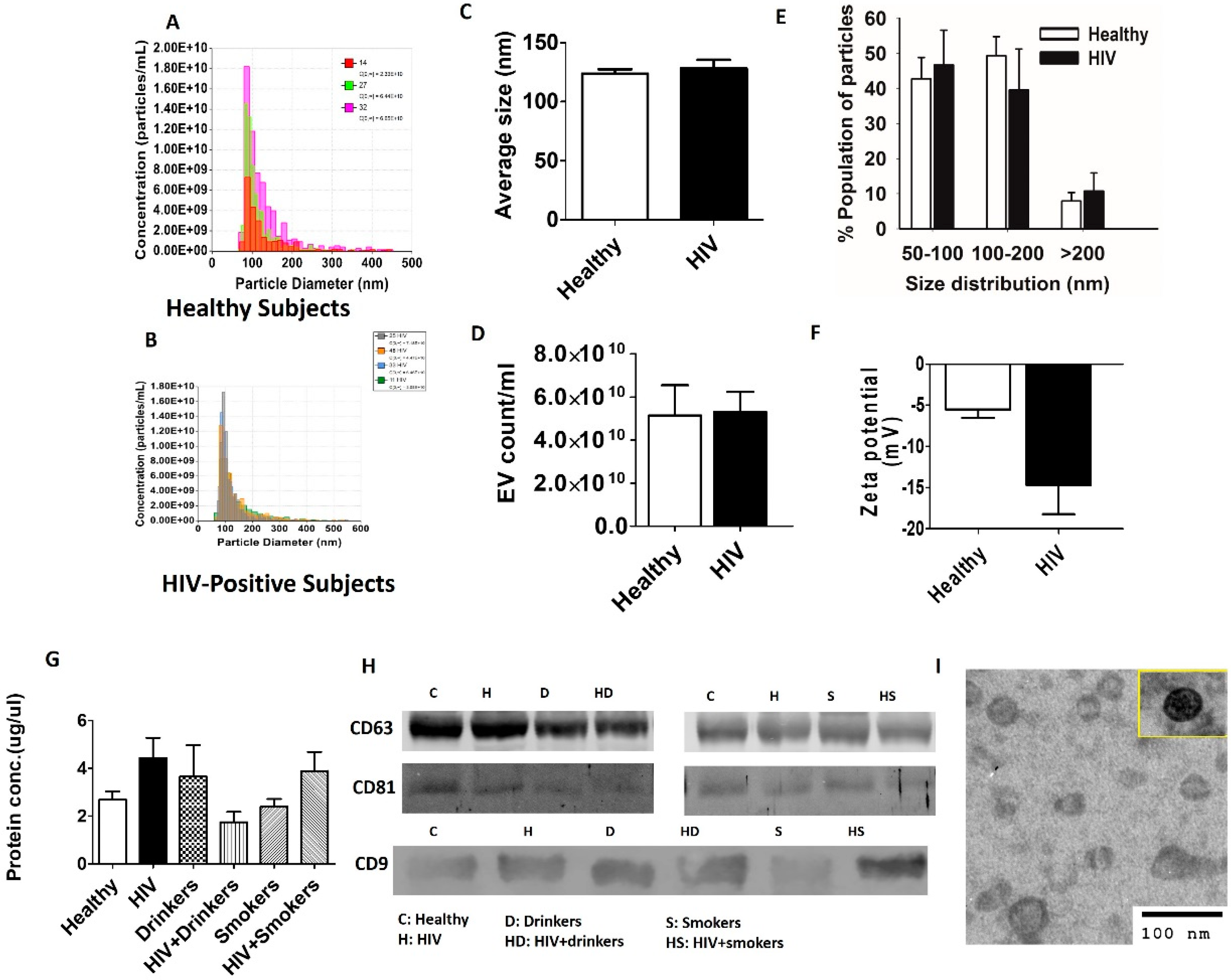
2.2. Plasma EV Isolation and Characterization
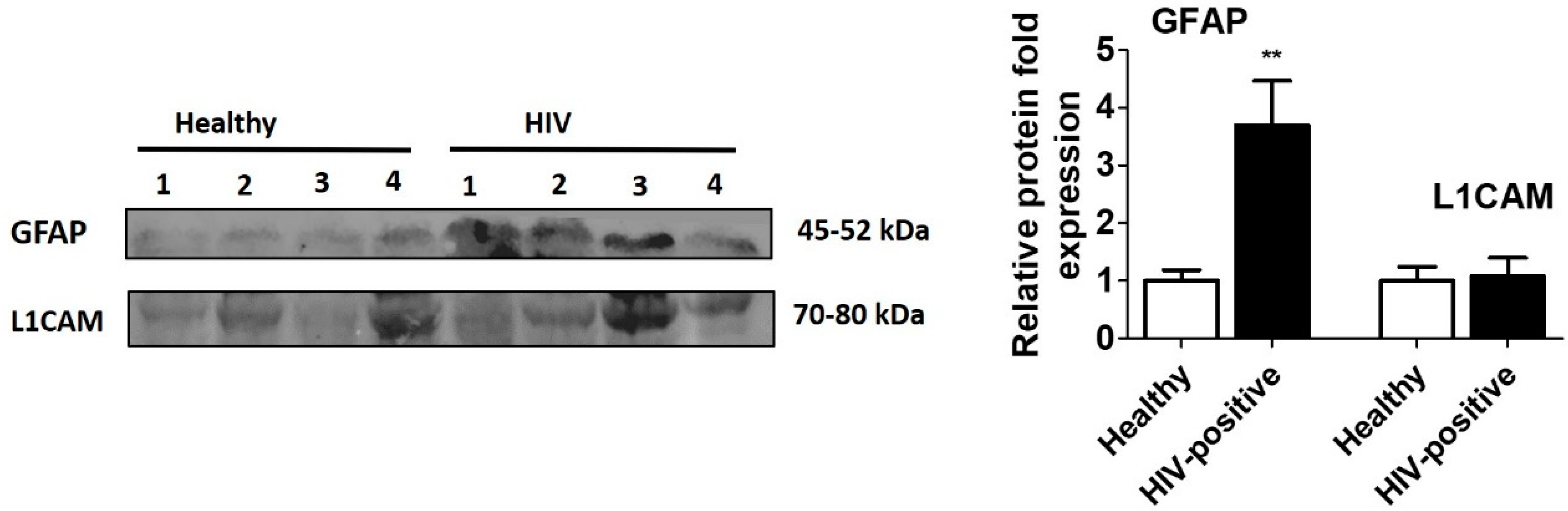
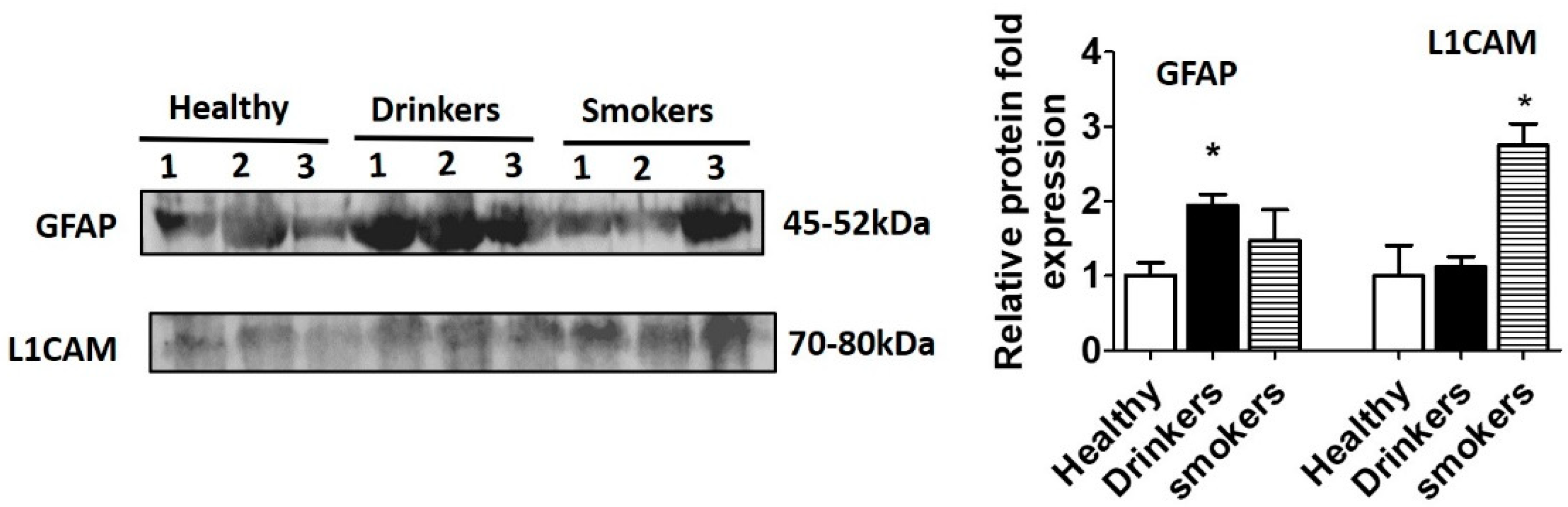
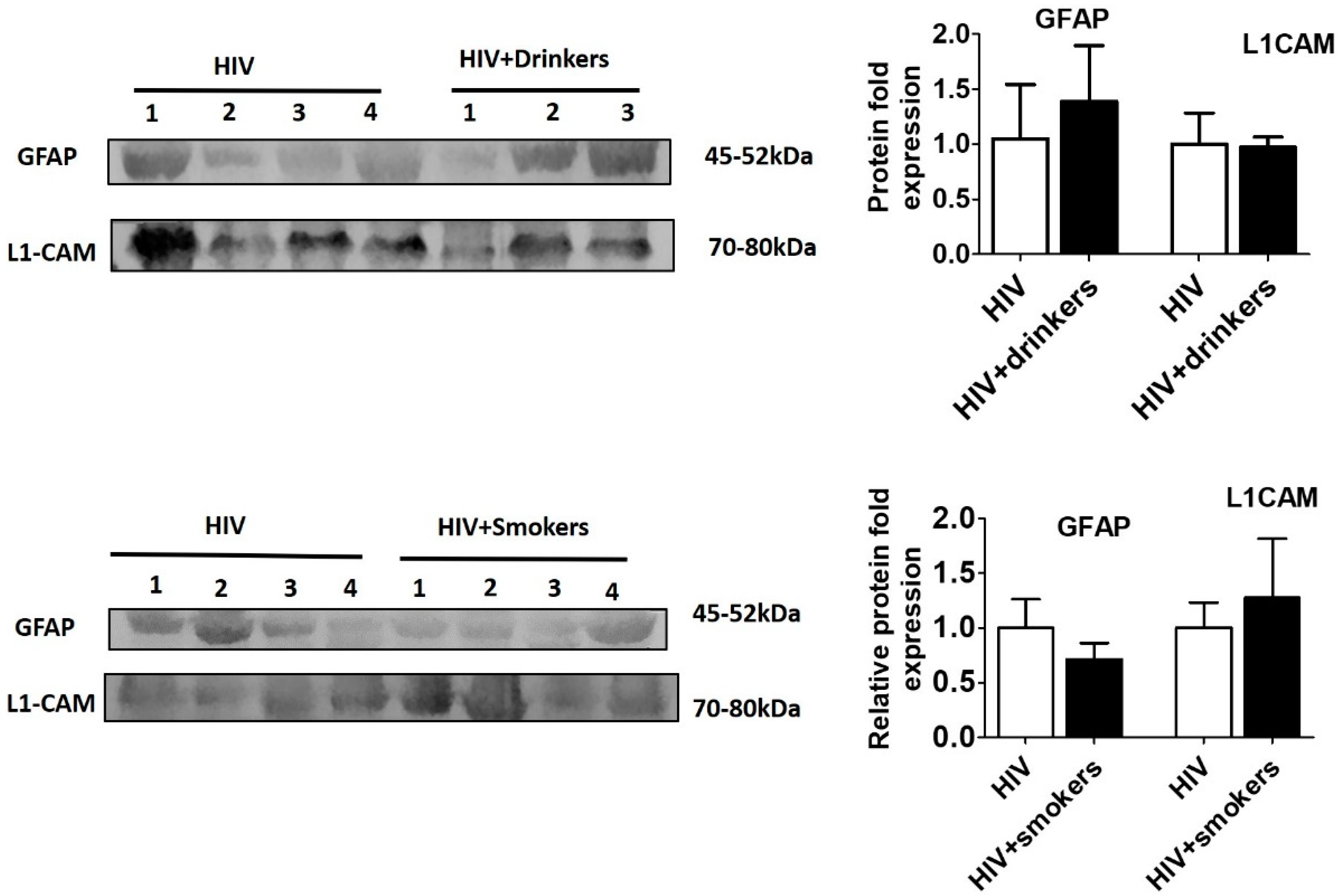
2.3. Western Blotting
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ellis, R.; Calero, P.; Stockin, M.D. HIV Infection and the Central Nervous System: A Primer. Neuropsychol. Rev. 2009, 19, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Banks, W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2014, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- HIV Treatment Overview. Available online: https://www.hiv.gov/hiv-basics/staying-in-hiv-care/hiv-treatment/hiv-treatment-overview (accessed on 8 April 2020).
- Ene, L.; Duiculescu, D.; Ruta, S.M. How much do antiretroviral drugs penetrate the central nervous system? J. Med. Life 2011, 4, 432–439. [Google Scholar]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Gisslén, M.; Cinque, P.; Price, R.W. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr. HIV/AIDS Rep. 2015, 12, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Valero, I.P.; Ellis, R.; Heaton, R.; Deutsch, R.; Franklin, D.; Clifford, D.B.; Collier, A.; Gelman, B.; Marra, C.; McCutchan, J.A.; et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy. AIDS 2019, 33, 475–481. [Google Scholar] [CrossRef]
- Spudich, S.S.; Ances, B.M. Central Nervous System Complications of HIV Infection. Top. Antivir. Med. 2016, 19, 48–57. [Google Scholar]
- Spudich, S.; González-Scarano, F. HIV-1-Related Central Nervous System Disease: Current Issues in Pathogenesis, Diagnosis, and Treatment. Cold Spring Harb. Perspect. Med. 2012, 2, a007120. [Google Scholar] [CrossRef]
- Nabha, L.; Duong, L.; Timpone, J.G. HIV-associated neurocognitive disorders: Perspective on management strategies. Drugs 2013, 73, 893–905. [Google Scholar] [CrossRef]
- Clifford, D.B.; Ances, B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; Leblanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2010, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Atluri, V.; Nair, M. Drugs of Abuse in HIV infection and neurotoxicity. Front. Microbiol. 2015, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.A.; Zerbo, E. HIV-Related Neurocognitive Disorders and Drugs of Abuse: Mired in Confound, Surrounded by Risk. Curr. Addict. Rep. 2014, 1, 229–236. [Google Scholar] [CrossRef]
- Kumar, S.; Jin, M.; Ande, A.; Sinha, N.; Silverstein, P.S.; Kumar, A. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Ande, A.; McArthur, C.; Ayuk, L.; Awasom, C.; Achu, P.N.; Njinda, A.; Sinha, N.; Rao, P.S.S.; Agudelo, M.; Nookala, A.R.; et al. Effect of Mild-to-Moderate Smoking on Viral Load, Cytokines, Oxidative Stress, and Cytochrome P450 Enzymes in HIV-Infected Individuals. PLoS ONE 2015, 10, e0122402. [Google Scholar] [CrossRef]
- Rao, P.; Ande, A.; Sinha, N.; Kumar, A.; Kumar, S. Effects of Cigarette Smoke Condensate on Oxidative Stress, Apoptotic Cell Death, and HIV Replication in Human Monocytic Cells. PLoS ONE 2016, 11, e0155791. [Google Scholar] [CrossRef]
- Ande, A.; McArthur, C.; Kumar, A.; Kumar, S. Tobacco smoking effect on HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1453–1464. [Google Scholar] [CrossRef]
- Ranjit, S.; Sinha, N.; Kodidela, S.; Kumar, S. Benzo(a)pyrene in Cigarette Smoke Enhances HIV-1 Replication through NF-κB Activation via CYP-Mediated Oxidative Stress Pathway. Sci. Rep. 2018, 8, 10394. [Google Scholar] [CrossRef]
- Kodidela, S.; Kumar, S. Choosing the right pharmacotherapeutic strategy for HIV maintenance in patients with alcohol addiction. Expert Opin. Pharmacother. 2019, 20, 631–633. [Google Scholar] [CrossRef]
- Shi, M.; Sheng, L.; Stewart, T.; Zabetian, C.P.; Zhang, J. New windows into the brain: Central nervous system-derived extracellular vesicles in blood. Prog. Neurobiol. 2019, 175, 96–106. [Google Scholar] [CrossRef]
- Bavisotto, C.C.; Scalia, F.; Gammazza, A.M.; Carlisi, D.; Bucchieri, F.; De Macario, E.C.; Macario, A.J.L.; Cappello, F.; Campanella, C. Extracellular Vesicle-Mediated Cell–Cell Communication in the Nervous System: Focus on Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 434. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Lan, L.; Kumar, S.; Wang, X.; Yue, J.; et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflammat. 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Kodidela, S.; Ranjit, S.; Sinha, N.; McArthur, C.; Kumar, A.; Kumar, S. Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers. PLoS ONE 2018, 13, e0201144. [Google Scholar] [CrossRef] [PubMed]
- Kodidela, S.; Wang, Y.; Patters, B.J.; Gong, Y.; Sinha, N.; Ranjit, S.; Gerth, K.; Haque, S.; Cory, T.; McArthur, C.; et al. Proteomic Profiling of Exosomes Derived from Plasma of HIV-Infected Alcohol Drinkers and Cigarette Smokers. J. Neuroimmune Pharmacol. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kodidela, S.; Sinha, N.; Haque, S.; Shukla, P.K.; Rao, R.; Kumar, S. Plasma exosomes exacerbate alcohol- and acetaminophen-induced toxicity via CYP2E1 pathway. Sci. Rep. 2019, 9, 6571. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Dalvi, P.; Abadjian, L.; Tang, N.; Pulliam, L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 2017, 31, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jean, L.; Ali, A.A.; Johnny, A.; Bob, S.C.; Michael, H.; Clark, C.C. A Pilot Proof-Of-Principle Analysis Demonstrating Dielectrophoresis (DEP) as a Glioblastoma Biomarker Platform. Sci. Rep. 2019, 9, 10279. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Boil. 2006, 30, 1–29. [Google Scholar] [CrossRef]
- Zadjali, F.; Kumar, P.; Yao, Y.; Johnson, D.; Astrinidis, A.; Vogel, P.; Gross, K.; Bissler, J. Tuberous Sclerosis Complex Axis Controls Renal Extracellular Vesicle Production and Protein Content. Int. J. Mol. Sci. 2020, 21, 1729. [Google Scholar] [CrossRef]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef]
- Willis, C.; Ménoret, A.; Jellison, E.R.; Nicaise, A.M.; Vella, A.T.; Crocker, S.J. A Refined Bead-Free Method to Identify Astrocytic Exosomes in Primary Glial Cultures and Blood Plasma. Front. Mol. Neurosci. 2017, 11, 11. [Google Scholar] [CrossRef]
- Mustapic, M.; Eitan, E.; Werner, K.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Mol. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.-C.; Goldman, J.E. Regulation of glial fibrillary acidic protein (GFAP) expression in CNS development and in pathological states. J. Neuroimmunol. 1985, 8, 283–292. [Google Scholar] [CrossRef]
- Oswald, M.J.; Palmer, D.N.; Kay, G.W.; Shemilt, S.; Rezaie, P.; Cooper, J.D.; A Shemilt, S.J. Glial activation spreads from specific cerebral foci and precedes neurodegeneration in presymptomatic ovine neuronal ceroid lipofuscinosis (CLN6). Neurobiol. Dis. 2005, 20, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Vitković, L.; Da Cunha, A. Role for Astrocytosis in HIV-1-Associated Dementia. Mol. Asp. Myeloid Stem Cell Dev. 1995, 202, 105–116. [Google Scholar] [CrossRef]
- Fan, Y.; He, J.J. HIV-1 Tat Induces Unfolded Protein Response and Endoplasmic Reticulum Stress in Astrocytes and Causes Neurotoxicity through Glial Fibrillary Acidic Protein (GFAP) Activation and Aggregation. J. Boil. Chem. 2016, 291, 22819–22829. [Google Scholar] [CrossRef]
- Sporer, B.; Missler, U.; Magerkurth, O.; Koedel, U.; Wiesmann, M.; Pfister, H.W. Evaluation of CSF Glial Fibrillary Acidic Protein (GFAP) as a Putative Marker for HIV-Associated Dementia. Infection 2004, 32, 20–23. [Google Scholar] [CrossRef]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflammat. 2019, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Kiefel, H.; Bondong, S.; Hazin, J.; Ridinger, J.; Schirmer, U.; Riedle, S.; Altevogt, P. L1CAM. Cell Adhes. Migr. 2012, 6, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Mukerji, S.S.; Chettimada, S.; Misra, V.; Lorenz, D.R.; Morgello, S.; Gabuzda, D. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS 2019, 33, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Guerri, C.; Pascual, M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 2010, 44, 15–26. [Google Scholar] [CrossRef]
- Harper, C.G. The neurotoxicity of alcohol. Hum. Exp. Toxicol. 2007, 26, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Benarroch, E.E. Astrocyte-neuron interactions: Implications for epilepsy. Neurology 2009, 73, 1323–1327. [Google Scholar] [CrossRef]
- Eng, L.F.; Yu, A.C.; Lee, Y.L. Chapter 30: Astrocytic response to injury. Prog. Brain Res. 1992, 94, 353–365. [Google Scholar] [CrossRef]
- Burda, J.; Bernstein, A.M.; Sofroniew, M. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2015, 275, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Arrastia, R.; Wang, K.K.; Papa, L.; Sorani, M.D.; Yue, J.K.; Puccio, A.M.; McMahon, P.J.; Inoue, T.; Yuh, E.L.; Lingsma, H.F.; et al. Acute Biomarkers of Traumatic Brain Injury: Relationship between Plasma Levels of Ubiquitin C-Terminal Hydrolase-L1 and Glial Fibrillary Acidic Protein. J. Neurotrauma 2014, 31, 19–25. [Google Scholar] [CrossRef]
- Udomuksorn, W.; Mukem, S.; Kumarnsit, E.; Vongvatcharanon, S.; Vongvatcharanon, U. Effects of alcohol administration during adulthood on parvalbumin and glial fibrillary acidic protein immunoreactivity in the rat cerebral cortex. Acta Histochem. 2011, 113, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Kittner, H.; Berger, P.; Wirkner, K.; Schramek, J. The reaction of astrocytes and neurons in the hippocampus of adult rats during chronic ethanol treatment and correlations to behavioral impairments. Alcohol 1997, 14, 445–454. [Google Scholar] [CrossRef]
- Vongvatcharanon, U.; Mukem, S.; Udomuksorn, W.; Kumarsit, E.; Vongvatcharanon, S. Alcohol administration during adulthood induces alterations of parvalbumin and glial fibrillary acidic protein immunoreactivity in rat hippocampus and cingulate cortex. Acta Histochem. 2010, 112, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Abreu-Villaça, Y. Chapter 39—Developmental Neurotoxicity of Nicotine and Tobacco. In Handbook of Developmental Neurotoxicology; Slikker, W., Paule, M.G., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 439–452. ISBN 978-0-12-809405-1. [Google Scholar]
- Lobo-Torres, L.H.; Tamborelli-Garcia, R.C.; Camarini, R.; Marcourakis, T. Chapter 24—Tobacco Smoke and Nicotine: Neurotoxicity in Brain Development. In Addictive Substances and Neurological Disease; Watson, R.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 273–280. ISBN 978-0-12-805373-7. [Google Scholar]
- Baek, R.; Varming, K.; Jorgensen, M. Does smoking, age or gender affect the protein phenotype of extracellular vesicles in plasma? Transfus. Apher. Sci. 2016, 55, 44–52. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodidela, S.; Gerth, K.; Sinha, N.; Kumar, A.; Kumar, P.; Kumar, S. Circulatory Astrocyte and Neuronal EVs as Potential Biomarkers of Neurological Dysfunction in HIV-Infected Subjects and Alcohol/Tobacco Users. Diagnostics 2020, 10, 349. https://doi.org/10.3390/diagnostics10060349
Kodidela S, Gerth K, Sinha N, Kumar A, Kumar P, Kumar S. Circulatory Astrocyte and Neuronal EVs as Potential Biomarkers of Neurological Dysfunction in HIV-Infected Subjects and Alcohol/Tobacco Users. Diagnostics. 2020; 10(6):349. https://doi.org/10.3390/diagnostics10060349
Chicago/Turabian StyleKodidela, Sunitha, Kelli Gerth, Namita Sinha, Asit Kumar, Prashant Kumar, and Santosh Kumar. 2020. "Circulatory Astrocyte and Neuronal EVs as Potential Biomarkers of Neurological Dysfunction in HIV-Infected Subjects and Alcohol/Tobacco Users" Diagnostics 10, no. 6: 349. https://doi.org/10.3390/diagnostics10060349
APA StyleKodidela, S., Gerth, K., Sinha, N., Kumar, A., Kumar, P., & Kumar, S. (2020). Circulatory Astrocyte and Neuronal EVs as Potential Biomarkers of Neurological Dysfunction in HIV-Infected Subjects and Alcohol/Tobacco Users. Diagnostics, 10(6), 349. https://doi.org/10.3390/diagnostics10060349