Can MRI Biomarkers Predict Triple-Negative Breast Cancer?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Technique
2.3. MR Imaging Evaluation
2.4. Histopathological Analysis
2.5. Statistical Analysis
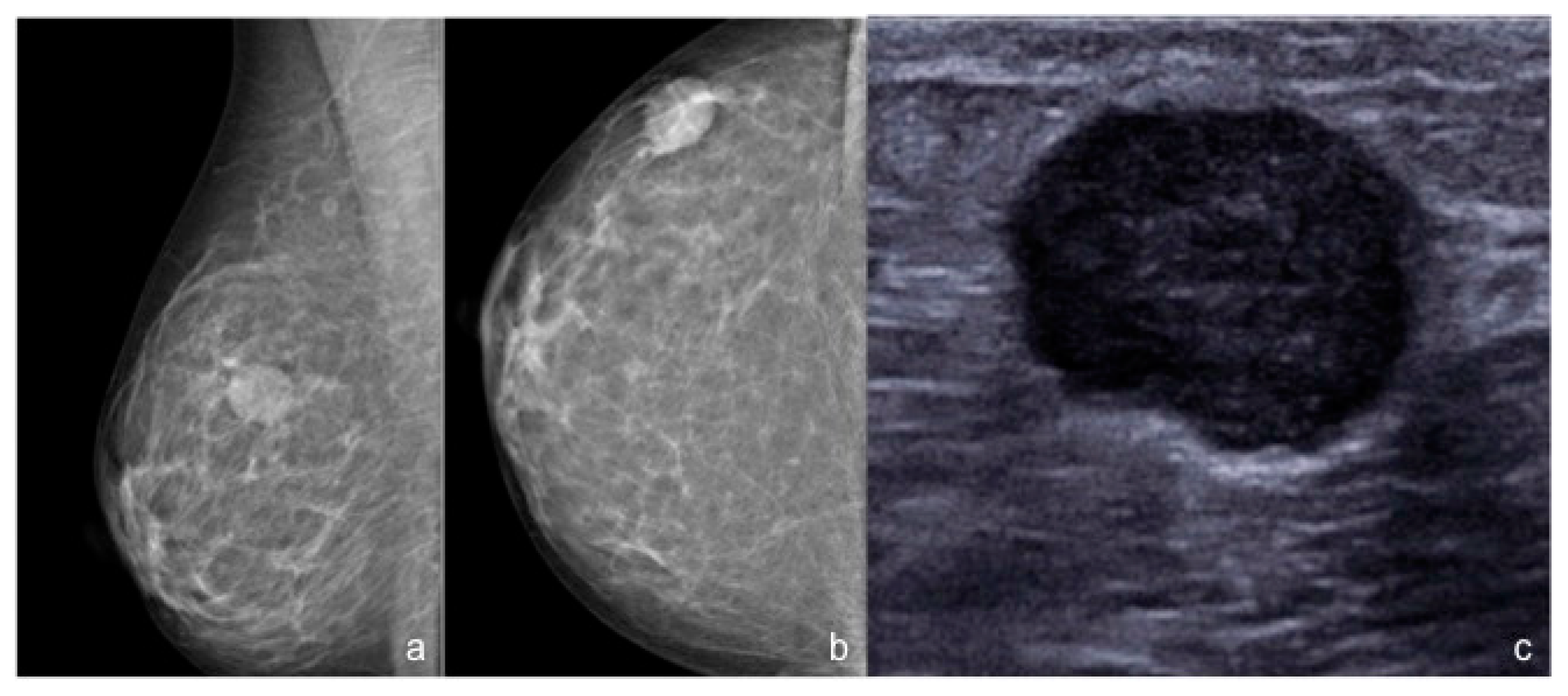
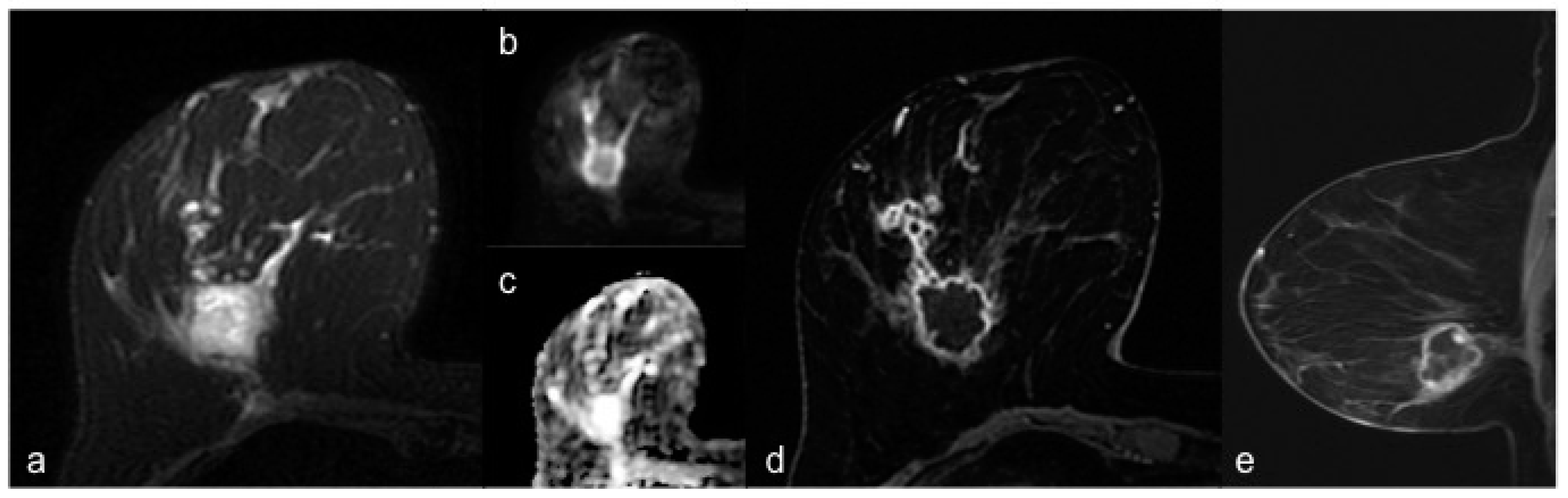
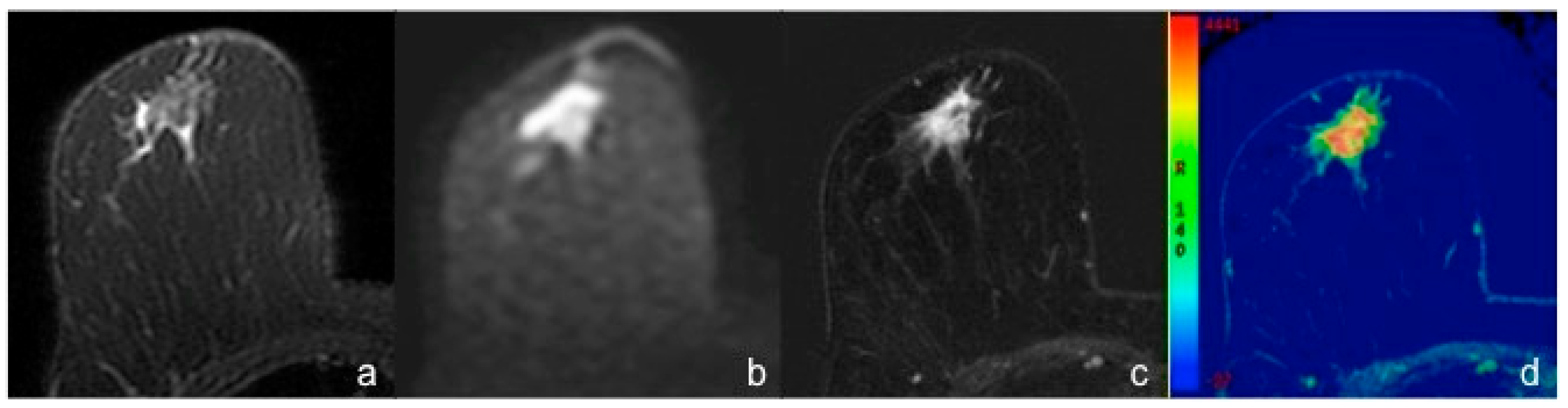
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Panel Members. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Vuong, D.; Simpson, P.T.; Green, B.; Cummings, M.C.; Lakhani, S.R. Molecular classification of breast cancer. Virchows Arch. 2014, 465, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Panel Members. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Atchley, D.P.; Albarracin, C.T.; Lopez, A.; Valero, V.; Amos, C.I.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Arun, B.K. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J. Clin. Oncol. 2008, 26, 4282–4288. [Google Scholar] [CrossRef]
- Boisserie-Lacroix, M.; Mac Grogan, G.; Debled, M.; Ferron, S.; Asad-Syed, M.; Brouste, V.; Mathoulin-Pelissier, S.; Hurtevent-Labrot, G. Radiological features of triple-negative breast cancers (73 cases). Diagn. Interv. Imaging 2012, 93, 183–190. [Google Scholar] [CrossRef]
- Dogan, B.E.; Turnbull, L.W. Imaging of triple-negative breast cancer. Ann. Oncol. 2012, 23 (Suppl. S66), 23–29. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Qin, Y.; Cai, J. Systematic review and meta-analysis of the malignant ultrasound features of triple negative breast cancer. J. Ultrasound Med. 2020, 39, 2013–2025. [Google Scholar] [CrossRef]
- Sung, J.S.; Jochelson, M.S.; Brennan, S.; Joo, S.; Wen, Y.H.; Moskowitz, C.; Zheng, J.; Dershaw, D.D.; Morris, E.A. MR imaging features of triple-negative breast cancers. Breast J. 2013, 19, 643–649. [Google Scholar] [CrossRef]
- Uematsu, T. MR imaging of triple-negative breast cancer. Breast Cancer 2011, 18, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.E.; Gonzalez-Angulo, A.M.; Gilcrease, M.; Dryden, M.J.; Yang, W.T. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am. J. Roentgenol. 2010, 194, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Agrawal, G.; Feig, B.; Baek, H.M.; Carpenter, P.M.; Mehta, R.S.; Nalcioglu, O.; Su, M.Y. Triple-negative breast cancer: MRI features in 29 patients. Ann. Oncol. 2007, 18, 2042–2043. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.H.; Son, E.J.; Chung, J.; Kim, J.A.; Kim, E.K. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: Comparison with other breast cancer subtypes. Eur. Radiol. 2012, 22, 1724–1734. [Google Scholar] [CrossRef]
- Martincich, L.; Deantoni, V.; Bertotto, I.; Redana, S.; Kubatzki, F.; Sarotto, I.; Rossi, V.; Liotti, M.; Ponzone, R.; Aglietta, M.; et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur. Radiol. 2012, 22, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Gigli, S.; Amabile, M.I.; David, E.; De Luca, A.; Grippo, C.; Manganaro, L.; Monti, M.; Ballesio, L. Morphological and semiquantitative kinetic analysis on dynamic contrast enhanced MRI in triple negative breast cancer Patients. Acad. Radiol. 2019, 26, 620–625. [Google Scholar] [CrossRef]
- Angelini, G.; Marini, C.; Iacconi, C.; Mazzotta, D.; Moretti, M.; Picano, E.; Morganti, R. Magnetic resonance (MR) features in triple negative breast cancer (TNBC) vs receptor positive cancer (nTNBC). Clin. Imaging 2018, 49, 12–16. [Google Scholar] [CrossRef]
- Vilar, L.N.; Alandete Germán, S.P.; Medina García, R.; Blanc García, E.; Camarasa Lillo, N.; Vilar Samper, J. MR Imaging findings in molecular subtypes of breast cancer according to BIRADS System. Breast J. 2017, 23, 421–428. [Google Scholar] [CrossRef]
- Bae, M.S.; Seo, M.; Kim, K.G.; Park, I.A.; Moon, W.K. Quantitative MRI morphology of invasive breast cancer: Correlation with immunohistochemical biomarkers and subtypes. Acta Radiol. 2015, 56, 269–275. [Google Scholar] [CrossRef]
- Boisserie-Lacroix, M.; Macgrogan, G.; Debled, M.; Ferron, S.; Asad-Syed, M.; McKelvie-Sebileau, P.; Mathoulin-Pélissier, S.; Brouste, V.; Hurtevent-Labrot, G. Triple-negative breast cancers: Associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Oncologist 2013, 18, 802–811. [Google Scholar] [CrossRef]
- Choi, J.J.; Kim, S.H.; Cha, E.S.; Kang, B.J.; Lee, J.H.; Lee, S.Y.; Jeong, S.H.; Yim, H.W.; Song, B.J. MRI findings of triple negative breast cancer: A comparison with non-triple negative breast cancer. JKSMRM J. Korean Soc. Magn. Reson. Med. 2010, 14, 95–102. [Google Scholar] [CrossRef]
- Mann, R.M.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Colin, C.; Cornford, E.; Evans, A.; Fallenberg, E.; Forrai, G.; Fuchsjäger, M.H.; et al. European Society of Breast Imaging (EUSOBI), with language review by Europa Donna—The European Breast Cancer Coalition. Breast MRI: EUSOBI recommendations for women’s information. Eur. Radiol. 2015, 25, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A.; Comstock, C.E.; Lee, C.H. ACR BI-RADS Magnetic Resonance Imaging. In ACR BI-RADS Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Dilorenzo, G.; Telegrafo, M.; La Forgia, D.; Stabile Ianora, A.A.; Moschetta, M. Breast MRI background parenchymal enhancement as an imaging bridge to molecular cancer sub-type. Eur. J. Radiol. 2019, 113, 148–152. [Google Scholar] [CrossRef]
- Li, J.; Han, X. Research and progress in magnetic resonance imaging of triple-negative breast cancer. Magn. Reson. Imaging 2014, 32, 392–396. [Google Scholar] [CrossRef]
- Alili, C.; Pages, E.; Curros Doyon, F.; Perrochia, H.; Millet, I.; Taourel, P. Correlation between MR imaging—Prognosis factors and molecular classification of breast cancers. Diagn. Interv. Imaging 2014, 95, 235–242. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Zhu, K.; Tian, J.; Li, Z.; Du, G.; Guo, Q.; Wu, T.; Li, J. Clinicopathological and ultrasonic features of triple-negative breast cancers: A comparison with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Ultrasound Med. Biol. 2018, 44, 1124–1132. [Google Scholar] [CrossRef]
- Yang, W.T.; Dryden, M.; Broglio, K.; Gilcrease, M.; Dawood, S.; Dempsey, P.J.; Valero, V.; Hortobagyi, G.; Atchley, D.; Arun, B. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res. Treat. 2008, 111, 405–410. [Google Scholar] [CrossRef]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, H.H.; Huh, M.O.; Kim, M.J.; Yi, A.; Kim, H.; Son, B.H.; Ahn, S.H. Correlation between mammographic and sonographic findings and prognostic factors in patients with node-negative invasive breast cancer. Br. J. Radiol. 2011, 84, 19–30. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Yuen, S. Triple-negative breast cancer: Correlation between MR imaging and pathologic findings. Radiology 2009, 250, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Youn, I.K.; Kim, S.H.; Kang, B.J.; Park, W.C.; Lee, A. Triple-negative breast cancer: Pretreatment magnetic resonance imaging features and clinicopathological factors associated with recurrence. Magn. Reson. Imaging 2020, 66, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Su, M.Y.; Nalcioglu, O.; Feig, S.A.; Chen, J.H. Significance of breast lesion descriptors in the ACR BI-RADS MRI lexicon. Cancer 2009, 115, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.M.; Loo, C.E.; Wesseling, J.; Pijnappel, R.M.; Gilhuijs, K.G. Association between rim enhancement of breast cancer on dynamic contrast-enhanced MRI and patient outcome: Impact of subtype. Breast Cancer Res. Treat. 2014, 148, 541–551. [Google Scholar] [CrossRef]
- Chang, Y.W.; Kwon, K.H.; Choi, D.L.; Lee, D.W.; Lee, M.H.; Lee, H.K.; Yang, S.B.; Kim, Y.; Seo, D.Y. Magnetic resonance imaging of breast cancer and correlation with prognostic factors. Acta Radiol. 2009, 50, 990–998. [Google Scholar] [CrossRef]
- Panzironi, G.; Moffa, G.; Galati, F.; Marzocca, F.; Rizzo, V.; Pediconi, F. Peritumoral edema as a biomarker of the aggressiveness of breast cancer: Results of a retrospective study on a 3 T scanner. Breast Cancer Res. Treat. 2020, 181, 53–60. [Google Scholar] [CrossRef]
- Costantini, M.; Belli, P.; Distefano, D.; Bufi, E.; Matteo, M.D.; Rinaldi, P.; Giuliani, M.; Petrone, G.; Magno, S.; Bonomo, L. Magnetic resonance imaging features in triple-negative breast cancer: Comparison with luminal and HER2-overexpressing tumors. Clin. Breast Cancer 2012, 12, 331–339. [Google Scholar] [CrossRef]
- Tang, P.; Tse, G.M. Immunohistochemical surrogates for molecular classification of breast carcinoma: A 2015 update. Arch. Pathol. Lab. Med. 2016, 140, 806–814. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Sun, X.; He, B.; Luo, X.; Li, Y.; Cao, J.; Wang, J.; Dong, J.; Sun, X.; Zhang, G. Preliminary study on molecular subtypes of breast cancer based on magnetic resonance imaging texture analysis. J. Comput. Assist. Tomogr. 2018, 42, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Harowicz, M.R.; Grimm, L.J.; Kim, C.E.; Ghate, S.V.; Walsh, R.; Mazurowski, M.A. A machine learning approach to radiogenomics of breast cancer: A study of 922 subjects and 529 DCE-MRI features. Br. J. Cancer 2018, 119, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J.; Zhang, J.; Baker, J.A.; Soo, M.S.; Johnson, K.S.; Mazurowski, M.A. Relationships between MRI Breast Imaging-Reporting and Data System (BI-RADS) lexicon descriptors and breast cancer molecular subtypes: Internal enhancement is associated with luminal B subtype. Breast J. 2017, 23, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, H.; Wang, S.; Zheng, B.; Zhang, J.; Li, L. Radiomic analysis reveals DCE-MRI features for prediction of molecular subtypes of breast cancer. PLoS ONE 2017, 12, e0171683. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Burnside, E.S.; Huang, E.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Zuley, M.; Net, J.M.; et al. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2016, 2, 1–10. [Google Scholar] [CrossRef]
| TNBC | Non-TNBC | p-Value | |
|---|---|---|---|
| Number of Patients | 26 | 24 | |
| Tumor size | 0.308 | ||
| ≥2 cm | 15 (57.7%) | 11 (45.8%) | |
| <2 cm | 11 (42.3%) | 13 (54.2%) | |
| Enhancement | 0.192 | ||
| Mass | 24 (92.3%) | 22 (91.7%) | |
| Non-mass | 2 (7.7%) | 2 (8.3%) | |
| Shape (masses) | 0.005 | ||
| Round | 9 (37.5%) | 3 (13.6%) | |
| Oval | 5 (20.8%) | 0 | |
| Irregular | 10 (41.7%) | 19 (86.4%) | |
| Margins (masses) | 0.001 | ||
| Circumscribed | 14 (58.4%) | 2 (9.1%) | |
| Irregular/Spiculated | 10 (41.6%) | 20 (90.9%) | |
| Rim enhancement (masses) | <0.001 | ||
| Yes | 18 (75.0%) | 2 (9.1%) | |
| No | 6 (25.0%) | 20 (90.9%) | |
| T2-signal intensity | 0.301 | ||
| Hyperintensity | 6 (23.1%) | 2 (8.3%) | |
| Isointensity/Hypointensity | 20 (76.9%) | 22 (91.7%) | |
| Intralesional necrosis | 0.016 | ||
| Yes | 11 (42.3%) | 2 (8.3%) | |
| No | 15 (57.7%) | 22 (91.7%) | |
| Perilesional edema | 0.88 | ||
| Yes | 6 (23.1%) | 5 (20.8%) | |
| No | 20 (76.9%) | 19 (79.2%) | |
| Multifocality/Multicentricity | 0.064 | ||
| Yes | 13 (50.0%) | 5 (20.8%) | |
| No | 13 (50.0%) | 19 (79.2%) | |
| Abnormal lymph nodes | 0.262 | ||
| Yes | 16 (61.5%) | 10 (41.7%) | |
| No | 10 (38.5%) | 14 (58.3%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (CI 95%) * | p-Value | OR (CI 95%) * | p-Value | |
| Tumor size ≥ 2 cm | 1.61 (0.53–4.93) | 0.4 | - | - |
| Enhancement | - | - | ||
| Mass | 1.10 (0.14–8.42) | 0.93 | ||
| Non-mass | 1.44 (0.22–9.42) | 0.7 | ||
| Shape (masses) | - | - | ||
| Round | 3.0 (0.30–31.63) | 0.4 | ||
| Oval | Out of scale | 1 | ||
| Irregular | 0.53 (0.06–4–32) | 0.55 | ||
| Irregular/Spiculated margins (masses) | 0.03 (0.01–0.17) | <0.001 | 0.03 (0.002–0.34) | 0.005 |
| Rim enhancement (masses) | 29.86 (5.53–161.35) | <0.001 | 33.08 (1.59–687.55) | 0.02 |
| T2-signal intensity | - | - | ||
| Hyperintensity | 0.22 (0.04–1.24) | 0.09 | ||
| Isointensity/Hypointensity | 1.20 (0.12–11.0) | 0.89 | ||
| Intralesional necrosis | 8.10 (1.56–41.72) | 0.01 | 0.52 (0.02–11.04) | 0.67 |
| Perilesional edema | 1.14 (0.30–4.37) | 0.85 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moffa, G.; Galati, F.; Collalunga, E.; Rizzo, V.; Kripa, E.; D’Amati, G.; Pediconi, F. Can MRI Biomarkers Predict Triple-Negative Breast Cancer? Diagnostics 2020, 10, 1090. https://doi.org/10.3390/diagnostics10121090
Moffa G, Galati F, Collalunga E, Rizzo V, Kripa E, D’Amati G, Pediconi F. Can MRI Biomarkers Predict Triple-Negative Breast Cancer? Diagnostics. 2020; 10(12):1090. https://doi.org/10.3390/diagnostics10121090
Chicago/Turabian StyleMoffa, Giuliana, Francesca Galati, Emmanuel Collalunga, Veronica Rizzo, Endi Kripa, Giulia D’Amati, and Federica Pediconi. 2020. "Can MRI Biomarkers Predict Triple-Negative Breast Cancer?" Diagnostics 10, no. 12: 1090. https://doi.org/10.3390/diagnostics10121090
APA StyleMoffa, G., Galati, F., Collalunga, E., Rizzo, V., Kripa, E., D’Amati, G., & Pediconi, F. (2020). Can MRI Biomarkers Predict Triple-Negative Breast Cancer? Diagnostics, 10(12), 1090. https://doi.org/10.3390/diagnostics10121090





