MRI-Derived Tumour-to-Breast Volume Is Associated with the Extent of Breast Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Patient Cohort
2.3. Image Acquisition
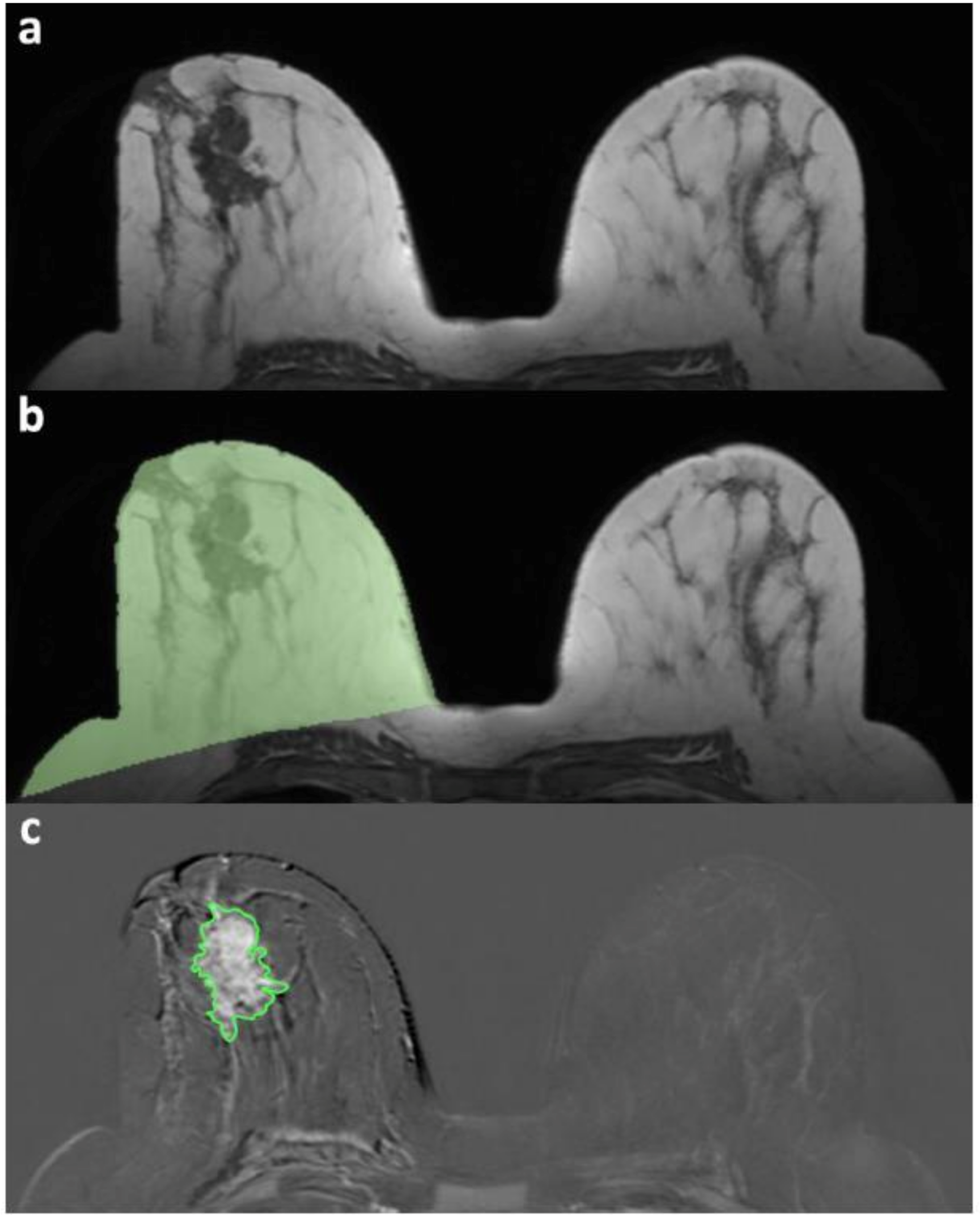
2.4. Image Analysis and Processing
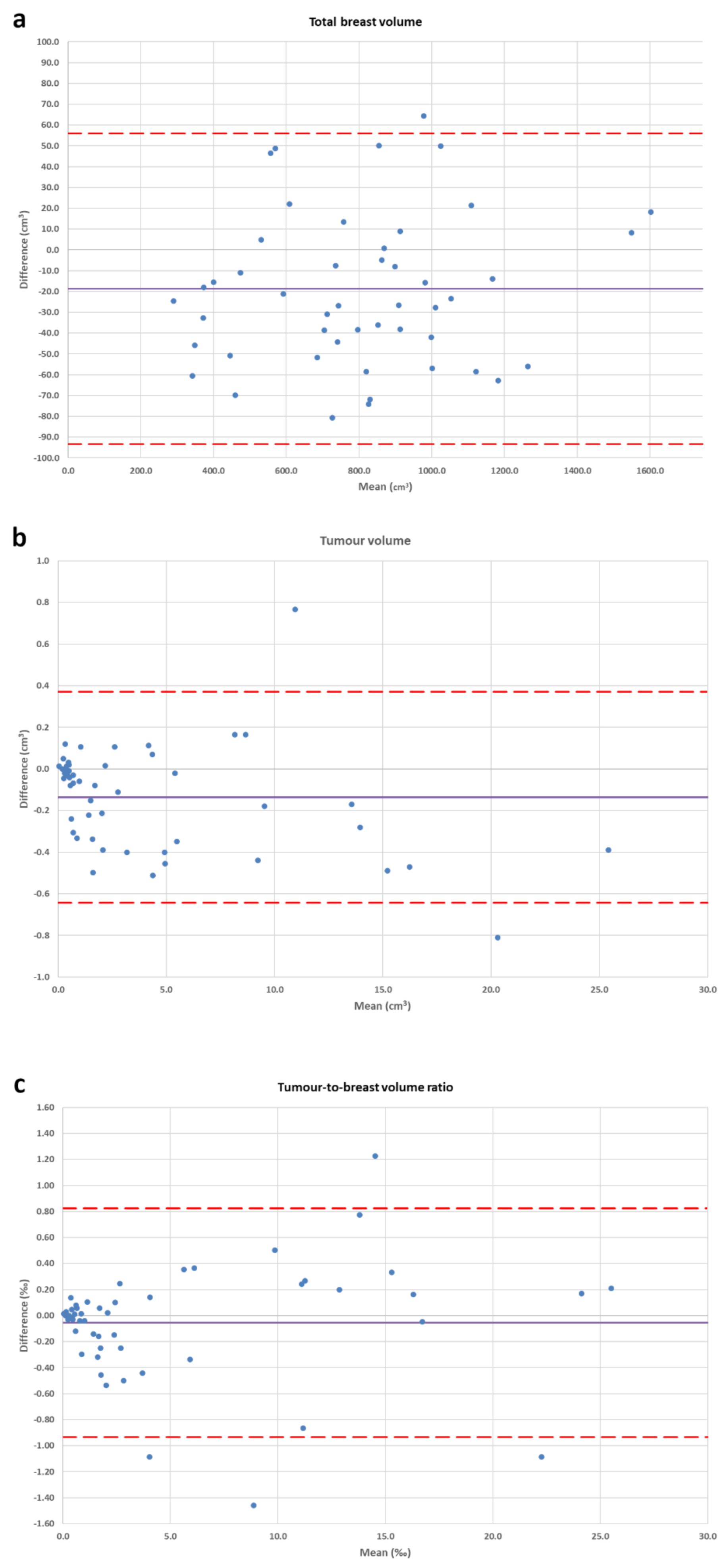
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, R.M.; Rossi, P.G.; Battisti, N.M.L.; Cozzi, A.; Magni, V.; Zanardo, M.; Sardanelli, F. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Saccozzi, R.; Del Vecchio, M.; Banfi, A.; Clemente, C.; De Lena, M.; Gallus, G.; Greco, M.; Luini, A.; Marubini, E.; et al. Comparing Radical Mastectomy with Quadrantectomy, Axillary Dissection, and Radiotherapy in Patients with Small Cancers of the Breast. N. Engl. J. Med. 1981, 305, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Lichter, A.S.; Lippman, M.E.; Danforth, D.N.; D’Angelo, T.; Steinberg, S.M.; DeMoss, E.; MacDonald, H.D.; Reichert, C.M.; Merino, M.; Swain, S.M. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J. Clin. Oncol. 1992, 10, 976–983. [Google Scholar] [CrossRef]
- Fisher, B.; Bauer, M.; Margolese, R.; Poisson, R.; Pilch, Y.; Redmond, C.; Fisher, E.; Wolmark, N.; Deutsch, M.; Montague, E.; et al. Five-Year Results of a Randomized Clinical Trial Comparing Total Mastectomy and Segmental Mastectomy with or without Radiation in the Treatment of Breast Cancer. N. Engl. J. Med. 1985, 312, 665–673. [Google Scholar] [CrossRef]
- Al-Ghazal, S.K.; Fallowfield, L.; Blamey, R.W. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur. J. Cancer 2000, 36, 1938–1943. [Google Scholar] [CrossRef]
- Curran, D.; van Dongen, J.P.; Aaronson, N.K.; Kiebert, G.; Fentiman, I.S.; Mignolet, F.; Bartelink, H. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: Results of EORTC trial 10801. Eur. J. Cancer 1998, 34, 307–314. [Google Scholar] [CrossRef]
- Schwartz, G.F.; Veronesi, U.; Clough, K.B.; Dixon, J.M.; Fentiman, I.S.; Heywang-Kobrunner, S.H.; Holland, R.; Hughes, K.S.; Mansel, R.E.; Margolese, R.; et al. Consensus Conference on Breast Conservation, Milan, Italy, April 28–May 1, 2005. Breast J. 2006, 12, 398–407. [Google Scholar] [CrossRef]
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kühn, T.; Mansel, R.E.; Ponti, A.; Poortmans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer 2017, 86, 59–81. [Google Scholar] [CrossRef]
- Brachtel, E.F.; Rusby, J.E.; Michaelson, J.S.; Chen, L.L.; Muzikansky, A.; Smith, B.L.; Koerner, F.C. Occult Nipple Involvement in Breast Cancer: Clinicopathologic Findings in 316 Consecutive Mastectomy Specimens. J. Clin. Oncol. 2009, 27, 4948–4954. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Benyahi, D.; Nos, C.; Charles, C.; Sarfati, I. Oncoplastic Surgery: Pushing the Limits of Breast-Conserving Surgery. Breast J. 2015, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Günay, S.; Gürsel, Ö.K.; Gökçek, B.; Yalçın, O.; Akan, A. Effect of Tumor Size and Tumor Location in the Breast on Late Cosmetic Outcomes in Patients with Early-Stage Breast Cancer Who Underwent Breast-Conserving Surgery and Intraoperative Boost Radiotherapy. Indian J. Surg. 2020. [Google Scholar] [CrossRef]
- Chan, S.W.W.; Chueng, P.S.Y.; Lam, S.H. Cosmetic Outcome and Percentage of Breast Volume Excision in Oncoplastic Breast Conserving Surgery. World J. Surg. 2010, 34, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Vos, E.; Koppert, L.; van Lankeren, W.; Verhoef, C.; Koerkamp, B.G.; Hunink, M. A preliminary prediction model for potentially guiding patient choices between breast conserving surgery and mastectomy in early breast cancer patients; a Dutch experience. Qual. Life Res. 2018, 27, 545–553. [Google Scholar] [CrossRef]
- Lagendijk, M.; Vos, E.L.; Koning, A.H.J.; Hunink, M.G.M.; Pignol, J.P.; Corten, E.M.L.; de Monye, C.; van Deurzen, C.H.M.; van Dam, J.H.; Vrijland, W.W.; et al. TUmor-volume to breast-volume RAtio for improving COSmetic results in breast cancer patients (TURACOS); a randomized controlled trial. BMC Cancer 2017, 17, 336. [Google Scholar] [CrossRef]
- Vos, E.L.; Koning, A.H.J.; Obdeijn, I.-M.; van Verschuer, V.M.T.; Verhoef, C.; van der Spek, P.J.; Menke-Pluijmers, M.B.; Koppert, L.B. Preoperative prediction of cosmetic results in breast conserving surgery. J. Surg. Oncol. 2015, 111, 178–184. [Google Scholar] [CrossRef]
- Lin, X.; Wang, J.; Han, F.; Fu, J.; Li, A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur. J. Radiol. 2012, 81, 873–878. [Google Scholar] [CrossRef]
- Clauser, P.; Londero, V.; Como, G.; Girometti, R.; Bazzocchi, M.; Zuiani, C. Comparison between different imaging techniques in the evaluation of malignant breast lesions: Can 3D ultrasound be useful? Radiol. Med. 2014, 119, 240–248. [Google Scholar] [CrossRef]
- Faermann, R.; Sperber, F.; Schneebaum, S.; Barsuk, D. Tumor-to-breast volume ratio as measured on MRI: A possible predictor of breast-conserving surgery versus mastectomy. Isr. Med. Assoc. J. 2014, 16, 101–105. [Google Scholar]
- Liu, Q.; Liu, Y.; Xu, L.; Duan, X.; Li, T.; Qin, N.; Kang, H.; Jiang, H.; Yang, D.; Qu, X.; et al. Multicenter prospective study of magnetic resonance imaging prior to breast-conserving surgery for breast cancer. Chin. Med. J. 2014, 127, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Dolen, U.; Thornton, M.; Tenenbaum, M.M.; Aripoli, A.; Patel, A.; Cyr, A.E.; Yan, Y.; Appleton, C.M.; Margenthaler, J.A.; Myckatyn, T.M. A prospective cohort study to analyze the interaction of tumor-to-breast volume in breast conservation therapy versus mastectomy with reconstruction. Breast Cancer Res. Treat. 2020, 181, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Pukancsik, D.; Kelemen, P.; Újhelyi, M.; Kovács, E.; Udvarhelyi, N.; Mészáros, N.; Kenessey, I.; Kovács, T.; Kásler, M.; Mátrai, Z. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: An aesthetic and functional prospective cohort study. Eur. J. Surg. Oncol. 2017, 43, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Rella, R.; Bufi, E.; Belli, P.; Conti, M.; Scaldaferri, A.; Grippo, C.; Franceschini, G.; Terribile, D.; Giuliani, M.; Manfredi, R. Magnetic Resonance Imaging prediction of large volume displacement oncoplastic surgery versus mastectomy in the treatment of breast cancer. Ann. Ital. Chir. 2020, 91, 248–256. [Google Scholar] [PubMed]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.-F.; Stanciu-Pop, C.; Drisis, S.; Radermeker, M.; Vandemerckt, C.; Noterman, D.; Moreau, M.; Larsimont, D.; Nogaret, J.-M.; Veys, I. The impact of breast MRI workup on tumor size assessment and surgical planning in patients with early breast cancer. Breast J. 2018, 24, 927–933. [Google Scholar] [CrossRef]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An Open-Source Software for Navigating in Multidimensional DICOM Images. J. Digit. Imaging 2004, 17, 205–216. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Di Leo, G.; Sardanelli, F. Statistical significance: P value, 0.05 threshold, and applications to radiomics—Reasons for a conservative approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, S.A.; Strasser, J. Updates in the Treatment of Breast Cancer with Radiotherapy. Surg. Oncol. Clin. N. Am. 2017, 26, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.; Weaver, D.L. Accurate determination of breast cancer size: The role of histopathology and imaging. Curr. Diagn. Pathol. 2005, 11, 435–442. [Google Scholar] [CrossRef]
- Gu, J.; Delisle, M.; Engler-Stringer, R.; Groot, G. Mastectomy versus breast-conservation therapy: An examination of how individual, clinicopathologic, and physician factors influence decision-making. Curr. Oncol. 2019, 26, e522–e534. [Google Scholar] [CrossRef] [PubMed]
- Association of Breast Surgery at BASO 2009. Surgical guidelines for the management of breast cancer. Eur. J. Surg. Oncol. 2009, 35, S1–S22. [Google Scholar] [CrossRef]
- Pilewskie, M.; Morrow, M. Margins in breast cancer: How much is enough? Cancer 2018, 124, 1335–1341. [Google Scholar] [CrossRef]
- de Koning, S.G.B.; Peeters, M.-J.T.F.D.V.; Jóźwiak, K.; Bhairosing, P.A.; Ruers, T.J.M. Tumor Resection Margin Definitions in Breast-Conserving Surgery: Systematic Review and Meta-analysis of the Current Literature. Clin. Breast Cancer 2018, 18, e595–e600. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; Marinovich, M.L.; Morrow, M. The Association of Surgical Margins and Local Recurrence in Women with Early-Stage Invasive Breast Cancer Treated with Breast-Conserving Therapy: A Meta-Analysis. Ann. Surg. Oncol. 2014, 21, 717–730. [Google Scholar] [CrossRef]
- McCahill, L.E.; Single, R.M.; Bowles, E.J.A.; Feigelson, H.S.; James, T.A.; Barney, T.; Engel, J.M.; Onitilo, A.A. Variability in Reexcision Following Breast Conservation Surgery. JAMA 2012, 307, 467–475. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Codari, M.; Schiaffino, S.; Sardanelli, F.; Trimboli, R.M. Artificial Intelligence for Breast MRI in 2008–2018: A Systematic Mapping Review. Am. J. Roentgenol. 2019, 212, 280–292. [Google Scholar] [CrossRef]

| Sequence | TR (ms) | TE (ms) | Flip Angle | b Values (s/mm2) | Spatial Resolution (mm) | Matrix (Voxels) | Slices | Field of View (mm3) | Time (mm:ss) |
|---|---|---|---|---|---|---|---|---|---|
| STIR | 9670 | 75.00 | 150° | - | 0.7 × 0.7 × 2.0 | 512 × 512 | 60 | 358.4 × 358.4 × 120 | 08:24 |
| DWI | 4900 | 76.00 | 90° | 0, 750 | 2.7 × 2.7 × 4.0 | 60 × 172 | 30 | 162 × 464.4 × 120 | 03:31 |
| DCE-MRI | 11 | 4.89 | 45° | - | 0.8 × 0.8 × 1.3 | 512 × 512 | 120 | 409.6 × 409.6 × 156 | 09:55 |
| Variables | Breast-Conserving Surgery (31 Patients) | Mastectomy (20 Patients) | |
|---|---|---|---|
| Median age (IQR) | 59.0 years (47.5–68.0 years) | 52.5 years (45.0–59.7 years) | |
| MRI staging | |||
| T1a (%) | 1 (3%) | - | |
| T1b (%) | 7 (23%) | 2 (10%) | |
| T1c (%) | 14 (45%) | 4 (20%) | |
| T2 (%) | 8 (26%) | 12 (60%) | |
| T3 (%) | - | 2 (10%) | |
| T4b (%) | 1 (3%) | - | |
| Final pathology * | |||
| Median tumour size at final pathology (IQR) | 1.35 cm (1.00–1.80 cm) | 2.20 cm (1.50–2.50 cm) | |
| Pathology diagnosis | IDC (%) | 6 (21%) | 10 (50%) |
| DCIS (%) | - | 1 (5%) | |
| ILC (%) | - | 3 (15%) | |
| IDC + DCIS (%) | 16 (55%) | 5 (25%) | |
| ILC + LCIS (%) | 6 (21%) | 1 (5%) | |
| Micropapillary carcinoma (%) | 1 (3%) | - | |
| Molecular subtypes ** | Luminal A type (%) | 7 (24%) | 5 (28%) |
| Luminal B type (%) | 19 (66%) | 9 (50%) | |
| HER2 overexpression type (%) | 1 (3%) | 1 (5%) | |
| Triple negative type (%) | 2 (7%) | 3 (17%) | |
| Variables | Breast-Conserving Surgery (31 Patients) | Mastectomy (20 Patients) | |
|---|---|---|---|
| Breast MRI Volume Metrics | Median breast volume Reader 1 (IQR) | 789.05 cm3 (587.12–1022.83 cm3) | 837.43 cm3 (687.38–982.93 cm3) |
| Median breast volume Reader 2 (IQR) | 863.20 cm3 (539.50–1022.10 cm3) | 857.54 cm3 (723.52–999.46 cm3) | |
| Median tumour volume Reader 1 (IQR) | 0.69 cm3 (0.40–1.91 cm3) | 6.81 cm3 (2.00–11.95 cm3) | |
| Median tumour volume Reader 2 (IQR) | 0.85 cm3 (0.39–2.21 cm3) | 6.74 cm3 (2.07–11.45 cm3) | |
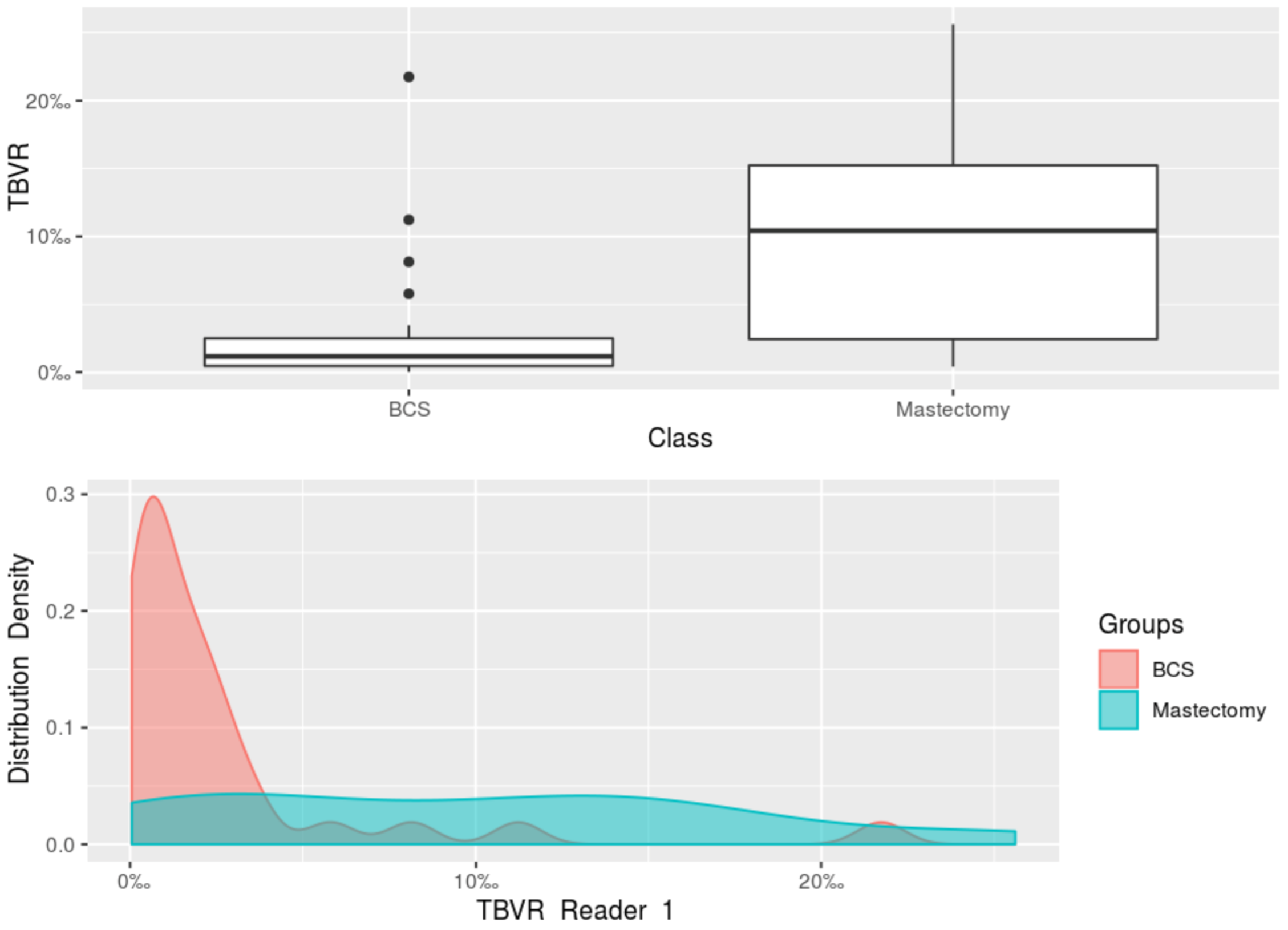
| Median TBVR Reader 1 (IQR) | 1.19‰ (0.49–2.51‰) | 10.43‰ (2.46–15.23‰) | |
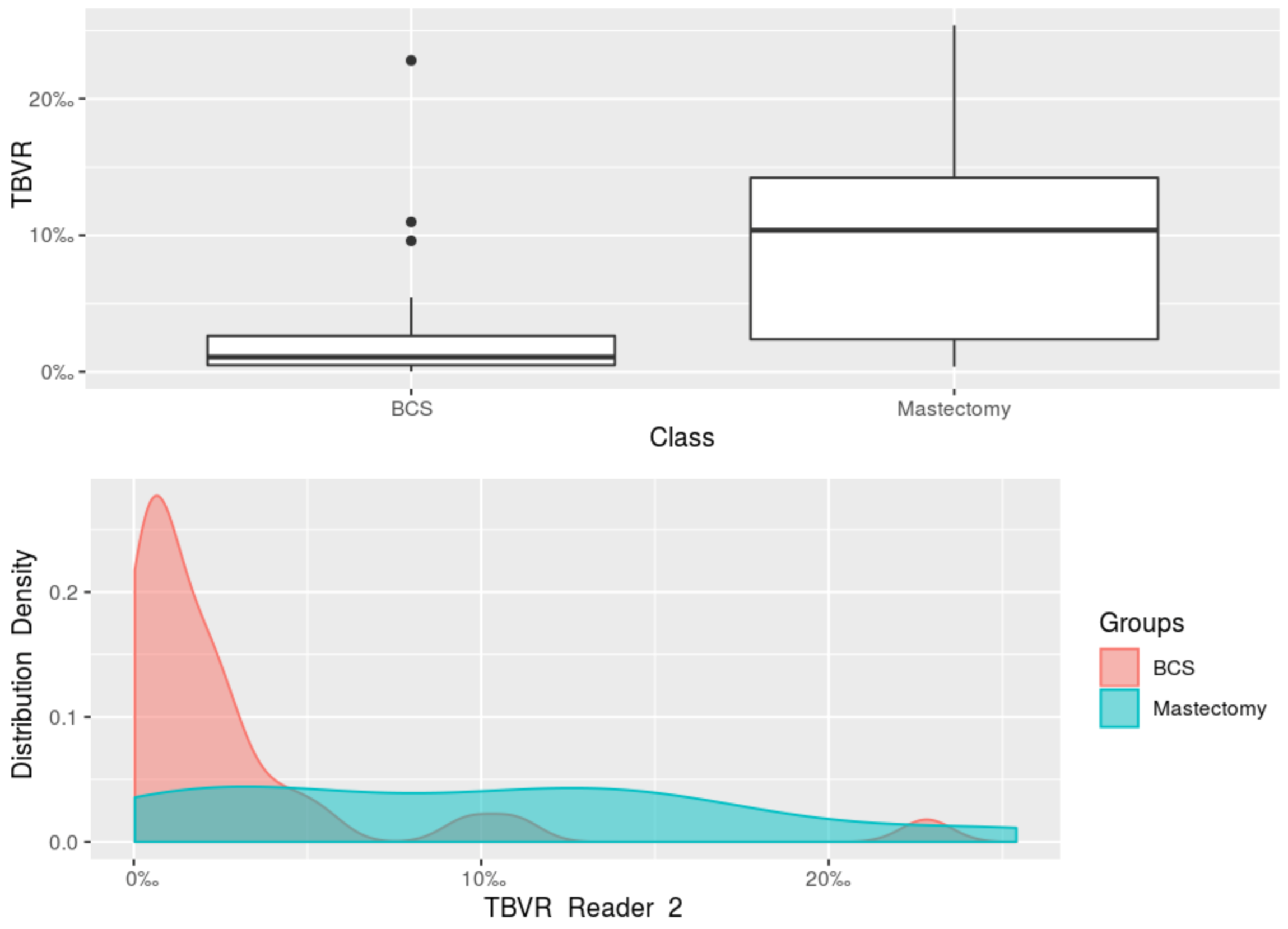
| Median TBVR Reader 2 (IQR) | 1.09‰ (0.49–2.64‰) | 10.37‰ (2.39–14.23‰) | |
| Segmentation Times | Breast volume Reader 1 (IQR) | 38 s (31–44 s) | |
| Breast volume Reader 2 (IQR) | 69 s (54–82 s) | ||
| Tumour volume Reader 1 (IQR) | 129 s (91–191 s) | ||
| Tumour volume Reader 2 (IQR) | 153 s (95–293 s) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzi, A.; Schiaffino, S.; Della Pepa, G.; Carriero, S.; Magni, V.; Spinelli, D.; Carbonaro, L.A.; Sardanelli, F. MRI-Derived Tumour-to-Breast Volume Is Associated with the Extent of Breast Surgery. Diagnostics 2021, 11, 204. https://doi.org/10.3390/diagnostics11020204
Cozzi A, Schiaffino S, Della Pepa G, Carriero S, Magni V, Spinelli D, Carbonaro LA, Sardanelli F. MRI-Derived Tumour-to-Breast Volume Is Associated with the Extent of Breast Surgery. Diagnostics. 2021; 11(2):204. https://doi.org/10.3390/diagnostics11020204
Chicago/Turabian StyleCozzi, Andrea, Simone Schiaffino, Gianmarco Della Pepa, Serena Carriero, Veronica Magni, Diana Spinelli, Luca A. Carbonaro, and Francesco Sardanelli. 2021. "MRI-Derived Tumour-to-Breast Volume Is Associated with the Extent of Breast Surgery" Diagnostics 11, no. 2: 204. https://doi.org/10.3390/diagnostics11020204
APA StyleCozzi, A., Schiaffino, S., Della Pepa, G., Carriero, S., Magni, V., Spinelli, D., Carbonaro, L. A., & Sardanelli, F. (2021). MRI-Derived Tumour-to-Breast Volume Is Associated with the Extent of Breast Surgery. Diagnostics, 11(2), 204. https://doi.org/10.3390/diagnostics11020204






