Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Collection, Processing and Storage of Saliva Samples
2.3. Biochemical Analysis of Saliva Samples
2.4. Statistical Analysis
3. Results
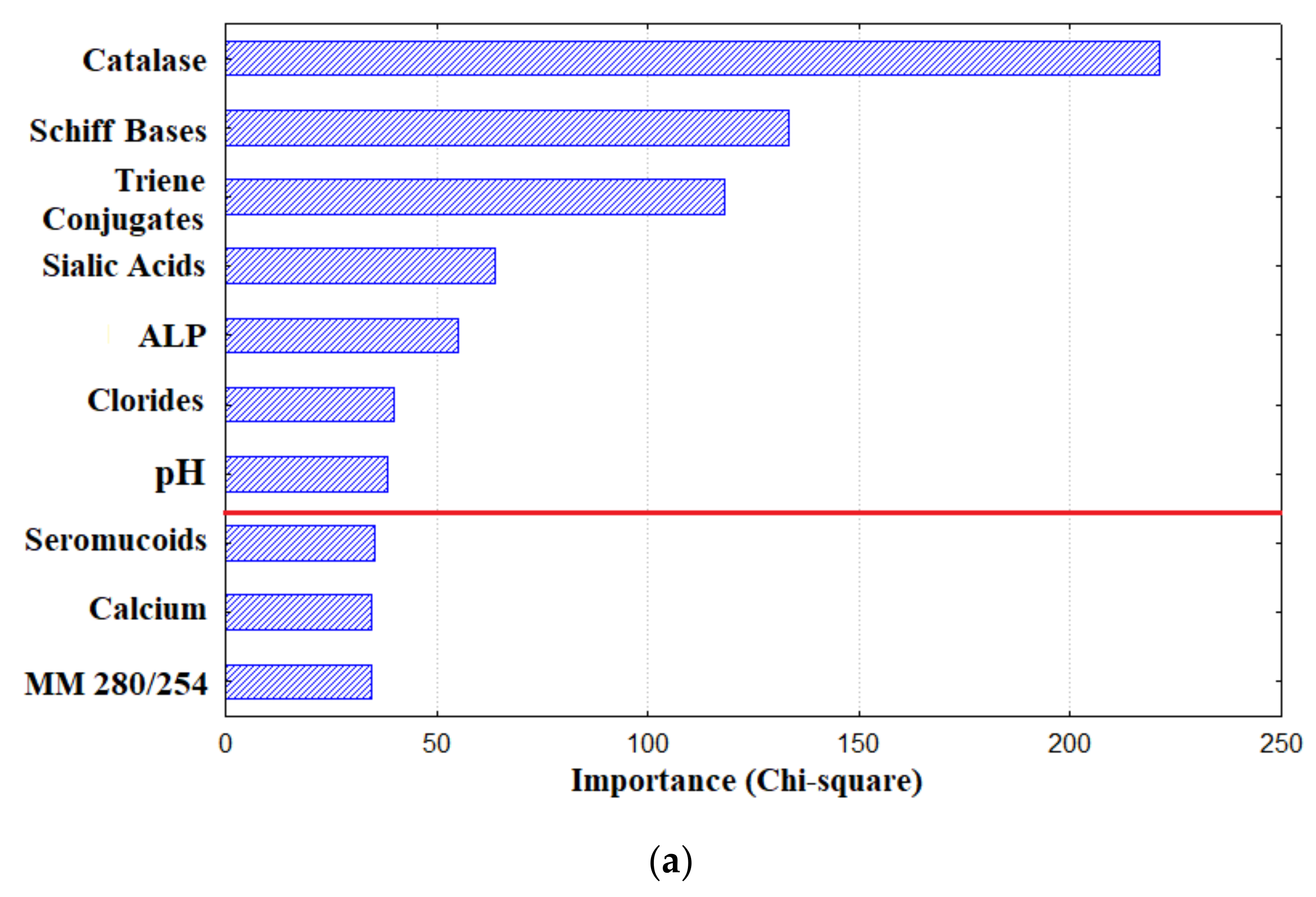
3.1. Metabolic Features of Saliva Composition in Patients with Lung Cancer
3.2. Diagnostic Capabilities of Saliva for Lung Cancer
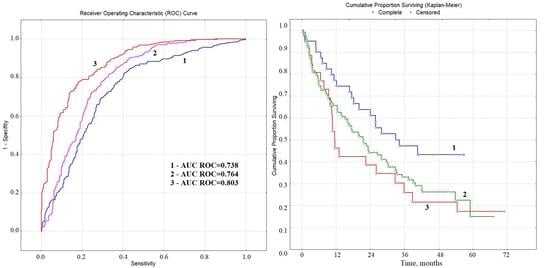
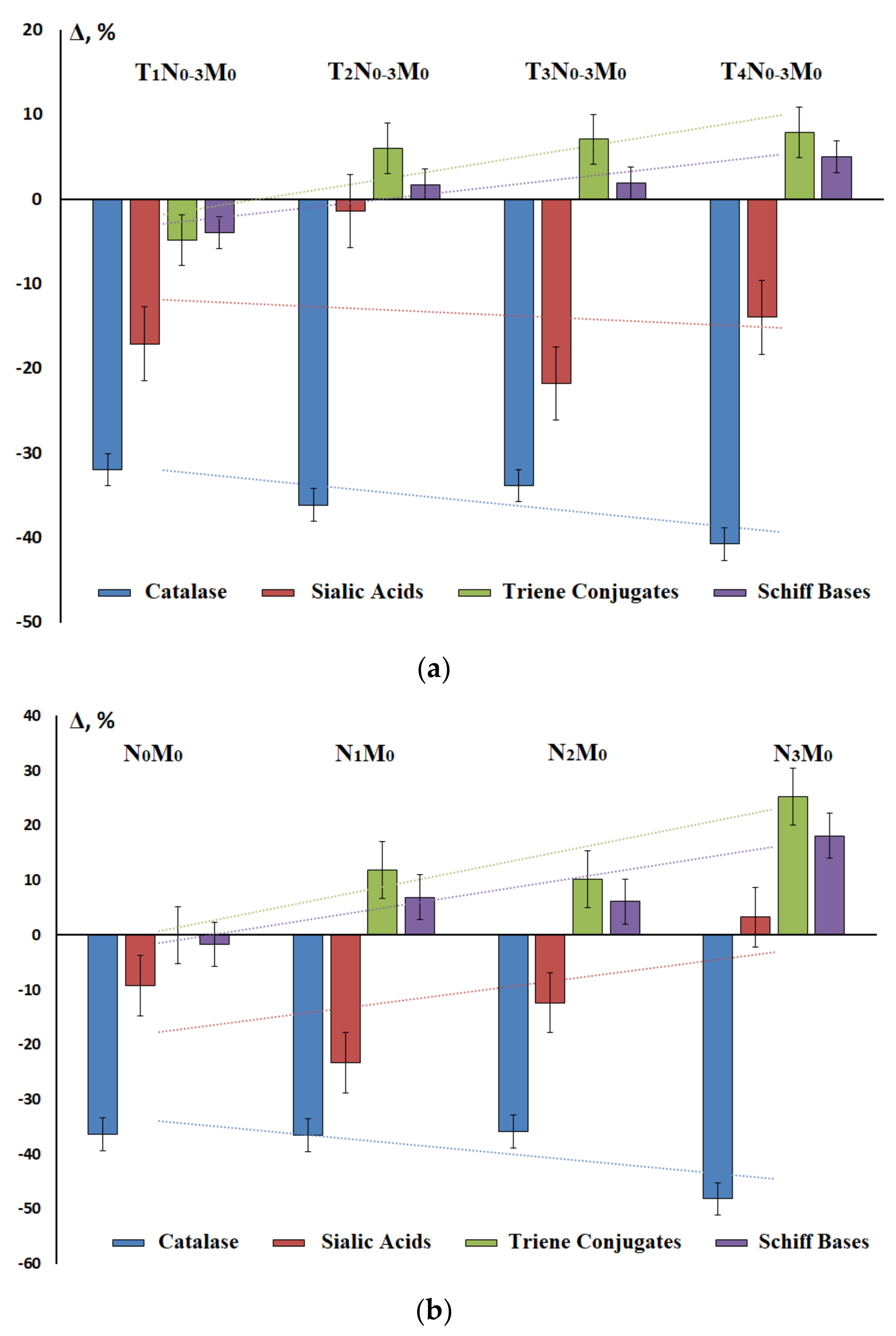
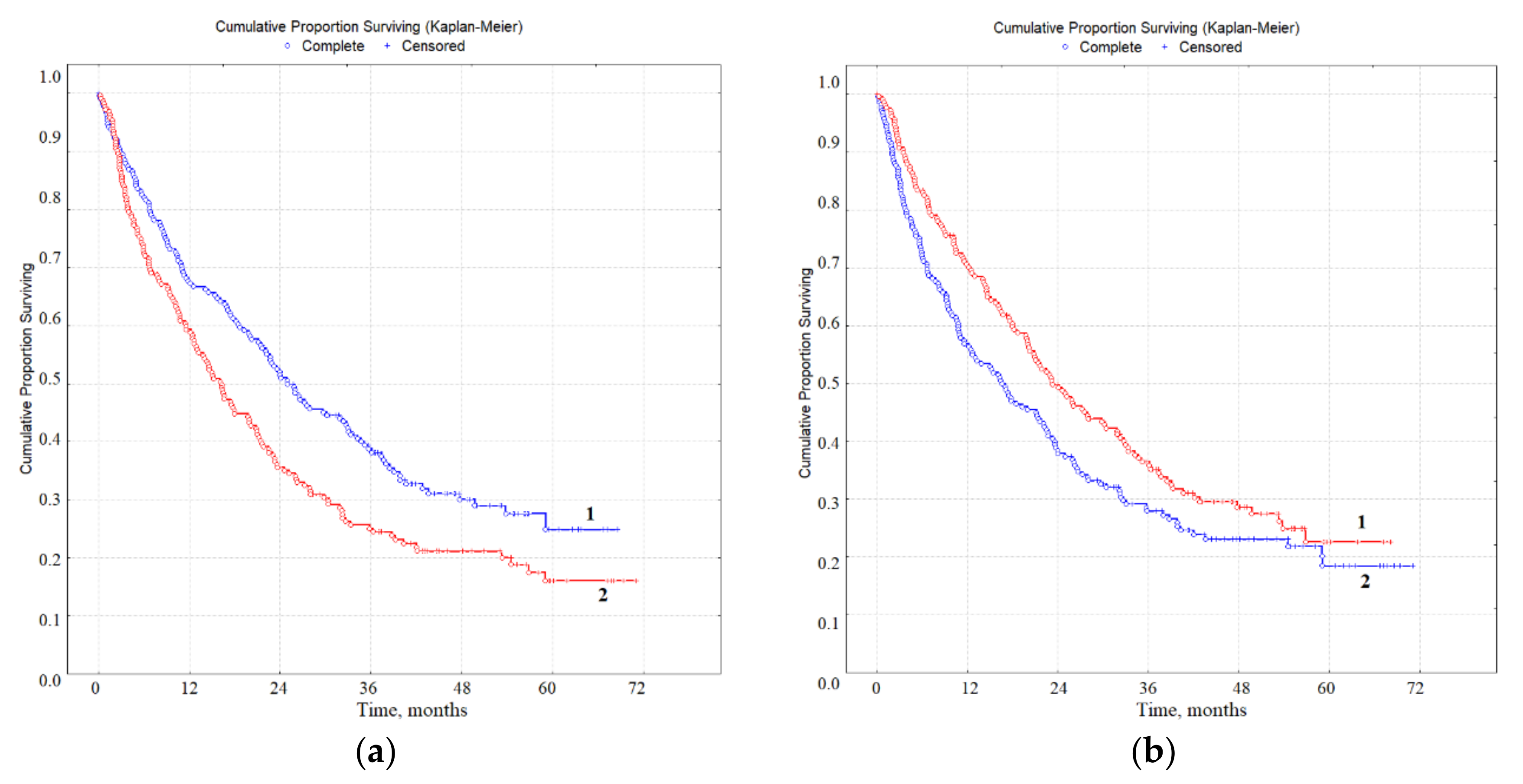
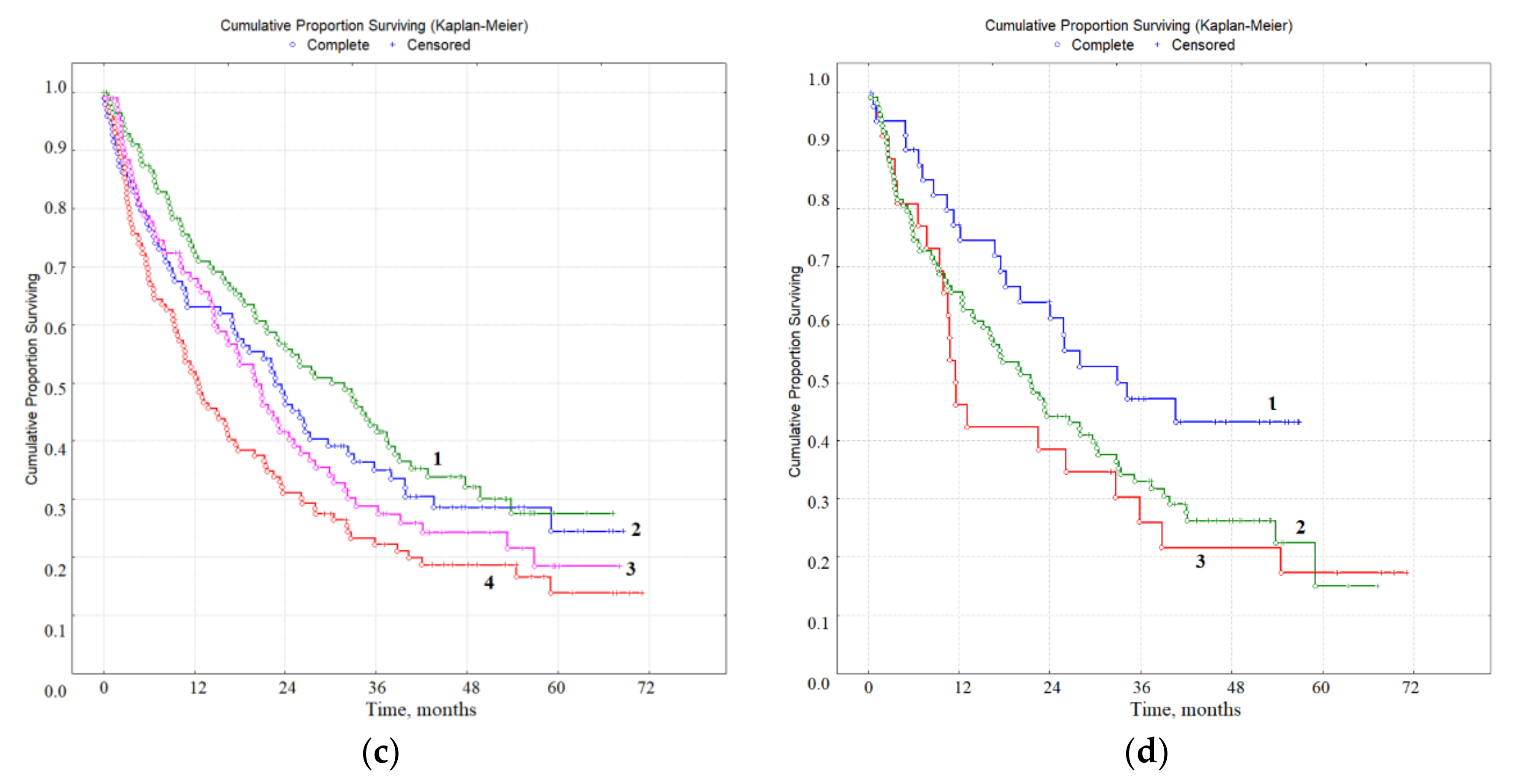
3.3. Prognostic Value of Biochemical Markers of Lung Cancer in Saliva
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Kaprin, A.D.; Starinskiy, V.V.; Petrova, G.V. The Status of Cancer Care for the Population of Russia in 2018; MNIOI im. P.A. Gertsena: Moscow, Russia, 2019; p. 236. [Google Scholar]
- Kosenok, V.К.; Bel’skaya, L.V.; Massard, Z.; Zavyalov, А.А. Lung cancer incidence in Omsk region. Sib. J. Oncol. 2016, 15, 21–25. [Google Scholar] [CrossRef][Green Version]
- Okiror, L.; Harling, L.; Toufektzian, L.; King, J.; Routledge, T.; Harrison-Phipps, K.; Pilling, J.; Veres, L.; Lal, R.; Bille, A. Prognostic factors including lymphovascular invasion on survival for resected non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 156, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in lung cancer screening: Achievements, promises, and challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Davydov, M.I.; Zaridze, D.G. Screening for malignant tumors. J. N.N. Blokhin Russ. Cancer Res. Center 2014, 25, 5–16. [Google Scholar]
- Sergeeva, N.S.; Marshutina, N.V.; Solokhina, M.P.; Alentov, I.I.; Parilova, N.K.; Zenkina, E.V.; Skashchkova, T.E. Modern conceptions of serological tumor markers and their role in oncology. Adv. Mol. Oncol. 2014, 1, 69–80. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Lazar, L.; Fang, Z.; Tang, C.; Zhao, J. Metabolomics workflow for lung cancer: Discovery of biomarkers. Clin. Chim. Acta 2019, 495, 436–445. [Google Scholar] [CrossRef]
- Hoseok, I.; Cho, J.-Y. Lung cancer biomarkers. Adv. Clin. Chem. 2015, 72, 107–170. [Google Scholar]
- Wu, Y.; Wang, J.; Yan, Z.; Qu, L.; Xiang, B.; Zhang, Y. An optimal tumor marker group-coupled artificial neural network for diagnostics of lung cancer. Expert Syst. Appl. 2011, 38, 11329–11334. [Google Scholar] [CrossRef]
- Liloglou, T.; Bediaga, N.G.; Brown, B.R.B.; Field, J.K.; Davies, M.P.A. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Lewis, K.E.; Beckmann, M.; Allison, G.G.; Ghosal, R.; Lewis, P.D.; Mur, L.A.J. The metabolomics detection of lung cancer biomarkers in sputum. Lung Cancer 2016, 94, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gessner, C.; Rechner, B.; Hammerschmidt, S.; Kuhn, H.; Hoheisel, G.; Sack, U.; Ruschpler, P.; Wirtz, H. Angiogenic markers in breath condensate identify non-small cell lung cancer. Lung Cancer 2010, 68, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.P.; Lewis, C.; Thomas, P.S. Exhaled breath analysis: Novel approach for early detection of lung cancer. Lung Cancer 2009, 63, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Callejón-Leblic, B.; García-Barrera, T.; Pereira-Vega, A.; Gómez-Ariza, J.L. Metabolomic study of serum, urine and bronchoalveolar lavage fluidbased on gas chromatography mass spectrometry to delve into thepathology of lung cancer. J. Pharm. Biomed. Anal. 2019, 163, 122–129. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Ponnusamy, M.P.; Macha, M.A.; Haridas, D.; Majhi, P.D.; Kaur, S.; Jain, M.; Batra, S.K.; Ganti, A.K. Mucins in lung cancer. J. Thorac. Oncol. 2015, 10, 19–27. [Google Scholar] [CrossRef]
- Soini, Y.; Kaarteenaho-Wiik, R.; Paakko, P.; Kinnula, V. Expression of antioxidant enzymes in bronchial metaplastic and dysplastic epithelium. Lung Cancer 2003, 39, 15–22. [Google Scholar] [CrossRef]
- Vannini, I.; Fanini, F.; Fabbri, M. MicroRNAs as lung cancer biomarkers and key players in lung carcinogenesis. Clin. Biochem. 2013, 46, 918–925. [Google Scholar] [CrossRef]
- Castro, J.; Rodríguez, M.C.; Martínez-Zorzano, V.S.; Llanillo, M.; Sánchez-Yagüe, J. Platelet linoleic acid is a potential biomarker of advanced non-small cell lung cancer. Exp. Mol. Pathol. 2009, 87, 226–233. [Google Scholar] [CrossRef]
- Schapher, M.; Wendler, O.; Gröschl, M. Salivary cytokines in cell proliferation and cancer. Clin. Chim. Acta 2011, 412, 1740–1748. [Google Scholar] [CrossRef]
- Chudasama, D.; Katopodis, P.; Stone, N.; Haskell, J.; Sheridan, H.; Gardner, B.; Urnovitz, H.; Schuetz, E.; Beck, J.; Hall, M.; et al. Liquid biopsies in lung cancer: Four emerging technologies and potential clinical applications. Cancers 2019, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Strom, C.M.; Cheng, J.; Lin, C.-C.; Hsu, C.-Y.; Soo Hoo, G.W.; Chia, D.; Kim, Y.; Li, F.; Elashoff, D.; et al. Electric Fielde Induced Release and Measurement Liquid Biopsy for Noninvasive Early Lung Cancer Assessment. J. Mol. Diagn. 2018, 20, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Rocco, G.; Pennazza, G.; Santonico, M.; Longo, F.; Rocco, R.; Crucitti, P.; Incalzi, R.A. Breathprinting and early diagnosis of lung cancer. J. Thorac. Oncol. 2018, 13, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Zhou, H.; Santiago, S.; Lee, J.M.; Garon, E.B.; Yang, J.; Brinkmann, O.; Yan, X.; Akin, D.; et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 2012, 69, 3341–3350. [Google Scholar] [CrossRef]
- Kisluk, J.; Ciborowski, M.; Niemira, M.; Kretowski, A.; Niklinski, J. Proteomics biomarkers for non-small cell lung cancer. J. Pharm. Biomed. Anal. 2014, 101, 40–49. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Qiao, Z.; Shang, Z.; Xia, Z.; Niu, X.; Qian, L.; Zhang, Y.; Fan, L.; Cao, C.X.; et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal. Chim. Acta 2017, 982, 84–95. [Google Scholar] [CrossRef]
- Roblegg, E.; Coughran, A.; Sirjani, D. Saliva: An all-rounder of our body. Eur. J. Pharm. Biopharm. 2019, 142, 133–141. [Google Scholar] [CrossRef]
- Bel’skaya, L.V. Possibilities of using saliva for the diagnosis of cancer. Klin. Lab. Diagn. Russ. Сlin. Lab. Diagn. 2019, 64, 333–336. [Google Scholar] [CrossRef]
- Yang, J.; Mu, X.; Wang, Y.; Zhu, D.; Zhang, J.; Liang, C.; Chen, B.; Wang, J.; Zhao, C.; Zuo, Z.; et al. Dysbiosis of the salivary microbiome is associated with non-smoking female lung cancer and correlated with immunocytochemistry markers. Front. Oncol. 2018, 8, 520. [Google Scholar] [CrossRef]
- Balan, J.J.; Rao, R.S.; Premalatha, B.R.; Patil, S. Analysis of tumor markers CA 125 in saliva of normal and oral squamous cell carcinoma patients: A comparative study. J. Contemp. Dent. Pract. 2012, 13, 671–675. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Wong, D.T.W. Salivary microRNAs and oral cancer detection. Methods Mol. Biol. 2013, 936, 313–324. [Google Scholar] [PubMed]
- Punyadeera, C.; Slowey, P.D. Saliva as an emerging biofluid for clinical diagnosis and applications of MEMS/NEMS in salivary diagnostics. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: Norwich, USA, 2019; pp. 543–565. [Google Scholar]
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Loo, J.A.; Wong, D.T. Human saliva proteome analysis and disease biomarker discovery. Expert Rev. Proteom. 2007, 4, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhou, H.; Zhang, L.; Lee, J.W.; Zhou, Q.; Hu, S.; Wolinsky, L.E.; Farrell, J.; Eibl, G.; Wong, D.T. Systemic disease-induced salivary biomarker profiles in mouse models of melanoma and non-small cell lung cancer. PLoS ONE 2009, 4, e5875. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, F.; Schafer, C.; Wong, D.T. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS ONE 2014, 9, e110641. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, L.; Zhou, H.; Lee, J.M.; Garon, E.B.; Wong, D.T. Proteomic analysis of human saliva from lung cancer patients using two dimensional difference gel electrophoresis and mass spectrometry. Mol. Cell. Proteom. 2012, 11, M111.012112. [Google Scholar] [CrossRef]
- Wei, F.; Lin, C.C.; Joon, A.; Feng, Z.; Troche, G.; Lira, M.E.; Chia, D.; Mao, M.; Ho, C.L.; Su, W.C.; et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 190, 1117–1126. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.W.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. BBA Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A. Chronophysiological features of the normal mineral composition of human saliva. Arch. Oral Biol. 2017, 82, 286–292. [Google Scholar] [CrossRef]
- Dos Santos, D.R.; Souza, R.O.; Dias, L.B.; Ribas, T.B.; Farias de Oliveira, L.C.; Sumida, D.H.; Dornelles, R.C.M.; Nakamune, A.C.S.; Chaves-Neto, A.H. The effects of storage time and temperature on the stability of salivary phosphatases, transaminases and dehydrogenase. Arch. Oral Biol. 2018, 85, 160–165. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solonenko, A.P. Morphology of dried drop patterns of saliva from a healthy individual depending on the dynamics of its surface tension. Surfaces 2019, 2, 395–414. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. Endogenous Intoxication and Saliva Lipid Peroxidation in Patients with Lung Cancer. Diagnostics 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. The system of lipid peroxidation and antioxidant protection of saliva in lung cancer. Klin. Lab. Diagn. Russ. Сlin. Lab. Diag. 2018, 63, 530–537. [Google Scholar]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. Antioxidant protection system in the saliva of patients with non-small cell lung cancer. Biomed. Chem. Res. Methods 2019, 2, e00061. [Google Scholar] [CrossRef]
- Gundyrev, I.A.; Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A. The use of synthetic images for solving the classification problem by the example of lung cancer diagnosis. Ann. Russ. Acad. Med. Sci. 2018, 73, 96–104. [Google Scholar] [CrossRef]
- Flakh, P. Machine Learning. The Science and Art of Building Algorithms that Extract Knowledge from Data; DMK Press: Moscow, Russia, 2015. [Google Scholar]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Gadbail, A.R.; Sharma, A.; Tekade, S. Oxidative and antioxidative mechanism in oral cancer and precancer: A review. Oral Oncol. 2014, 50, 10–18. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G.; Zav’yalov, A.A. Status indicators of lipid peroxidation and endogenous intoxication in lung cancer patients. Ann. Russ. Acad. Med. Sci. 2016, 71, 313–322. [Google Scholar]
- Gȩgotek, A.; Nikliński, J.; Žarković, N.; Žarković, K.; Waeg, G.; Łuczaj, W.; Charkiewicz, R.; Skrzydlewska, E. Lipid mediators involved in the oxidative stress and antioxidant defense of human lung cancer cells. Redox Biol. 2016, 9, 210–219. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Oxidant metabolism in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 22, 14s–27s. [Google Scholar] [CrossRef] [PubMed]
- Makarova, Y.V.; Vakhlamov, V.A.; Shoniya, M.L.; Men’kov, N.V.; Solov’yeva, T.I.; Arkhipova, Y.V.; Varvarina, G.N.; Novikov, V.V. Identifying the predictors of the development of the inflammatory process in the bronchi of beginner smokers. Modern Technol. Med. 2015, 7, 77–83. [Google Scholar]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Fermoselle, C.; Mateu-Jimenez, M.; Sánchez-Font, A.; Pijuan, L.; Gea, J.; Curull, V. Oxidative stress and inflammation in the normal airways and blood of patients with lung cancer and CORD. Free Radic. Biol. Med. 2013, 65, 859–871. [Google Scholar] [CrossRef]
- Filaire, E.; Dupuis, C.; Galvaing, G.; Aubreton, S.; Laurent, H.; Richard, R.; Filaire, M. Lung cancer: What are the links with oxidative stress, physical activity and nutrition. Lung Cancer 2013, 82, 383–389. [Google Scholar] [CrossRef]
- Lin, J.L.; Thomas, P.S. Current perspectives of oxidative stress and its measurement in chronic obstructive pulmonary disease. COPD 2010, 7, 291–306. [Google Scholar] [CrossRef]
- Smolyakova, R.M.; Prokhorova, V.I.; Zharkov, V.V.; Lappo, S.V. Assessment of the binding capacity and transport function of serum albumin in patients with lung cancer. Nov. Khirurgii 2005, 13, 78–84. [Google Scholar]
- Chesnokova, N.P.; Barsukov, V.Y.; Ponukalina, E.V.; Agabekov, A.I. Regularities of changes in free radical destabilization processes of biological membranes in cases of colon ascendens adenocarcinoma and the role of such regularities in neoplastic proliferation development. Fundam. Res. 2015, 1, 164–168. [Google Scholar]
- Pankova, O.V.; Perelmuter, V.M.; Savenkova, O.V. Characteristics of proliferation marker expression and apoptosis regulation depending on the character of disregenerator changes in bronchial epithelium of patients with squamous cell lung cancer. Sib. Oncol. J. 2010, 41, 36–41. [Google Scholar]
- Sato, E.F.; Choudhury, T.; Nishikawa, T.; Inoue, M. Dynamic aspect of reactive oxygen and nitric oxide in oral cavity. J. Clin. Biochem. Nutr. 2008, 42, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nurtur. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, F.; Martinez-Outschoorn, U.E.; Lisanti, M.P. Mitochondrial oxidative stress drives tumor progression and metastasis. BMC Med. 2011, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A.; Zhuchkov, S.A. The state of lipid peroxidation and endogenous intoxication under non-small-cell lung cancer. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1247–1253. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A.; Zhuchkov, S.A. Impact of distant and regional metastasis of non-small-cell lung cancer on endotoxicosis development. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1235–1241. [Google Scholar] [CrossRef]
- Smriti, K.; Pai, K.M.; Ravindranath, V.; Pentapati, K.C. Role of salivary malondialdehyde in assessment of oxidative stress among diabetics. J. Oral Biol. Craniofac. Res. 2016, 6, 42–45. [Google Scholar] [CrossRef]
- Su, H.; Gornitsky, M.; Velly, A.M.; Yu, H.; Benarroch, M.; Schipper, H.M. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic. Biol. Med. 2009, 46, 914–921. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ngo, L.Q.; Promsudthi, A.; Surarit, R. Salivary Lipid Peroxidation in Patients with Generalized Chronic Periodontitis and Acute Coronary Syndrome. J. Periodontol. 2016, 87, 134–141. [Google Scholar] [CrossRef]
- Sobaniec, H.; Sobaniec, W.; Sendrowski, K.; Sobaniec, S.; Pietruska, M. Antioxidant activity of blood serum and saliva in patients with periodontal disease treated due to epilepsy. Adv. Med. Sci. 2007, 52, 204–206. [Google Scholar]
- Rai, B.; Kharb, S.; Jain, R.; Anand, S.C. Salivary lipid peroxidation product malondialdehyde in precancer and cancer. Adv. Med. Dent. Sci. 2008, 2, 7–8. [Google Scholar]
- Shivashankara, A.R.; Kavya, P.M. Salivary total protein, sialic acid, lipid peroxidation and glutathione in oral squamous cell carcinoma. Biomed. Res. 2011, 22, 12–19. [Google Scholar]
- Shetty, S.R.; Babu, S.; Kumari, S.; Shetty, P.; Hegde, S.; Castelino, R. Status of salivary lipid peroxidation in oral cancer and precancer. Indian J. Med. Pediatr. Oncol. 2014, 35, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.; Kumari, S.N.; Hegde, M.N.; Chandra, P.M.; Nireeksha, R. Lipid peroxidation and vitamin C level in saliva of oral precancerous patients—An In-vitro study. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 13–18. [Google Scholar]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Nikolaev, I.V.; Kolobkova, L.N.; Landesman, E.O.; Stepanova, E.V.; Koroleva, O.V. The antioxidant and peroxidase activities of saliva in patients with inflammatory periodontal diseases and possibility of their correction. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2008, 2, 426. [Google Scholar] [CrossRef]
- Khan, A.; Tania, M.; Zhang, D.; Chen, H. Antioxidant enzymes and cancer. Chin. J. Cancer Res. 2010, 22, 87–92. [Google Scholar] [CrossRef]
- Abiaka, C.; Al-Awadi, F.; Al-Sayer, H.; Gulshan, S.; Behbehani, A.; Farghally, M. Activities of erythrocyte antioxidant enzymes in cancer patients. J. Clin. Lab. Anal. 2002, 16, 167–171. [Google Scholar] [CrossRef]
- Chanchaeva, E.A.; Aizman, R.I.; Gerasev, A.D. The modern idea of the antioxidant system of the human body. Hum. Ecol. 2013, 7, 50–58. [Google Scholar]
- Sozarukova, M.M.; Proskurina, E.V.; Vladimirov, Y.A. Serum albumin as a source of and a target for free radicals in pathology. Bull. Russ. State Med. Univ. 2016, 1, 61–67. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K. The activity of metabolic enzymes in the saliva of lung cancer patients. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 646–653.40. [Google Scholar] [CrossRef]
- Savchenko, A.A.; Lapeshin, P.V.; Dykhno, Y.A. The dependence of the activity of metabolic enzymes of blood lymphocytes in the cells of healthy and tumor lung tissue in patients with lung cancer. Russ. Biother. J. 2004, 4, 70–75. [Google Scholar]
- Elf, S.E.; Chen, J. Targeting glucose metabolism in patients with cancer. Cancer 2014, 120, 774. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K. The level of sialic acids and imidazole compounds in the saliva of patients with lung cancer of different histological types. Sib. J. Oncol. 2018, 17, 84–91. [Google Scholar] [CrossRef]
- Speransky, V.V.; Alekhin, E.K.; Petrova, I.V.; Alekhin, V.E. On the role of histamine and antihistamines in oncogenesis. Med. Bull. Bashkortostan 2010, 5, 151–156. [Google Scholar]
- Fleming, M.V.; Klimov, V.V.; Cherdyntseva, N.V. On the interaction of allergic reactions and malignant processes. Sib. Oncol. J. 2005, 13, 96–101. [Google Scholar]
- Keskinege, A.; Elgun, S.; Yitmaz, E. Possible implications of arginase and diamine oxidase in prostatic carcinoma. Cancer Detect. Prev. 2001, 25, 76–79. [Google Scholar]
- Manina, I.V.; Peretolchina, N.M.; Saprykina, N.S.; Kozlov, A.M.; Mikhailova, I.N.; Zhordania, K.I.; Baryshnikov, A.Y. Prospects for the use of an H2-histamine receptor antagonist (cimetidine) as an adjuvant of melanoma biotherapy. Immunopathol. Allergol. Infectol. 2010, 4, 42–51. [Google Scholar]
- Faverio, F.; Guzzetti, A.; Mereghetti, A.; Jemoli, R. Hiperhistaminemia nelle neoplasie della mammilla. Chir. Ital. 1982, 34, 727–734. [Google Scholar]
- Moshkovskii, S.A. Omics biomarkers and early diagnostics. Biomed. Khimiya 2017, 63, 369–372. [Google Scholar] [CrossRef]
- Kushlinskiy, N.E.; Lyubimova, N.V. Tumor markers. General characteristics, clinical significance and recommendations for use. Poliklinika 2016, 8, 62–77. [Google Scholar]
- Chen, S.; Yan, H.; Du, J.; Li, J.; Shen, B.; Ying, H.; Zhang, Y.; Chen, S. Prognostic significance of pre-resection albumin/fibrinogen ratio in patients with non-small cell lung cancer: A propensity score matching analysis. Clin. Chim. Acta 2018, 482, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.W.; Lee, S.J.; Park, S.Y. Prognostic significance of lactate dehydrogenase B according to histologic type of non-small-cell lung cancer and its association with serum lactate dehydrogenase. Pathol. Res. Pract. 2017, 213, 1134–1138. [Google Scholar] [CrossRef]
- Hermes, A.; Gatzemeier, U.; Waschki, B.; Reck, M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer—A retrospective single institution analysis. Respir. Med. 2010, 104, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Lv, W.-Y.; Du, H.; Li, Y.-J.; Zhang, W.-B.; Che, G.-W.; Liu, L.-X. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: Propensity score matching analysis using a prospective database. Int. J. Surg. 2019, 69, 32–42. [Google Scholar] [CrossRef]
- Hanagiri, T.; Sugaya, M.; Takenaka, M.; Oka, S.; Baba, T.; Shigematsu, Y.; Nagata, Y.; Shimokawa, H.; Uramoto, H.; Takenoyama, M.; et al. Preoperative CYFRA 21–1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung Cancer 2011, 74, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, S.; Wojcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wojcik, N.; Dębniak, T.; et al. Influence of the selenium level on overall survival in lung cancer. J. Trace Elem. Med. Biol. 2019, 56, 46–51. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Correlation interrelations between the composition of saliva and blood plasma in norm. Klin. Lab. Diagn. Russ. Сlin. Lab. Diagn. 2018, 63, 477–482. [Google Scholar]

| Feature | Lung Cancer, n (%) | Non-Malignant Lung Diseases, n = 168 | ||
|---|---|---|---|---|
| ADC, n = 189 | SCC, n = 135 | NEC, n = 68 | ||
| Age, Years | 61.0 [56.0; 65.0] | 59.0 [55.0; 66.5] | 55.0 [52.0; 60.0] | 55.0 [45.5; 60.5] |
| Gender | ||||
| Male | 129 (68.3) | 128 (94.8) | 50 (73.5) | 98 (58.3) |
| Female | 60 (31.7) | 7 (5.2) | 18 (26.5) | 70 (41.7) |
| pT | ||||
| T1 | 21 (11.1) | 4 (3.0) | 5 (7.3) | - |
| T2 | 105 (55.6) | 47 (34.8) | 31 (45.6) | - |
| T3 | 30 (15.9) | 53 (39.3) | 8 (11.8) | - |
| T4 | 33 (17.4) | 31 (22.9) | 24 (35.3) | - |
| pN | ||||
| N0 | 82 (43.4) | 51 (37.8) | 19 (27.9) | - |
| N1 | 34 (18.0) | 34 (25.2) | 13 (19.2) | - |
| N2 | 51 (27.0) | 45 (33.3) | 24 (35.3) | - |
| N3 | 22 (11.6) | 5 (3.7) | 12 (17.6) | - |
| pM | ||||
| M0 | 133 (70.4) | 108 (80.0) | 48 (70.6) | - |
| M1 | 56 (29.6) | 27 (20.0) | 20 (29.4) | - |
| Indicators | Control Group | Comparison Group | Lung Cancer | Kruskal–Wallis test (H, p) |
|---|---|---|---|---|
| Electrolytes | ||||
| pH | 6.50 [6.30; 6.72] | 6.50 [6.25; 6.82] | 6.49 [6.23; 6.79] | 0.5756, 0.7500 |
| Calcium, mmol/L | 1.33 [1.05; 1.66] | 1.38 [1.02; 1.75] | 1.42 [1.00; 1.85] | 4.6847, 0.0961 |
| - | - | p1-3 = 0.0306 | ||
| Phosphorus, mmol/L | 4.53 [3.58; 5.85] | 4.73 [3.25; 5.65] | 4.56 [3.37; 5.77] | 1.9933, 0.3691 |
| Sodium, mmol/L | 8.4 [5.5; 12.4] | 8.2 [5.6; 11.1] | 9.1 [5.8; 14.6] | 3.7291, 0.1550 |
| Potassium, mmol/L | 11.8 [9.3; 14.7] | 12.0 [8.7; 14.8] | 12.9 [9.5; 16.3] | 7.6743, 0.0216 |
| - | p1-2 = 0.0352 | p1-3 = 0.0118 | ||
| Chlorides, mmol/L | 26.1 [21.2; 32.2] | 23.4 [18.1; 31.1] | 28.3 [21.7; 36.3] | 18.5965, 0.00009 |
| - | p1-2 = 0.0012 | p1-3 = 0.0059 | ||
| Magnesium, mmol/L | 0.300 [0.246; 0.350] | 0.293 [0.223; 0.366] | 0.300 [0.230; 0.372] | 1.4687, 0.4798 |
| Nitric oxide (NO), nmol/mL | 23.5 [13.5; 38.1] | 21.7 [11.9; 37.0] | 24.0 [14.2; 42.3] | 1.5079, 0.4705 |
| Protein Metabolism | ||||
| Protein, g/L | 0.80 [0.50; 1.23] | 0.68 [0.48; 1.00] | 0.65 [0.34; 1.04] | 20.6393, 0.00003 |
| - | p1-2 = 0.0140 | p1-3 = 0.0000 | ||
| Albumin, g/L | 0.26 [0.17; 0.44] | 0.31 [0.17; 0.51] | 0.30 [0.16; 0.48] | 3.937, 0.1397 |
| - | p1-2 = 0.0121 | - | ||
| Urea, mmol/L | 7.84 [5.40; 11.03] | 7.44 [4.93; 10.31] | 8.00 [5.76; 11.86] | 5.8911, 0.0526 |
| - | p1-2 = 0.0001 | - | ||
| Uric acid, nmol/mL | 86.49 [28.18; 154.77] | 79.64 [32.18; 151.38] | 83.33 [36.54; 166.67] | 1.8995, 0.3868 |
| α-amino acids, mmol/L | 4.12 [3.83; 4.50] | 4.21 [3.94; 4.57] | 4.16 [3.88; 4.61] | 5.4938, 0.0641 |
| - | p1-2 = 0.0487 | p1-3 = 0.0270 | ||
| Imidazole compounds (ICs), mmol/L | 0.281 [0.175; 0.379] | 0.364 [0.220; 0.501] | 0.311 [0.197; 0.478] | 34.1462, 0.00000 |
| - | p1-2 = 0.0000 | p1-3 = 0.0000 | ||
| Seromucoids, c.u. | 0.090 [0.060; 0.130] | 0.101 [0.062; 0.147] | 0.098 [0.055; 0.154] | 1.5811, 0.4536 |
| Sialic acids, mmol/L | 0.195 [0.134; 0.299] | 0.153 [0.088; 0.211] | 0.171 [0.095; 0.281] | 36.2003, 0.00000 |
| - | p1-2 = 0.0000 | p1-3 = 0.0000 | ||
| Metabolic enzymes | ||||
| Alanine aminotransferase (ALT), U/L | 3.62 [2.54; 4.92] | 3.69 [2.77; 5.31] | 4.01 [2.77; 5.62] | 13.3327, 0.0013 |
| - | p1-2 = 0.0035 | p1-3 = 0.0003 | ||
| Aspartate aminotransferase (AST), U/L | 5.50 [3.67; 7.33] | 5.42 [3.58; 7.75] | 5.25 [3.17; 7.50] | 1.6532, 0.4375 |
| AST/ALT | 1.42 [1.13; 1.92] | 1.36 [1.01; 1.79] | 1.25 [0.96; 1.60] | 34.349, 0.00000 |
| - | p1-2 = 0.0026 | p1-3 = 0.0000 | ||
| Alkaline phosphatase (ALP), U/L | 58.67 [41.29; 82.57] | 71.71 [52.15; 117.34] | 73.88 [49.98; 117.34] | 46.515, 0.00000 |
| - | p1-2 = 0.0000 | p1-3 = 0.0000 | ||
| Lactate dehydrogenase (LDH), U/L | 1127.5 [652.1; 1838.0] | 1140.0 [541.5; 1802.0] | 1133.0 [545.5; 1748.9] | 1.4225, 0.4910 |
| Gamma-glutamyl transpeptidase (GGT), U/L | 20.3 [17.5; 24.0] | 20.5 [16.8; 25.0] | 21.8 [18.2; 25.7] | 12.8134, 0.0017 |
| - | - | p1-3 = 0.0003 | ||
| α-amylase, U/L | 201.6 [100.5; 404.4] | 312.4 [138.0; 514.4] | 312.1 [175.2; 650.4] | 23.6106, 0.00001 |
| - | - | p1-3 = 0.0000 | ||
| Antioxidant enzymes | ||||
| Catalase, mcat/L | 4.32 [3.20; 5.57] | 2.94 [2.24; 4.28] | 2.68 [2.02; 4.01] | 175.453, 0.00000 |
| - | p1-2 = 0.0000 | p1-3 = 0.0000 | ||
| Superoxide dismutase (SOD), c.u. | 57.9 [34.2; 104.0] | 60.5 [34.2; 102.6] | 65.8 [29.0; 121.1] | 1.4605, 0.4818 |
| Antioxidant activity (АОА), mmol/L | 2.36 [1.61; 3.48] | 2.66 [1.85; 3.24] | 2.48 [1.61; 3.62] | 2.4126, 0.2993 |
| Peroxidase, c.u. | 0.320 [0.170; 0.610] | 0.540 [0.300; 1.080] | 0.440 [0.240; 0.820] | 13.7029, 0.0011 |
| - | p1-2 = 0.0009 | p1-3 = 0.0010 | ||
| Lipoperoxidation Products | ||||
| Diene conjugates, c.u. | 3.92 [3.78; 4.06] | 3.93 [2.89; 4.13] | 3.98 [3.79; 4.16] | 14.972, 0.0006 |
| - | - | p1-3 = 0.0004 | ||
| Triene conjugates, c.u. | 0.870 [0.793; 0.944] | 0.979 [0.843; 1.233] | 0.891 [0.787; 1.000] | 9.8454, 0.0073 |
| - | p1-2 = 0.0000 | p1-3 = 0.0250 | ||
| Schiff bases, c.u. | 0.528 [0.492; 0.565] | 0.558 [0.495; 0.682] | 0.557 [0.489; 0.669] | 41.5333, 0.00000 |
| - | p1-2 = 0.0000 | p1-3 = 0.0000 | ||
| Malondialdehyde (MDA), nmol/mL | 6.84 [5.81; 8.38] | 7.35 [5.64; 9.32] | 7.18 [5.73; 9.49] | 5.6906, 0.0581 |
| Endogenous intoxication rates | ||||
| MM 254 nm, c.u. | 0.271 [0.187; 0.381] | 0.261 [0.151; 0.407] | 0.252 [0.164; 0.398] | 3.3012, 0.1919 |
| MM 280 nm, c.u. | 0.224 [0.157; 0.324] | 0.225 [0.135; 0.359] | 0.226 [0.147; 0.348] | 0.0782, 0.9617 |
| MM 280/254 nm | 0.847 [0.749; 0.948] | 0.900 [0.797; 1.004] | 0.897 [0.801; 1.011] | 28.723, 0.00000 |
| - | p1-2 = 0.0007 | p1-3 = 0.0000 | ||
| Stage | Correctly Classified Patients | Incorrectly Classified Patients | Sensitivity at the Appropriate Stage, % |
|---|---|---|---|
| T1-2N0M0, n = 114 | 78 | 37 | 67.9 |
| T2N1-3M0, n = 59 | 38 | 21 | 65.0 |
| T3N0-3M0, n = 78 | 60 | 18 | 77.4 |
| T4N0-3M0, n = 59 | 38 | 21 | 65.0 |
| T1-4N0-3M1, n = 116 | 84 | 32 | 72.2 |
| Total, n = 426 | 298 | 128 | 69.5 |
| Feature | Category | Hazard Ratio (95% CI) | p Value | Overall Survival, Months |
|---|---|---|---|---|
| Median IC and LDH Concentrations | ||||
| IC, mmol/L | <0.311, n = 212 | 1 | 0.00244 | 23.0 |
| >0.311, n = 213 | 1.73 (1.14–2.62) * | 14.9 | ||
| LDH, U/L | <1133, n = 212 | 1 | 0.04720 | 15.6 |
| >1133, n = 210 | 0.72 (0.48–1.09) | 21.3 | ||
| IC, mmol/L + LDH, U/L | <0.311, >1133, n = 114 | 1 | 0.00000 | 24.9 |
| >0.311, <1133, n = 116 | 2.32 (1.29–4.14) * | 12.1 | ||
| Interquartile Range ofIC and LDH Concentrations | ||||
| IC, mmol/L | <0.197, n = 108 | 1 | 0.02742 | 21.7 |
| 0.197–0.478, n = 209 | 1.41 (0.87–2.28) | 17.9 | ||
| >0.478, n = 108 | 2.64 (1.42–4.82) * | 16.6 | ||
| LDH, U/L | <545, n = 106 | 1 | 0.00022 | 11.5 |
| 545–1748, n = 212 | 0.78 (0.46–1.34) | 19.2 | ||
| >1748, n = 104 | 0.44 (0.24–0.79) * | 23.9 | ||
| IC, mmol/L + LDH, U/L | <0.197, >1748, n = 52 | 1 | 0.00000 | 22.4 |
| >0.478, <575, n = 48 | 4.17 (1.36–12.51) * | 11.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K.; Gundyrev, I.A. Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics 2020, 10, 186. https://doi.org/10.3390/diagnostics10040186
Bel’skaya LV, Sarf EA, Kosenok VK, Gundyrev IA. Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics. 2020; 10(4):186. https://doi.org/10.3390/diagnostics10040186
Chicago/Turabian StyleBel’skaya, Lyudmila V., Elena A. Sarf, Victor K. Kosenok, and Ivan A. Gundyrev. 2020. "Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives" Diagnostics 10, no. 4: 186. https://doi.org/10.3390/diagnostics10040186
APA StyleBel’skaya, L. V., Sarf, E. A., Kosenok, V. K., & Gundyrev, I. A. (2020). Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics, 10(4), 186. https://doi.org/10.3390/diagnostics10040186