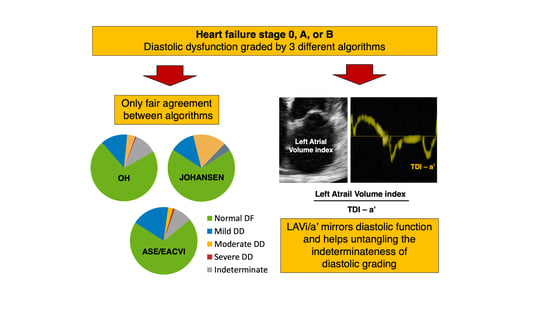
Discrepancies in Assessing Diastolic Function in Pre-Clinical Heart Failure Using Different Algorithms—A Primary Care Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Diastolic Function Algorithms
2.4. Assessment of Undetermined DF
2.5. Statistics
3. Results
3.1. ASE/EACVI
3.2. JOHANSEN
3.3. OH
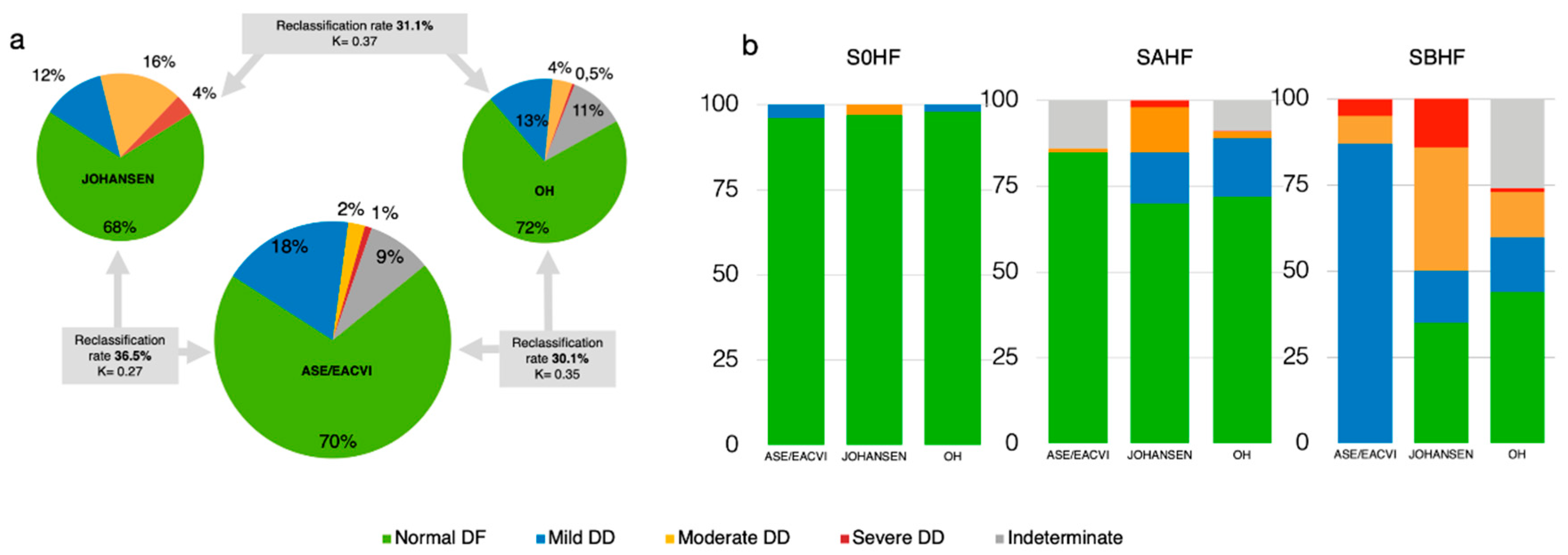
3.4. Concordance Among Methods
3.5. Sorting the Undetermined DF: A Hierarchical Approach
4. Discussion
4.1. Present Findings
4.2. Inconsistencies, Discrepancies, and Their Implications
4.3. Sorting the Undetermined DF
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Overall (n = 885) | SAHF (n = 644) | SBHF (n = 241) | p-Value | |
|---|---|---|---|---|
| Arterial Hypertension (n,%) | 569 (64%) | 377 (59%) | 192 (80%) | <0.0001 |
| Diabetes (n,%) | 125 (14%) | 69 (11%) | 56 (23%) | <0.0001 |
| Dyslipidemia (n,%) | 377 (43%) | 251 (39%) | 126 (43%) | <0.0001 |
| Smoke (n,%) | 112 (13%) | 85 (13%) | 27 (11%) | 0.4 |
| CAD (n,%) | 66 (7%) | 25 (4%) | 41 (17%) | <0.0001 |
| CKD (n,%) | 25 (3%) | 11 (2%) | 14 (6%) | 0.002 |
| Dyalisis (n,%) | 6 (0.7%) | 1 (0.1%) | 5 (2%) | 0.004 |
| Tyroid disease (n,%) | 88 (10%) | 60 (9%) | 28 (12%) | 0.4 |
| PAD (n,%) | 72 (8%) | 40 (6%) | 32 (13%) | 0.001 |
| Other (n,%) | 157 (18%) | 107 (17%) | 50 (21%) | 0.2 |
| ACEi (n,%) | 247 (28%) | 155 (24%) | 92 (38%) | <0.0001 |
| ARB (n,%) | 205 (23%) | 126 (20%) | 79 (33%) | <0.0001 |
| Beta blockers (n,%) | 197 (22%) | 121 (19%) | 76 (32%) | <0.0001 |
| Calcium channel blockers (n,%) | 151 (17%) | 92 (14%) | 59 (25%) | 0.0006 |
| Diuretics (n,%) | 243 (27%) | 143 (22%) | 100 (42%) | <0.0001 |
| Alpha blockers (n,%) | 39 (5%) | 24 (4%) | 15 (6%) | 0.1 |
| Acetylsalicylic acid (n,%) | 192 (22%) | 106 (17%) | 86 (36%) | <0.0001 |
| Statins (n,%) | 205 (23%) | 114 (18%) | 91 (38%) | <0.0001 |
| Digoxin (n,%) | 4 (0.4%) | 1 (0.2%) | 3 (1%) | 0.05 |
| Antiarrythmics (n,%) | 27 (3%) | 15 (2%) | 12 (5%) | 0.05 |
| NTG (n,%) | 23 (3%) | 3 (0.5%) | 20 (8%) | <0.0001 |
| (a) | ||||||
|---|---|---|---|---|---|---|
| JOHANSEN | ||||||
| ASE/EACVI | Normal | Grade 1 | Grade 2 | Grade 3 | Total | |
| Normal | 692 * | 108 | 9 | 0 | 809 | |
| Grade 1 | 82 | 35 * | 85 | 7 | 209 | |
| Grade 2 | 1 | 0 | 0 | 26 | 27 | |
| Grade 3 | 2 | 0 | 1 | 8 * | 11 | |
| Indeterminate | 10 | 0 | 86 | 6 | 102 | |
| Total | 787 | 143 | 181 | 47 | 1158 | |
| (b) | ||||||
| OH | ||||||
| ASE/EACVI | Normal | Grade 1 | Grade 2 | Grade 3 | Indeterminate | Total |
| Normal | 692 * | 110 | 0 | 0 | 7 | 809 |
| Grade 1 | 100 | 38 * | 15 | 0 | 56 | 209 |
| Grade 2 | 1 | 0 | 22 * | 0 | 4 | 27 |
| Grade 3 | 6 | 0 | 0 | 2 * | 3 | 11 |
| Indeterminate | 30 | 9 | 7 | 1 | 55 * | 101 |
| Total | 829 | 157 | 44 | 3 | 125 | 1158 |
| (c) | ||||||
| JOHANSEN | ||||||
| OH | Normal | Grade 1 | Grade 2 | Grade 3 | Total | |
| Normal | 708 * | 69 | 46 | 6 | 829 | |
| Grade 1 | 71 | 71 * | 15 | 0 | 157 | |
| Grade 2 | 0 | 0 | 16* | 28 | 44 | |
| Grade 3 | 0 | 0 | 0 | 3 * | 3 | |
| Indeterminate | 8 | 3 | 104 | 10 | 125 | |
| Total | 787 | 143 | 181 | 47 | 1158 | |
References
- Nistri, S.; Ballo, P.; Mele, D.; Papesso, B.; Galderisi, M.; Mondillo, S.; Zito, G.B.; Henein, M.Y. Effect of Echocardiographic Grading of Left Ventricular Diastolic Dysfunction by Different Classifications in Primary Care. Am. J. Cardiol. 2015, 116, 1144–1152. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C., Jr.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003, 289, 194–202. [Google Scholar] [CrossRef]
- Abhayaratna, W.P.; Marwick, T.H.; Smith, W.T.; Becker, N.G. Characteristics of left ventricular diastolic dysfunction in the community: An echocardiographic survey. Heart 2006, 92, 1259–1264. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Prasad, S.B.; Holland, D.J.; Atherton, J.J.; Whalley, G. New Diastology Guidelines: Evolution, Validation and Impact on Clinical Practice. Heart Lung Circ. 2019, 28, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Johansen, N.D.; Biering-Sorensen, T.; Jensen, J.S.; Mogelvang, R. Diastolic dysfunction revisited: A new, feasible, and unambiguous echocardiographic classification predicts major cardiovascular events. Am. Heart J. 2017, 188, 136–146. [Google Scholar] [CrossRef]
- Oh, J.K.; Miranda, W.R.; Bird, J.G.; Kane, G.C.; Nagueh, S.F. The 2016 Diastolic Function Guideline: Is it Already Time to Revisit or Revise Them? JACC Cardiovasc. Imaging 2020, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.R.; Jessup, M. Stage B heart failure: Management of asymptomatic left ventricular systolic dysfunction. Circulation 2006, 113, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, W.; Jellis, C.L.; Marwick, T.H. Exercise limitation associated with asymptomatic left ventricular impairment: Analogy with stage B heart failure. J. Am. Coll. Cardiol. 2015, 65, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Borlaug, B.A. Stage B heart failure: Is it more common than we think? J. Am. Coll. Cardiol. 2015, 65, 267–269. [Google Scholar] [CrossRef]
- Nistri, S.; Galderisi, M.; Ballo, P.; Olivotto, I.; D’Andrea, A.; Pagliani, L.; Santoro, A.; Papesso, B.; Innelli, P.; Cecchi, F.; et al. Determinants of echocardiographic left atrial volume: Implications for normalcy. Eur. J. Echocardiogr. 2011, 12, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sieweke, J.T.; Biber, S.; Weissenborn, K.; Heuschmann, P.U.; Akin, M.; Zauner, F.; Gabriel, M.M.; Schuppner, R.; Berliner, D.; Bauersachs, J.; et al. Septal total atrial conduction time for prediction of atrial fibrillation in embolic stroke of unknown source: A pilot study. Clin. Res. Cardiol. 2020, 109, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Yamada, A.; Sugimoto, K.; Sugimoto, K.; Iwase, M.; Ishikawa, T.; Ishii, J.; Ozaki, Y. Clinical implication of LAVI over A’ ratio in patients with acute coronary syndrome. Heart Asia 2018, 10, e011038. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jung, H.O.; Min, J.; Park, M.W.; Park, C.S.; Shin, D.I.; Shin, W.S.; Kim, P.J.; Youn, H.J.; Seung, K.B. Left atrial volume index over late diastolic mitral annulus velocity (LAVi/A’) is a useful echo index to identify advanced diastolic dysfunction and predict clinical outcomes. Clin. Cardiol. 2011, 34, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ballo, P.; Nistri, S.; Mele, D.; Henein, M.Y. Simplified vs comprehensive echocardiographic grading of left ventricular diastolic dysfunction in primary care. Int. J. Cardiol. 2016, 214, 243–245. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Hurrell, D.G.; Nishimura, R.A.; Ilstrup, D.M.; Appleton, C.P. Utility of preload alteration in assessment of left ventricular filling pressure by Doppler echocardiography: A simultaneous catheterization and Doppler echocardiographic study. J. Am. Coll. Cardiol. 1997, 30, 459–467. [Google Scholar] [CrossRef]
- Bukachi, F.; Waldenstrom, A.; Morner, S.; Lindqvist, P.; Henein, M.Y.; Kazzam, E. Age dependency in the timing of mitral annular motion in relation to ventricular filling in healthy subjects: Umea General Population Heart Study. Eur. J. Echocardiogr. 2008, 9, 522–529. [Google Scholar] [CrossRef]
- Khankirawatana, B.; Khankirawatana, S.; Peterson, B.; Mahrous, H.; Porter, T.R. Peak atrial systolic mitral annular velocity by Doppler tissue reliably predicts left atrial systolic function. J. Am. Soc. Echocardiogr. 2004, 17, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Oki, T.; Yamada, H.; Tanaka, H.; Ishimoto, T.; Wakatsuki, T.; Tabata, T.; Ito, S. Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J. Am. Soc. Echocardiogr. 2003, 16, 333–339. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Borlaug, B.A. Diastology for the clinician. J. Cardiol. 2019, 73, 445–452. [Google Scholar] [CrossRef]
- Chetrit, M.; Cremer, P.C.; Klein, A.L. Imaging of Diastolic Dysfunction in Community-Based Epidemiological Studies and Randomized Controlled Trials of HFpEF. JACC Cardiovasc. Imaging 2020, 13, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Nolan, M.; Negishi, K.; Okin, P.M.; Marwick, T.H. Community Screening for Nonischemic Cardiomyopathy in Asymptomatic Subjects >/= 65 Years With Stage B Heart Failure. Am. J. Cardiol. 2016, 117, 1959–1965. [Google Scholar] [CrossRef]
- Miyoshi, T.; Addetia, K.; Citro, R.; Daimon, M.; Desale, S.; Fajardo, P.G.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; et al. Left Ventricular Diastolic Function in Healthy Adult Individuals: Results of the World Alliance Societies of Echocardiography Normal Values Study. J. Am. Soc. Echocardiogr. 2020. [Google Scholar] [CrossRef]
- Letnes, J.M.; Nes, B.; Vaardal-Lunde, K.; Slette, M.B.; Molmen-Hansen, H.E.; Aspenes, S.T.; Stoylen, A.; Wisloff, U.; Dalen, H. Left Atrial Volume, Cardiorespiratory Fitness, and Diastolic Function in Healthy Individuals: The HUNT Study, Norway. J. Am. Heart Assoc. 2020, 9, e014682. [Google Scholar] [CrossRef]
- Brinker, S.K.; Pandey, A.; Ayers, C.R.; Barlow, C.E.; DeFina, L.F.; Willis, B.L.; Radford, N.B.; Farzaneh-Far, R.; de Lemos, J.A.; Drazner, M.H.; et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: The Cooper Center Longitudinal Study. JACC Heart Fail. 2014, 2, 238–246. [Google Scholar] [CrossRef]
- Sorrentino, R.; Esposito, R.; Santoro, C.; Vaccaro, A.; Cocozza, S.; Scalamogna, M.; Lembo, M.; Luciano, F.; Santoro, A.; Trimarco, B.; et al. Practical Impact of New Diastolic Recommendations on Noninvasive Estimation of Left Ventricular Diastolic Function and Filling Pressures. J. Am. Soc. Echocardiogr. 2020, 33, 171–181. [Google Scholar] [CrossRef]
- Kosmala, W.; Marwick, T.H. Asymptomatic Left Ventricular Diastolic Dysfunction: Predicting Progression to Symptomatic Heart Failure. JACC Cardiovasc. Imaging 2020, 13, 215–227. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Sun, H.; Kopelen, H.A.; Middleton, K.J.; Khoury, D.S. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J. Am. Coll. Cardiol. 2001, 37, 278–285. [Google Scholar] [CrossRef]
- Fang, N.N.; Sui, D.X.; Yu, J.G.; Gong, H.P.; Zhong, M.; Zhang, Y.; Zhang, W. Strain/strain rate imaging of impaired left atrial function in patients with metabolic syndrome. Hypertens Res. 2015, 38, 758–764. [Google Scholar] [CrossRef]
- Kuwaki, H.; Takeuchi, M.; Chien-Chia Wu, V.; Otani, K.; Nagata, Y.; Hayashi, A.; Iwataki, M.; Fukuda, S.; Yoshitani, H.; Abe, H.; et al. Redefining diastolic dysfunction grading: Combination of E/A </=0.75 and deceleration time >140 ms and E/epsilon’ >/= 10. JACC Cardiovasc. Imaging 2014, 7, 749–758. [Google Scholar] [CrossRef]
- Hansen, S.; Brainin, P.; Sengelov, M.; Jorgensen, P.G.; Bruun, N.E.; Olsen, F.J.; Fritz-Hansen, T.; Schou, M.; Gislason, G.; Biering-Sorensen, T. Prognostic utility of diastolic dysfunction and speckle tracking echocardiography in heart failure with reduced ejection fraction. ESC Heart Fail. 2020, 7, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Benfari, G.; Miller, W.L.; Antoine, C.; Rossi, A.; Lin, G.; Oh, J.K.; Roger, V.L.; Thapa, P.; Enriquez-Sarano, M. Diastolic Determinants of Excess Mortality in Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2019, 7, 808–817. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Schiros, C.G.; Aban, I.; Denney, T.S.; Gupta, H. Diagnostic Accuracy of Tissue Doppler Index E/e’ for Evaluating Left Ventricular Filling Pressure and Diastolic Dysfunction/Heart Failure With Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Mazzone, C.; Cioffi, G.; Barbati, G.; Gentile, P.; Ballo, P.; Borca, E.C.; Faganello, G.; Cherubini, A.; Bussani, R.; et al. Tissue Doppler indices of diastolic function as prognosticator in patients without heart failure in primary care. J. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kane, G.C.; Karon, B.L.; Mahoney, D.W.; Redfield, M.M.; Roger, V.L.; Burnett, J.C., Jr.; Jacobsen, S.J.; Rodeheffer, R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011, 306, 856–863. [Google Scholar] [CrossRef] [PubMed]


| Overall (n = 1158) | S0HF (n = 273) | SAHF (n = 644) | SBHF (n = 241) | p-Value | |
|---|---|---|---|---|---|
| Male gender (n, %) | 617 (53%) | 162 (59%) | 354 (55%) | 101 (42%) | 0.0002 |
| Age (years) | 58.4 ± 12.9 | 50.4 ± 11.0 | 58.8 ± 12.1 | 66.4 ± 11.4 | <0.0001 |
| Height (cm) | 166.7 ± 10.0 | 170.2 ± 8.6 | 166.6 ± 10.0 | 162.8 ± 10.1 | <0.0001 |
| Weight (kg) | 74.6 ± 14.4 | 70.9 ± 11.5 | 75.2 ± 15.1 | 77.0 ± 15.0 | <0.0001 |
| BMI (kg/m2) | 26.8 ± 4.3 | 24.4 ± 3.2 | 27.0 ± 4.2 | 29.0 ± 4.5 | <0.0001 |
| BSA (m2) | 1.83 ± 0.21 | 1.82 ± 0.18 | 1.83 ± 0.22 | 1.82 ± 0.21 | 0.5 |
| Heart rate (bpm) | 69.1 ± 11.2 | 71.1 ± 12.1 | 68.9 ± 10.6 | 67.3 ± 11.2 | 0.0005 |
| Systolic Blood Pressure (mmHg) | 138.6 ± 19.3 | 127.1 ± 12.9 | 139.6 ± 18.2 | 149.2 ± 21.6 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 80.3 ± 8.6 | 77.7 ± 7.4 | 80.7 ± 8.4 | 81.8 ± 9.5 | <0.0001 |
| Left Ventricular Mass index (g/m2) | 90.0 ± 22.4 | 81.5 ± 17.5 | 83.5 ± 15.1 | 116.8 ± 23.1 | <0.0001 |
| Left Ventricular End Diastolic Volume index (mL/m2) | 58.1 ± 13.3 | 60.3 ± 13.2 | 54.8 ± 10.7 | 64.7 ± 16.4 | <0.0001 |
| Left Ventricular End Systolic Volume index (mL/m2) | 20.1 ± 7.3 | 19.6 ± 5.1 | 18.6 ± 4.7 | 24.3 ± 11.8 | <0.0001 |
| LV ejection fraction (%) | 65.7 ± 6.3 | 67.4 ± 4.7 | 65.9 ± 5.5 | 63.0 ± 8.8 | <0.0001 |
| LAVi (cm/m2) | 33.4 ± 10.7 | 32.1 ± 9.0 | 31.6 ± 9.7 | 39.7 ± 12.2 | <0.0001 |
| E velocity (m/s) | 0.76 ± 0.17 | 0.79 ± 0.14 | 0.75 ± 0.17 | 0.76 ± 0.20 | 0.001 |
| A velocity (m/s) | 0.71 ± 0.23 | 0.63 ± 0.15 | 0.71 ± 0.25 | 0.79 ± 0.22 | <0.0001 |
| A duration (ms) | 124.1 ± 22.0 | - | 123.9 ± 22.3 | 124.7 ± 21.1 | 0.6 |
| E/A ratio | 1.22 ± 0.81 | 1.32 ± 0.39 | 1.22 ± 0.94 | 1.09 ± 0.73 | 0.004 |
| Deceleration time E (ms) | 206.1 ± 56.5 | 187.5 ± 44.5 | 208.4 ± 52.3 | 221.3 ± 71.8 | <0.0001 |
| Average s’ (cm/s) | 9.6 ± 1.9 | 10.7 ± 1.9 | 9.5 ± 1.7 | 8.5 ± 1.7 | <0.0001 |
| Average e’ (cm/s) | 10.7 ± 3.1 | 13.1 ± 2.8 | 10.6 ± 2.8 | 8.3 ± 2.3 | <0.0001 |
| Average a’ (cm/s) | 11.0 ± 2.2 | 10.7 ± 2.0 | 11.2 ± 2.1 | 10.6 ± 2.4 | 0.0001 |
| Average E/e’ ratio | 7.6 ± 2.7 | 6.3 ± 1.3 | 7.4 ± 2.3 | 9.7 ± 3.6 | <0.0001 |
| LAVi/a’ ratio | 3.23 ± 1.62 | 3.10 ± 1.15 | 2.93 ± 1.19 | 4.16 ± 2.54 | <0.0001 |
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | p-Value | |
|---|---|---|---|---|---|
| ASE/EACVI | 2.86 ± 1.07 | 3.87 ± 2.35 | 5.22 ± 2.08 | 7.00 ± 3.94 | <0.0001 |
| OH | 3.12 ± 1.52 | 2.49 ± 0.80 | 5.28 ± 2.04 | 8.25 ± 5.39 | <0.0001 |
| JOHANSEN | 3.00 ± 1.15 | 2.41 ± 0.58 | 4.29 ± 2.36 | 5.59 ± 2.65 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setti, M.; Benfari, G.; Mele, D.; Rossi, A.; Ballo, P.; Galderisi, M.; Henein, M.; Nistri, S. Discrepancies in Assessing Diastolic Function in Pre-Clinical Heart Failure Using Different Algorithms—A Primary Care Study. Diagnostics 2020, 10, 850. https://doi.org/10.3390/diagnostics10100850
Setti M, Benfari G, Mele D, Rossi A, Ballo P, Galderisi M, Henein M, Nistri S. Discrepancies in Assessing Diastolic Function in Pre-Clinical Heart Failure Using Different Algorithms—A Primary Care Study. Diagnostics. 2020; 10(10):850. https://doi.org/10.3390/diagnostics10100850
Chicago/Turabian StyleSetti, Martina, Giovanni Benfari, Donato Mele, Andrea Rossi, Piercarlo Ballo, Maurizio Galderisi, Michael Henein, and Stefano Nistri. 2020. "Discrepancies in Assessing Diastolic Function in Pre-Clinical Heart Failure Using Different Algorithms—A Primary Care Study" Diagnostics 10, no. 10: 850. https://doi.org/10.3390/diagnostics10100850
APA StyleSetti, M., Benfari, G., Mele, D., Rossi, A., Ballo, P., Galderisi, M., Henein, M., & Nistri, S. (2020). Discrepancies in Assessing Diastolic Function in Pre-Clinical Heart Failure Using Different Algorithms—A Primary Care Study. Diagnostics, 10(10), 850. https://doi.org/10.3390/diagnostics10100850