Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics
Abstract
1. Introduction
2. Urinary EV Isolation
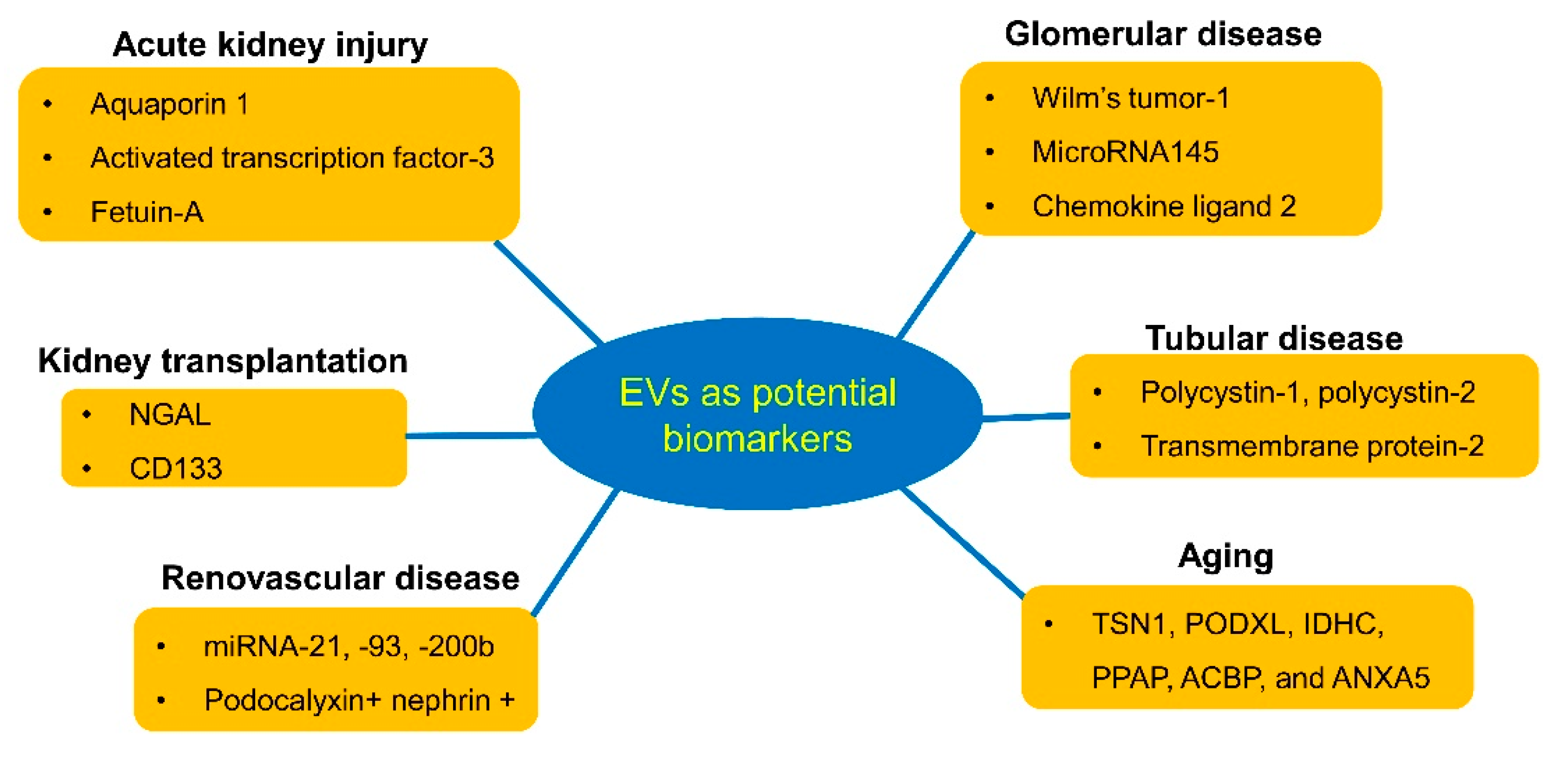
3. Urinary EVs as Diagnostic Biomarkers for Kidney Diseases
3.1. Acute Kidney Injury
3.2. Glomerular Disease
3.3. Tubular Disease
3.4. Aging
3.5. Renovascular Disease
3.6. Renal Transplantation
4. EVs as a Therapeutic Approach in Kidney Disease
5. Urinary EVs as Monitors of Drug Therapy
6. Urinary EVs as Vehicles for Drug Delivery
7. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| AQP1 | aquaporin-1 |
| CCL2 | chemokine ligand-2 |
| EV | extracellular vesicle |
| FSGS | focal segmental glomerulosclerosis |
| MiRNA | microRNA |
| MSC | mesenchymal stem cell |
| NCC | Na-Cl cotransporter |
| NGAL | neutrophil gelatinase-associated lipocalin |
| PC | polycystin |
| PKD | polycystic kidney disease |
| RVD | renovascular disease |
| SSNS | steroid-sensitive nephrotic syndrome |
| TMEM2 | transmembrane protein-2 |
| WT-1 | Wilm’s tumor-1 |
References
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and characterization of urinary vesicles: Implications for biomarker discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.L.; Khosroheidari, M.; Kanchi, R.R.; DiStefano, J.K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosomes isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012, 82, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Bontha, S.V.; La Salvia, S.; Fernandez-Piñeros, A.; Lannigan, J.; Le, T.H.; Erdbrügger, U. Rigorous characterization of urinary extracellular vesicles (uEVs) in the low centrifugation pellet—A neglected source for uEVs. Sci. Rep. 2020, 28, 3701. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemic-reperfusion injury. Am. J. Physiol. Renal Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef] [PubMed]
- dU Cheyron, D.; Daubin, C.; Poggioli, J.; Ramakers, M.; Houillier, P.; Charbonneau, P.; Paillard, M. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am. J. Kidney Dis. 2003, 42, 497–506. [Google Scholar] [CrossRef]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.S.; Hoffert, J.D.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.F.; Knepper, M.A.; et al. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006, 70, 1847–1857. [Google Scholar] [CrossRef]
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Panich, T.; Chancharoenthana, W.; Somparn, P.; Issara-Amphorn, J.; Hirankarn, N.; Leelahavanichkul, A. Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis induced acute kidney injury. BMC Nephrol. 2017, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kajiyama, H.; Tsuji, T.; Hu, X.; Leelahavanichkul, A.; Vento, S.; Frank, R.; Kopp, J.B.; Trachtman, H.; Star, R.A.; et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Renal Physiol. 2013, 305, F553–F559. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Mohan, A.; Godbole, M.M.; Bhatia, E.; Gupta, A.; Sharma, R.K.; Tiwari, S. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS ONE 2013, 8, e60177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Zhu, X.Y.; Eirin, A.; Nargesi, A.A.; Woollard, J.R.; Santelli, A.; Sun, I.O.; Textor, S.C.; Lerman, L.O. Early podocyte injury and elevated levels of urinary podocyte-derived extracellular vesicles in swin with metabolic syndrome: Role of podocyte mitochondria. Am. J. Physiol. Renal Physiol. 2019, 317, F12–F22. [Google Scholar] [CrossRef]
- Moon, P.G.; Lee, J.E.; You, S.; Kim, T.K.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Kim, C.D.; Park, S.H.; Hwang, D.; et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 2011, 11, 2459–2475. [Google Scholar] [CrossRef]
- Feng, Y.; Lv, L.L.; Wu, W.J.; Li, Z.L.; Chen, J.; Ni, H.F.; Zhou, L.T.; Tang, T.T.; Wang, F.M.; Wang, B.; et al. Urinary exosomes and exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA nephropathy. Am. J. Pathol. 2018, 188, 2542–2552. [Google Scholar] [CrossRef]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef]
- Joo, K.W.; Lee, J.W.; Jang, H.R.; Heo, N.J.; Jeon, U.S.; Oh, Y.K.; Lim, C.S.; Na, K.Y.; Kim, J.; Cheong, H.I.; et al. Reduced urinary excretion of thiazie-sensitive Na-Cl cotransporter in Gitelman syndrome: Preliminary data. Am. J. Kidney Dis. 2007, 50, 765–773. [Google Scholar] [CrossRef]
- Corbetta, S.; Raimondo, F.; Tedeschi, S.; Syren, M.L.; Rebora, P.; Savoia, A.; Baldi, L.; Bettinelli, A.; Pitto, M. Urinary exosomes in the diagnosis of Gitelman and Bartter syndromes. Nephrol. Dial. Transplant. 2015, 30, 621–630. [Google Scholar] [CrossRef]
- Hogan, M.C.; Manganelli, L.; Wollard, J.R.; Masyuk, A.I.; Masyuk, T.V.; Tammachote, R.; Huang, B.Q.; Leontovich, A.A.; Beito, T.G.; Madden, B.J.; et al. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 2009, 20, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Demmers, J.A.; Bezstarosti, K.; Leonhard, W.N.; Losekoot, M.; van Kooten, C.; Gansevoort, R.T.; Peters, D.J.; Zietse, R.; Hoorn, E.J.; et al. Proteomics of urinary vesicles links plakins and complement to polycystic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 3079–3092. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.A.; Fiume, I.; Capasso, G.; Pocsfalvi, G. A multiplex quantitative proteomics strategy for protein biomarker studies in urinary exosomes. Kidney Int. 2012, 81, 1263–1272. [Google Scholar] [CrossRef]
- Turco, A.E.; Lam, W.; Rule, A.D.; Denic, A.; Lieske, J.C.; Miller, V.M.; Larson, J.J.; Kremers, W.K.; Jayachandran, M. Specific renal parenchymal-derived urinary extracellular vesciles identify age-associated structural changes in living donor kidneys. J. Extracell. Vesicles 2016, 5, 29642. [Google Scholar] [CrossRef]
- Kim, S.R.; Eirin, A.; Zhang, X.; Lerman, A.; Lerman, L.O. Mitochondria protection partly mitigates kidney cellular senescence in swine atherosclerotic renal artery stenosis. Cell. Physiol. Biochem. 2019, 52, 617–632. [Google Scholar]
- Hansen, K.J.; Edwards, M.S.; Craven, T.E.; Cherr, G.S.; Jackson, S.A.; Appel, R.G.; Burke, G.L.; Dean, R.H. Prevalence of renovascular disease in the elderly: A population-based study. J. Vasc. Surg. 2002, 36, 443–451. [Google Scholar] [CrossRef]
- Kwon, S.H.; Tang, H.; Saad, A.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Lerman, L.O. Differential expression of microRNAs in urinary extracellular vesicles obtained from hypertensive patients. Am. J. Kidney Dis. 2016, 68, 331–332. [Google Scholar] [CrossRef][Green Version]
- Kwon, S.H.; Woollard, J.R.; Saad, A.; Garovic, V.D.; Zand, L.; Jordan, K.L.; Textor, S.C.; Lerman, L.O. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol. Dial. Transplant. 2017, 32, 800–807. [Google Scholar] [CrossRef]
- Santelli, A.; Sun, I.O.; Eirin, A.; Abumoawad, A.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Senescent kidney cells in hypertensive patients release urinary extracellular vesicles. J. Am. Heart Assoc. 2019, 8, e012584. [Google Scholar] [CrossRef]
- Sun, I.O.; Santelli, A.; Abumoawad, A.; Eirin, A.; Ferguson, C.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension 2018, 72, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Alvarz, S.; Suazo, C.; Boltansky, A.; Ursu, M.; Carvajal, D.; Innocenti, G.; Vukusich, A.; Hurtado, M.; Villanueva, S.; Carreno, J.E.; et al. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 2013, 45, 3719–3723. [Google Scholar] [CrossRef] [PubMed]
- Dimuccio, V.; Ranghino, A.; Pratico Barbato, L.; Fop, F.; Biancone, L.; Camussi, G.; Bussolati, B. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS ONE 2014, 9, e104490. [Google Scholar] [CrossRef]
- Park, J.; Lin, H.Y.; Assaker, J.P.; Jeong, S.; Huang, C.H.; Kurdi, T.; Lee, K.; Fraser, K.; Min, C.; Eskandari, S.; et al. Integrated kidney exosome analysis for the detection of kidney transplant rejection. ACS Nano 2017, 11, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, E.; Kahwaji, J.; Nast, C.C.; Li, P.; Mirocha, J.; Thomas, D.L.; Ge, S.; Vo, A.A.; Jordan, S.C.; et al. Plasma exosomes from HLA-sensitized kidney transplant recipients contain mRNA transcripts which predict development of antibody-mediated rejection. Transplantation 2017, 101, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Jonnada, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant. 2018, 27, 1080–1095. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.Y.; Zhao, Y.; Eirin, A.; Liu, L.; Ferguson, C.M.; Tang, H.; Lerman, A.; Lerman, L.O. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic Res. Cardiol. 2020, 115, 16. [Google Scholar] [CrossRef]
- Wu, R.; Huang, C.; Wu, Q.; Jia, X.; Liu, M.; Xue, Z.; Qiu, Z.; Niu, X.; Wang, Y. Exosomes secreted by urine-derived stem cells improve stress urinary incontience by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res. Ther. 2019, 10, 80. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Liu, Y.M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S.C.; Fan, Y.; Wang, Y.; Wang, N.S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of gliblastoma therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Aubertin, K.; Silva, A.K.; Luciani, N.; Espinosa, A.; Djmat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar]
- Pathare, G.; Tutakhel, O.A.Z.; van der Wel, M.C.; Shelton, L.M.; Deinum, J.; Lenders, J.W.M.; Hoenderop, J.G.J.; Bindels, R.J.M. Hydrochlorothiazide treatment increases the abundance of the NaCl cotransporter in urinary extracellular vesicles of essential hypertensive patients. Am. J. Physiol. Renal Physiol. 2017, 312, F1063–F1072. [Google Scholar] [CrossRef]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapetic drug carriers and delivery vehicles across biological membrane: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Villa, F.; Quarto, R.; Tasso, R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics 2019, 11, 557. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bahre, H.; von der Ohe, J.; Hass, R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef]
- Goh, W.J.; Lee, C.K.; Zou, S.; Woon, E.C.; Czarny, B.; Pastorin, G. Doxorubicin-loaded cell-derived nanovesicles: An alternative targeted approach for anti-tumor therapy. Int. Nanomed. 2017, 12, 2759–2767. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marban, E. Targeting extracellular vesicles to injuired tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, I.O.; Lerman, L.O. Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics. Diagnostics 2020, 10, 311. https://doi.org/10.3390/diagnostics10050311
Sun IO, Lerman LO. Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics. Diagnostics. 2020; 10(5):311. https://doi.org/10.3390/diagnostics10050311
Chicago/Turabian StyleSun, In O., and Lilach O. Lerman. 2020. "Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics" Diagnostics 10, no. 5: 311. https://doi.org/10.3390/diagnostics10050311
APA StyleSun, I. O., & Lerman, L. O. (2020). Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics. Diagnostics, 10(5), 311. https://doi.org/10.3390/diagnostics10050311




