Quantitative Measurement of Breast Density Using Personalized 3D-Printed Breast Model for Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subject: A Personalized 3D-Printed Breast Model
2.2. MR Scanning Protocol
2.3. Quantitative Measurement: Breast Volume, Fibroglandular Tissue Volume, and Percentage of Breast Density
2.4. Data Synthesis
2.5. Statistical Analysis
3. Results
3.1. Scanning of the Personalized 3D-Printed Breast Model
3.2. Quantitative Measurement of Breast Volume, Fibroglandular Tissue Volume, and Percentage of Breast Density
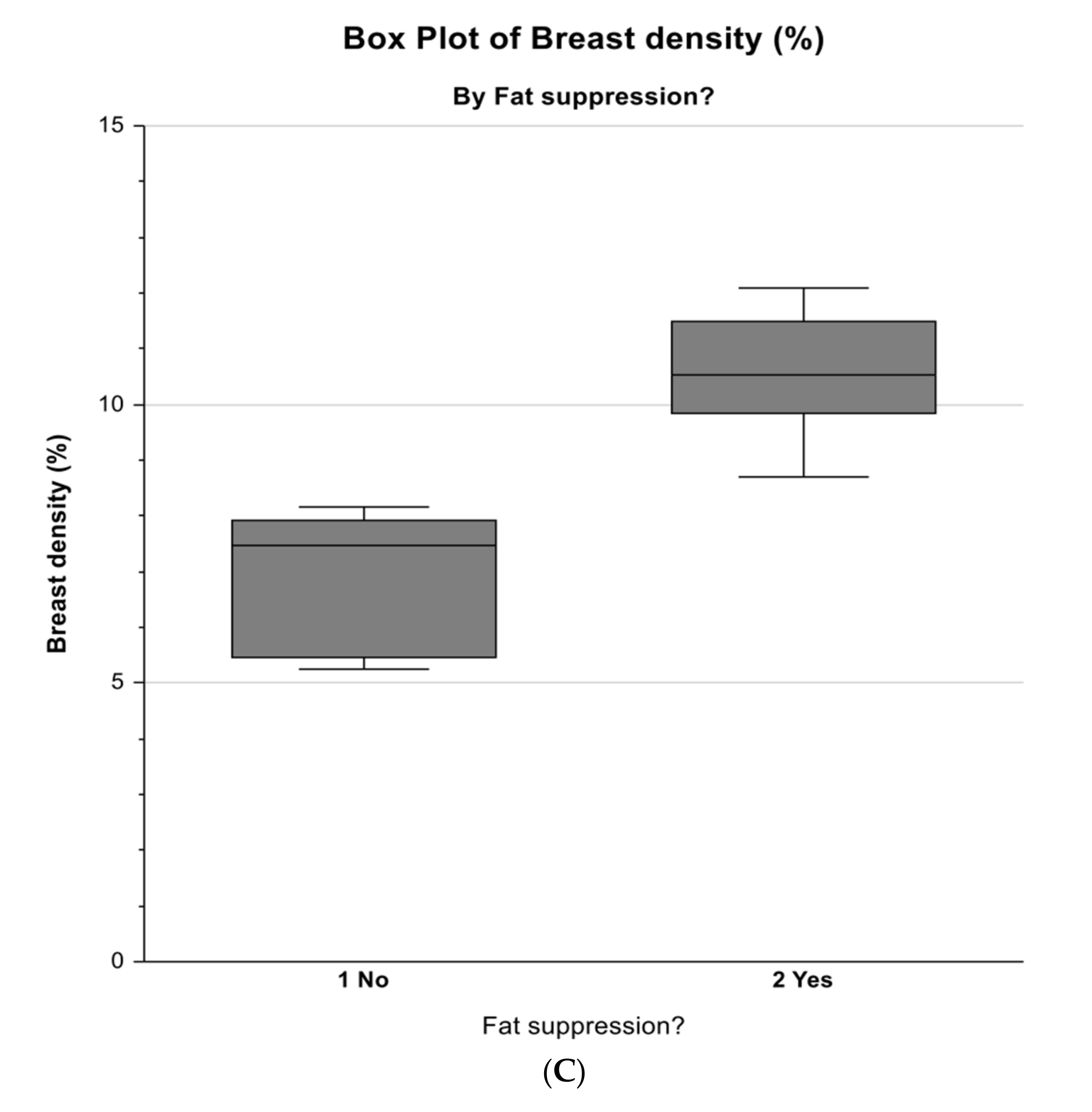
3.3. Comparison of Measurements Between Non-Fat-Suppression and Fat-Suppression Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.H.; Chang, Y.C.; Chang, D.; Wang, Y.T.; Nie, K.; Chang, R.F.; Nalcioglu, O.; Huang, C.S.; Su, M.Y. Reduction of breast density following tamoxifen treatment evaluated by 3-D MRI: Preliminary study. Magn. Reson. Imaging 2011, 29, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Azziz, A.; Fan, B.; Malkov, S.; Klifa, C.; Newitt, D.; Yitta, S.; Hylton, N.; Kerlikowske, K.; Shepherd, J.A. Agreement of mammographic measures of volumetric breast density to MRI. PLoS ONE 2013, 8, e81653. [Google Scholar] [CrossRef]
- Tagliafico, A.; Bignotti, B.; Tagliafico, G.; Astengo, D.; Martino, L.; Airaldi, S.; Signori, A.; Sormani, M.P.; Houssami, N.; Calabrese, M. Breast density assessment using a 3T MRI system: Comparison among different sequences. PLoS ONE 2014, 9, e99027. [Google Scholar] [CrossRef]
- Bu, Y.; Xia, J.; Joseph, B.; Zhao, X.; Xu, M.; Yu, Y.; Qi, S.; Shah, K.A.; Wang, S.; Hu, J. Non-contrast MRI for breast screening: Preliminary study on detectability of benign and malignant lesions in women with dense breasts. Breast Cancer Res. Treat. 2019, 177, 629–639. [Google Scholar] [CrossRef]
- Nelson, H.D.; Zakher, B.; Cantor, A.; Fu, R.; Griffin, J.; O’Meara, E.S.; Buist, D.S.; Kerlikowske, K.; Van Ravesteyn, N.T.; Trentham-Dietz, A.; et al. Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 635–648. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidem. Biomar. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerlikowske, K.; Ichikawa, L.; Miglioretti, D.L.; Buist, D.S.; Vacek, P.M.; Smith-Bindman, R.; Yankaskas, B.; Carney, P.A.; Ballard-Barbash, R. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J. Natl. Cancer Inst. 2007, 99, 386–395. [Google Scholar] [CrossRef]
- Sindi, R.; Sá dos Reis, C.; Bennett, C.; Stevenson, G.; Sun, Z. Quantitative measurements of breast density using magnetic resonance imaging: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 745. [Google Scholar] [CrossRef] [Green Version]
- Sindi, R.; Sun, Z. Optimal protocols for quantitative assessment of breast density using magnetic resonance imaging. Australas. Med. J. 2019, 12, 186–188. [Google Scholar] [CrossRef]
- Warner, E.; Messersmith, H.; Causer, P.; Eisen, A.; Shumak, R.; Plewes, D. Systematic review: Using magnetic resonance imaging to screen women at high risk for breast cancer. Ann. Intern. Med. 2008, 148, 671–679. [Google Scholar] [CrossRef]
- Nayeem, F.; Ju, H.; Brunder, D.G.; Nagamani, M.; Anderson, K.E.; Khamapirad, T.; Lu, L.J. Similarity of fibroglandular breast tissue content measured from magnetic resonance and mammographic images and by a mathematical algorithm. Int. J. Breast. Cancer 2014, 2014, 961679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Chan, S.; Chen, J.H.; Chang, D.; Nie, K.; Chen, S.T.; Lin, C.J.; Shih, T.C.; Nalcioglu, O.; Su, M.Y. A new bias field correction method combining N3 and FCM for improved segmentation of breast density on MRI. Med. Phys. 2011, 38, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doran, S.J.; Hipwell, J.H.; Denholm, R.; Eiben, B.; Busana, M.; Hawkes, D.J.; Leach, M.O.; Silva, I.D. Breast MRI segmentation for density estimation: Do different methods give the same results and how much do differences matter? Med. Phys. 2017, 44, 4573–4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliafico, A.; Tagliafico, G.; Tosto, S.; Chiesa, F.; Martinoli, C.; Derchi, L.E.; Calabrese, M. Mammographic density estimation: Comparison among BI-RADS categories, a semi-automated software and a fully automated one. Breast 2009, 18, 35–40. [Google Scholar] [CrossRef]
- Boston, R.C.; Schnall, M.D.; Englander, S.A.; Landis, J.R.; Moate, P.J. Estimation of the content of fat and parenchyma in breast tissue using MRI T1 histograms and phantoms. Magn. Reson. Imaging 2005, 23, 591–599. [Google Scholar] [CrossRef]
- Lu, L.J.; Nishino, T.K.; Johnson, R.F.; Nayeem, F.; Brunder, D.G.; Ju, H.; Leonard, M.H., Jr.; Grady, J.J.; Khamapirad, T. Comparison of breast tissue measurements using magnetic resonance imaging, digital mammography and a mathematical algorithm. Phys. Med. Biol. 2012, 57, 6903–6927. [Google Scholar] [CrossRef]
- Chang, D.H.; Chen, J.H.; Lin, M.; Bahri, S.; Hon, J.Y.; Mehta, R.S.; Nie, K.; Hsiang, D.J.; Nalcioglu, O.; Su, M.Y. Comparison of breast density measured on MR images acquired using fat-suppressed versus nonfat-suppressed sequences. Med. Phys. 2011, 38, 5961–5968. [Google Scholar] [CrossRef]
- Nie, K.; Chang, D.; Chen, J.H.; Hsu, C.C.; Nalcioglu, O.; Su, M.Y. Quantitative analysis of breast parenchymal patterns using 3D fibroglandular tissues segmented based on MRI. Med. Phys. 2010, 37, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Pertuz, S.; McDonald, E.S.; Weinstein, S.P.; Conant, E.F.; Kontos, D. Fully automated quantitative estimation of volumetric breast density from digital breast tomosynthesis images: Preliminary results and comparison with digital mammography and MR imaging. Radiology 2016, 279, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Ledger, A.E.; Scurr, E.D.; Hughes, J.; Macdonald, A.; Wallace, T.; Thomas, K.; Wilson, R.; Leach, M.O.; Schmidt, M.A. Comparison of Dixon sequences for estimation of percent breast fibroglandular tissue. PLoS ONE 2016, 11, e0152152. [Google Scholar] [CrossRef]
- Miyazaki, M.; Wheaton, A.; Kitane, S. Enhanced fat suppression technique for breast imaging. J. Magn. Reson. Imaging 2013, 38, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Kalovidouri, A.; Firmenich, N.; Delattre, B.M.; Picarra, M.; Becker, C.D.; Montet, X.; Botsikas, D. Fat suppression techniques for breast MRI: Dixon versus spectral fat saturation for 3D T1-weighted at 3 T. Radiol. Med. 2017, 122, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Le-Petross, H.; Kundra, V.; Szklaruk, J.; Wei, W.; Hortobagyi, G.N.; Ma, J. Fast three-dimensional dual echo Dixon technique improves fat suppression in breast MRI. J. Magn. Reson. Imaging 2010, 31, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.E.; Ma, J.; Hwang, K.; Liu, P.; Yang, W.T. T1-weighted 3D dynamic contrast-enhanced MRI of the breast using a dual-echo Dixon technique at 3 T. J. Magn. Reson. Imaging 2011, 34, 842–851. [Google Scholar] [CrossRef]
- Clauser, P.; Pinker, K.; Helbich, T.H.; Kapetas, P.; Bernathova, M.; Baltzer, P.A. Fat saturation in dynamic breast MRI at 3 Tesla: Is the Dixon technique superior to spectral fat saturation? A visual grading characteristics study. Eur. Radiol. 2014, 24, 2213–2219. [Google Scholar] [CrossRef]
- An, Y.Y.; Kim, S.H.; Kang, B.J. Differentiation of malignant and benign breast lesions: Added value of the qualitative analysis of breast lesions on diffusion-weighted imaging (DWI) using readout-segmented echo-planar imaging at 3.0 T. PLoS ONE 2017, 12, e0174681. [Google Scholar] [CrossRef]
- Beckett, K.R.; Moriarity, A.K.; Langer, J.M. Safe use of contrast media: What the radiologist needs to know. Radiographics 2015, 35, 1738–1750. [Google Scholar] [CrossRef]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.I.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Ramalho, M.; Ramalho, J.; Burke, L.M.; Semelka, R.C. Gadolinium retention and toxicity—An update. Adv. Chronic Kidney Dis. 2017, 24, 138–146. [Google Scholar] [CrossRef]
- Del Grande, F.; Santini, F.; Herzka, D.A.; Aro, M.R.; Dean, C.W.; Gold, G.E.; Carrino, J.A. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics 2014, 34, 217–233. [Google Scholar] [CrossRef] [Green Version]
- McRobbie, D.W.; Moore, E.A.; Graves, M.J.; Prince, M.R. MRI from Picture to Proton; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Brown, M.A.; Semelka, R.C.; Dale, B.M. MRI: Basic Principles and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European society of breast imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef] [Green Version]
- Hauer, M.P.; Uhl, M.; Allmann, K.H.; Laubenberger, J.; Zimmerhackl, L.B.; Langer, M. Comparison of turbo inversion recovery magnitude (TIRM) with T2-weighted turbo spin-echo and T1-weighted spin-echo MR imaging in the early diagnosis of acute osteomyelitis in children. Pediatr. Radiol. 1998, 28, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Freed, M.; Badal, A.; Jennings, R.J.; de Las, H.H.; Myers, K.J.; Badano, A. X-ray properties of an anthropomorphic breast phantom for MRI and x-ray imaging. Phys. Med. Biol. 2011, 56, 3513–3533. [Google Scholar] [CrossRef] [PubMed]
- Carton, A.K.; Bakic, P.; Ullberg, C.; Derand, H.; Maidment, A.D. Development of a physical 3D anthropomorphic breast phantom. Med. Phys. 2011, 38, 891–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzara, G.P.; Briggs, R.W.; Wu, Z.; Steinbach, B.G. Use of a modified polysaccharide gel in developing a realistic breast phantom for MRI. Magn. Reson. Imaging 1996, 14, 639–648. [Google Scholar] [CrossRef]
- Liney, G.P.; Tozer, D.J.; Turnbull, L.W. A simple and realistic tissue-equivalent breast phantom for MRI. J. Magn. Reson. Imaging 1999, 10, 968–971. [Google Scholar] [CrossRef]
- Freed, M.; de Zwart, J.A.; Loud, J.T.; El Khouli, R.H.; Myers, K.J.; Greene, M.H.; Duyn, J.H.; Badano, A. An anthropomorphic phantom for quantitative evaluation of breast MRI. Med. Phys. 2011, 38, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Burfeindt, M.J.; Colgan, T.J.; Mays, R.O.; Shea, J.D.; Behdad, N.; van Veen, B.D.; Hagness, S.C. MRI-derived 3-D-printed breast phantom for microwave breast imaging validation. IEEE. Antennas. Wirel. Propag. Lett. 2012, 11, 1610–1613. [Google Scholar] [CrossRef] [Green Version]
- Dantuma, M.; van Dommelen, R.; Manohar, S. Semi-anthropomorphic photoacoustic breast phantom. Biomed. Opt. Express. 2019, 10, 5921–5939. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Dyer, B.A.; Boone, J.M.; Liu, S.; Chen, T.; Zheng, F.; Zhu, Y.; Sun, Y.; Rong, Y.; et al. 3D printed breast phantom for multi-purpose and multi-modality imaging. Quant. Imaging Med. Surg. 2019, 9, 63–74. [Google Scholar] [CrossRef]
- Sindi, R.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Development of patient-specific 3D-printed breast phantom using silicone and peanut oils for magnetic resonance imaging. Quant. Imaging Med. Surg. 2020, 10, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Westra, C.; Dialani, V.; Mehta, T.S.; Eisenberg, R.L. Using T2-weighted sequences to more accurately characterize breast masses seen on MRI. AJR Am. J. Roentgenol. 2014, 202, 183–190. [Google Scholar] [CrossRef] [PubMed]

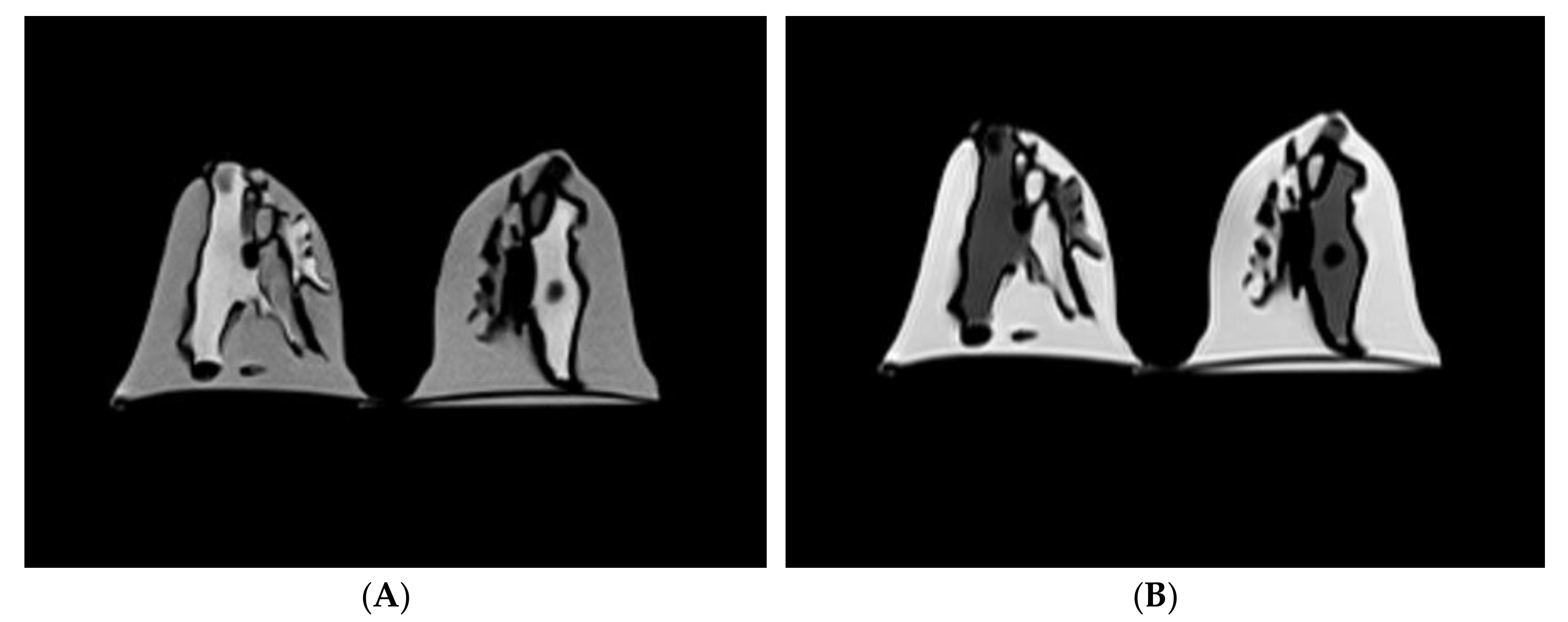
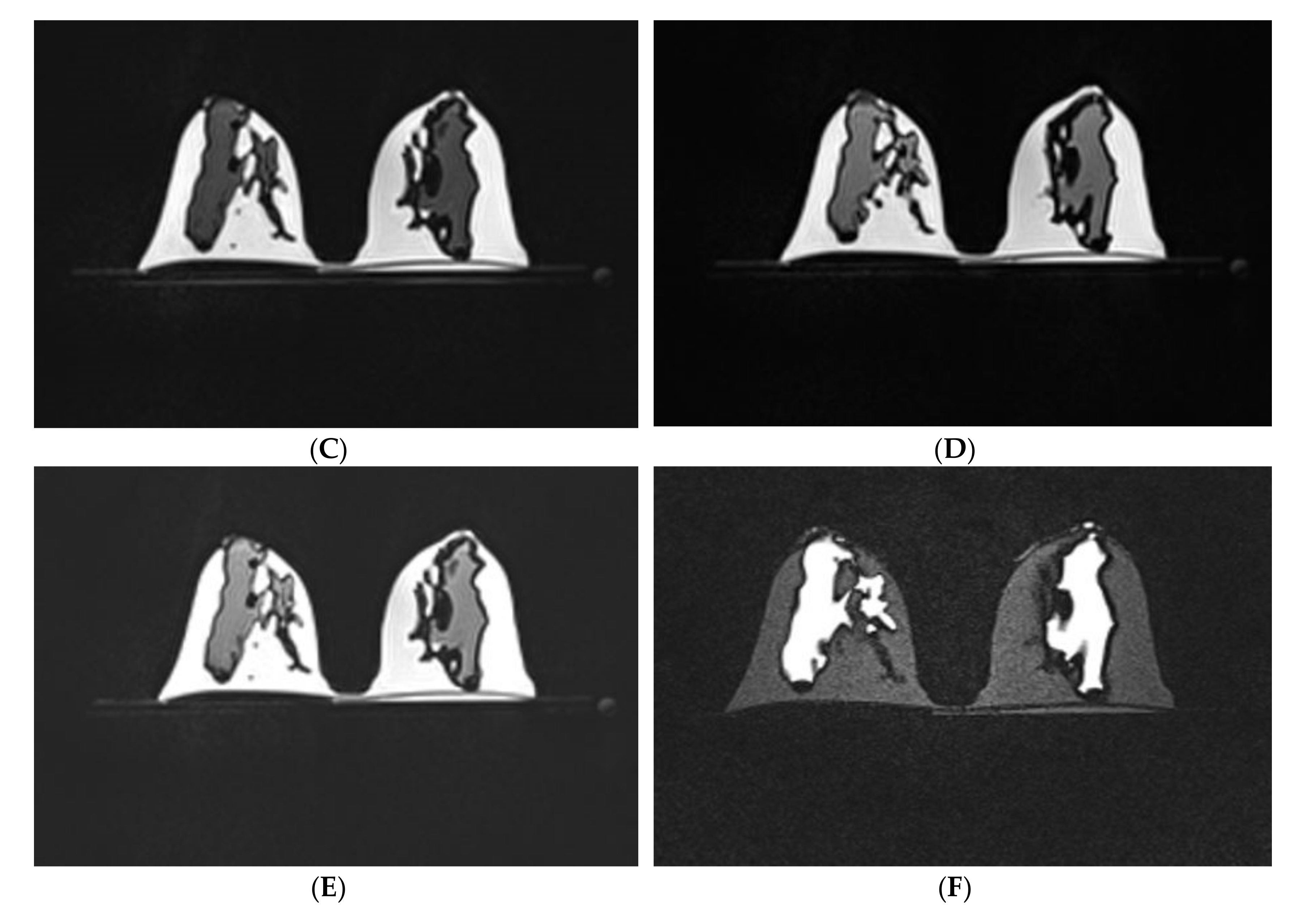

| No. | MRI Sequence | Acquisition Type | Orientation, Slice No. | TR (ms) | TE (ms) | TI (ms) | FOV (mm) | Matrix Size | Slice Thickness (mm) | Flip Angle (°) | NSA | Scan Time (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Non-fat-suppressed TSE (T2W) | 2D | Axial, 33 | 6080 | 78 | 350 × 350 | 336 × 448 | 4.0 | 80 | 1 | 1.10 | |
| 2. | Non-fat-suppressed TSE (T1W) | 2D | Axial, 37 | 709 | 10 | 350 × 350 | 224 × 320 | 2.9 | 130 | 2 | 2.38 | |
| 3. | Non-fat-suppressed TSE SPACE (T1W) | 3D | Axial, 88 | 600 | 3.4 | 400 × 400 | 256 × 256 | 1.6 | 120 | 2 | 2.47 | |
| 4. | Fat-suppressed TSE SPACE (T1W) | 3D | Axial, 88 | 1500 | 3.4 | 400 × 400 | 256 ×2 56 | 1.6 | 120 | 1 | 4.58 | |
| 5. | Fat-suppressed TSE SPACE SPAIR (T1W) | 3D | Axial, 88 | 1500 | 3.4 | 400 × 400 | 256 × 256 | 1.6 | 120 | 1 | 4.58 | |
| 6. | Fat-suppressed IR/PFP TIRM (T2W) | 2D | Axial, 37 | 4120 | 70 | 230 | 340 × 340 | 358 × 448 | 3.0 | 80 | 2 | 1.51 |
| MRI Sequence * | Breast Volume (cm3) | Fibroglandular Tissue Volume (cm3) | Breast Density (%) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Non-fat-suppression group (MR Sequences 1, 2, and 3) | ||||||
| MR Seq. 1 (N = 3) | 592.291 | 5.065 | 31.984 | 0.735 | 5.401 | 0.165 |
| MR Seq. 2 (N = 3) | 388.793 | 4.159 | 30.067 | 1.159 | 7.733 | 0.365 |
| MR Seq. 3 (N = 3) | 443.884 | 11.913 | 34.261 | 1.809 | 7.719 | 0.366 |
| Combined (N = 9) | 474.989 | 91.406 | 32.104 | 2.144 | 6.952 | 1.194 |
| Fat-suppression group (MR Sequences 4, 5, and 6) | ||||||
| MR Seq. 4 (N = 3) | 461.188 | 4.699 | 53.940 | 1.083 | 11.698 | 0.351 |
| MR Seq. 5 (N = 3) | 462.948 | 11.882 | 48.456 | 1.140 | 10.467 | 0.084 |
| MR Seq. 6 (N = 3) | 715.784 | 32.097 | 67.794 | 3.623 | 9.498 | 0.930 |
| Combined (N = 9) | 546.640 | 128.031 | 56.730 | 8.854 | 10.555 | 1.077 |
| Breast Density Parameter | Non-Fat-Suppressed (N = 9) | Fat-Suppressed (N = 9) | F-Ratio | Prob Level ** | ||
|---|---|---|---|---|---|---|
| Mean | SE (4 df *) | Mean | SE (4 df *) | |||
| Breast volume (cm3) | 474.989 | 73.639 | 546.640 | 73.639 | 0.47 | 0.5293 |
| Fibroglandular tissue volume (cm3) | 32.104 | 4.158 | 56.730 | 4.158 | 17.54 | 0.0138 |
| Breast density (%) | 6.952 | 0.709 | 10.555 | 0.709 | 12.90 | 0.0229 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindi, R.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Quantitative Measurement of Breast Density Using Personalized 3D-Printed Breast Model for Magnetic Resonance Imaging. Diagnostics 2020, 10, 793. https://doi.org/10.3390/diagnostics10100793
Sindi R, Wong YH, Yeong CH, Sun Z. Quantitative Measurement of Breast Density Using Personalized 3D-Printed Breast Model for Magnetic Resonance Imaging. Diagnostics. 2020; 10(10):793. https://doi.org/10.3390/diagnostics10100793
Chicago/Turabian StyleSindi, Rooa, Yin How Wong, Chai Hong Yeong, and Zhonghua Sun. 2020. "Quantitative Measurement of Breast Density Using Personalized 3D-Printed Breast Model for Magnetic Resonance Imaging" Diagnostics 10, no. 10: 793. https://doi.org/10.3390/diagnostics10100793
APA StyleSindi, R., Wong, Y. H., Yeong, C. H., & Sun, Z. (2020). Quantitative Measurement of Breast Density Using Personalized 3D-Printed Breast Model for Magnetic Resonance Imaging. Diagnostics, 10(10), 793. https://doi.org/10.3390/diagnostics10100793






