Post-Mortem Investigations for the Diagnosis of Sepsis: A Review of Literature
Abstract
:1. Introduction
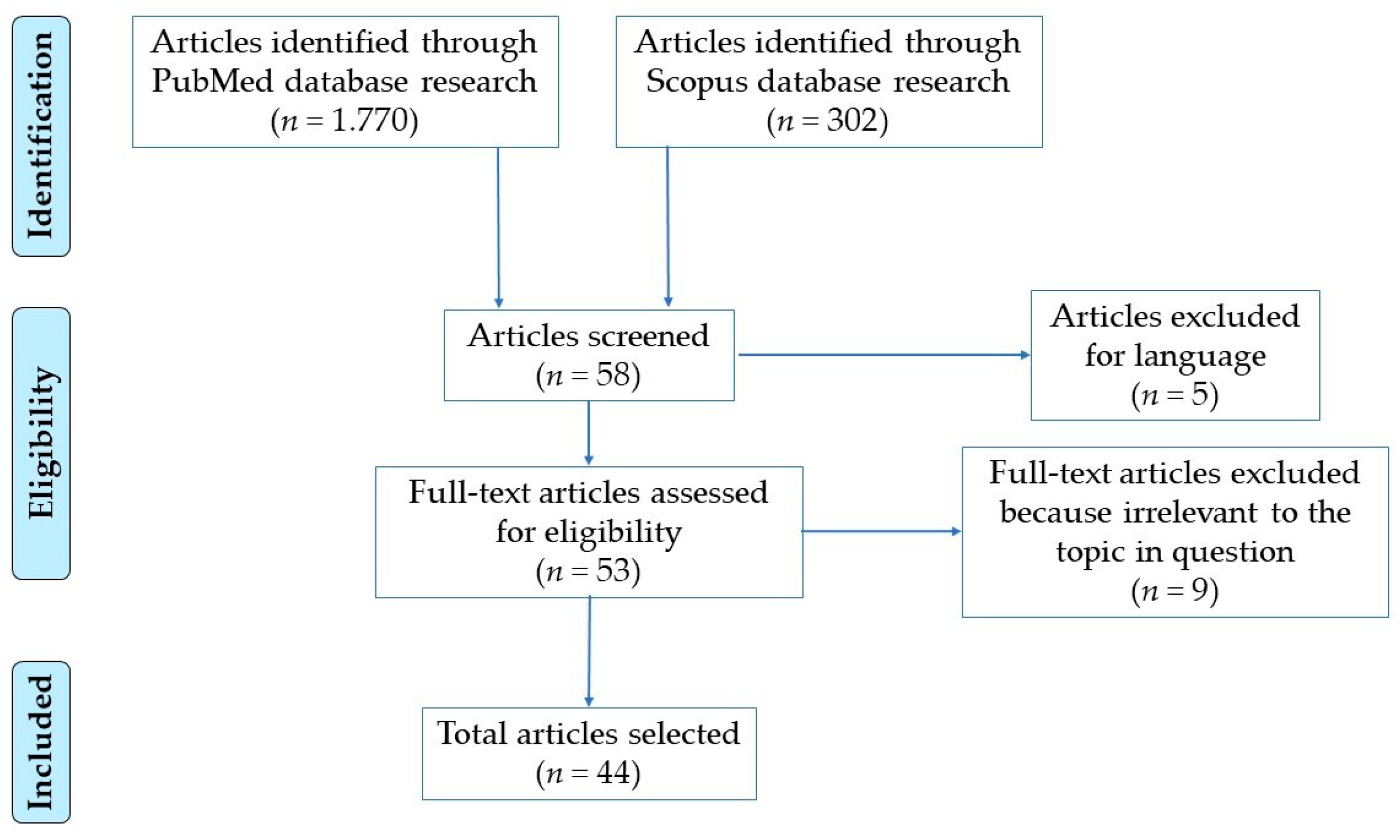
2. Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
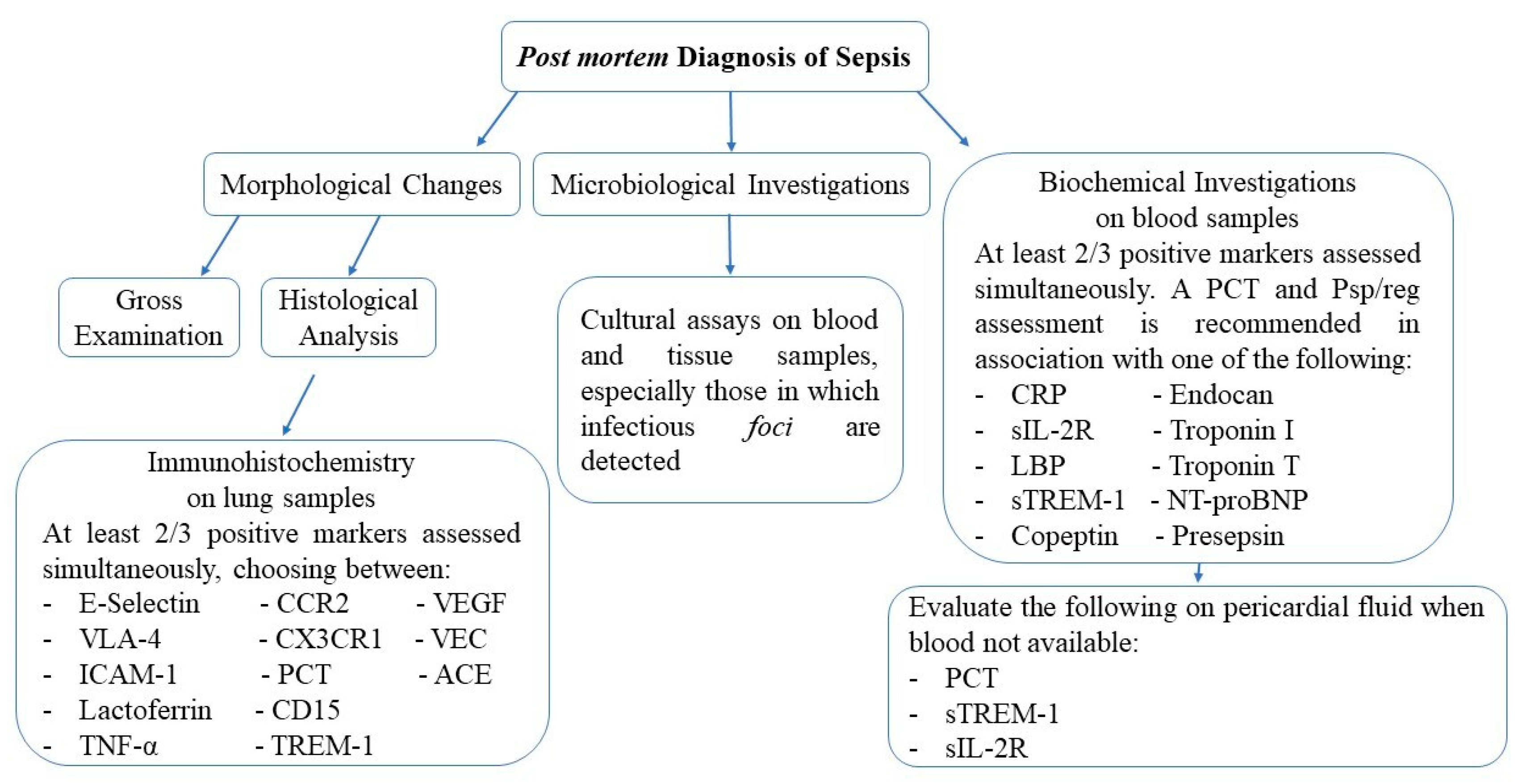
3.1. Macroscopic and Histological Findings
3.2. Microbial Isolation
3.3. Immunohistochemical Markers
3.4. Biochemical Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.M.; Annane, D.; Bauer, M.M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.J.; Coopersmith, C.C.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rorat, M.; Jurek, T.; Simon, K. Post-mortem diagnostics in cases of sepsis. Part 1. Aetiology, epidemiology and microbiological tests. Arch. Med. Sąd. Kryminol. 2014, 4, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Pomara, C.; Riezzo, I.; Bello, S.; De Carlo, D.; Neri, M.; Turillazzi, E. A Pathophysiological Insight into Sepsis and Its Correlation with Postmortem Diagnosis. Mediat. Inflamm. 2016, 2016, 4062829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dermengiu, D.; Curca, G.C.; Ceausu, M.; Hostiuc, S. Particularities Regarding the Etiology of Sepsis in Forensic Services. J. Forensic Sci. 2013, 58, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, M. Postmortem diagnosis of sepsis. Forensic Sci. Int. 2007, 165, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, E.V.; Stassi, C.; Mondello, C.; Zerbo, S.; Milone, L.; Argo, A. Forensic microbiology applications: A systematic review. Leg. Med. 2019, 36, 73–80. [Google Scholar] [CrossRef]
- Yuki, K.; Murakami, N. Sepsis Pathophysiology and Anesthetic Consideration. Cardiovasc. Hematol. Disord. Drug Targets 2015, 15, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.-P.; Liu, W.-P.; Guo, L.-X.; Jiang, Y.; Li, G.-D.; Bai, Y.-Q.; Li, S.-H.; Wu, T.; Jing, H.-Q. Autopsy report of four cases who died from Streptococcus suis infection, with a review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 447–453. [Google Scholar] [CrossRef]
- Torgersen, C.; Moser, P.; Luckner, G.; Mayr, V.; Jochberger, S.; Hasibeder, W.R.; Dünser, M.W. Macroscopic Postmortem Findings in 235 Surgical Intensive Care Patients with Sepsis. Anesth. Analg. 2009, 108, 1841–1847. [Google Scholar] [CrossRef]
- Śledzińska, A.; Mielech, A.; Krawczyk, B.; Samet, A.; Nowicki, B.; Nowicki, S.; Jankowski, Z.; Kur, J. Fatal sepsis in a pregnant woman with pyelonephritis caused by Escherichia coli bearing Dr and P adhesins: Diagnosis based on postmortem strain genotyping. BJOG 2011, 118, 266–269. [Google Scholar] [CrossRef]
- Rossi, M.A.; Santos, C.S. Sepsis-related microvascular myocardial damage with giant cell inflammation and calcification. Virchows Arch. 2003, 443, 87–92. [Google Scholar] [CrossRef]
- Sinicina, I.; Matevossian, E.; Fischer, F.; Mall, G.; Graw, M. The petrified heart in sepsis. Virchows Arch. 2005, 447, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Manetti, F.; La Russa, R.; Di Fazio, N.; De Matteis, A.; Frati, P.; Fineschi, V.; Aniello, M.; Federico, M.; Raffaele, L.R.; et al. Septic myocardial calcification: A case report. J. Forensic Leg. Med. 2019, 65, 45–47. [Google Scholar] [CrossRef] [PubMed]
- De Matos Soeiro, D.; Parra, E.; Canzian, M.; Farhat, C.; Capelozzi, V.L. Pulmonary histopathological alterations in patients with acute respiratory failure: An autopsy study. J. Bras. Pneumol. 2008, 34, 67–73. [Google Scholar]
- Suzuki, H.; Murata, K.; Sakamoto, A. An autopsy case of fulminant sepsis due to pneumatosis cystoides intestinalis. Leg. Med. 2009, 11, S528–S530. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; van den Heuvel, M.C.; Stegeman, C.A.; Popa, E.R.; Leliveld, A.; Molema, G.; Zijlstra, J.G.; Moser, J.; Van Meurs, M. Kidney histopathology in lethal human sepsis. Crit. Care 2018, 22, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, A.M.; Lorente-Ros, M.; Goncalvez, G.; Carriedo, D.; Ballén-Barragán, A.; Villar-Fernández, A.; Peñuelas, O.; Herrero, R.; Granados, R.; Lorente, J.A. Histopathological changes of organ dysfunction in sepsis. Intensive Care Med. Exp. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gunia, S.; Albrecht, K.; May, M.; Stosiek, P. The white pulp in the setting of the septic spleen caused by different bacteria: A comparative morphometric study. APMIS 2005, 113, 675–682. [Google Scholar] [CrossRef]
- Abernathy, K.; Fiester, S.; Fulcher, J.W. Miliary pattern MRSA sepsis following clandestine intravenous infusion. Forensic Sci. Med. Pathol. 2019, 15, 267–271. [Google Scholar] [CrossRef]
- Gioia, S.; Lancia, M.; Mencacci, A.; Bacci, M.; Suadoni, F. FatalClostridiumperfringensSepticemia After Colonoscopic Polypectomy, Without Bowel Perforation. J. Forensic Sci. 2016, 61, 1689–1692. [Google Scholar] [CrossRef]
- Rorat, M.; Jurek, T.; Simon, K. Post-mortem diagnostics in cases of sepsis. Part 2. Biochemical and morphological examinations. Arch. Med. Sąd. Kryminol. 2015, 1, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploy, M.-C.; Garnier, F.; Languepin, J.; Fermeaux, V.; Martin, C.; Denis, F. Interest of postmortem-collected specimens in the diagnosis of fulminant meningococcal sepsis. Diagn. Microbiol. Infect. Dis. 2005, 52, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Hamaa, M.; Abe, M.; Suenaga, T.; Ishida, Y.; Nosaka, M.; Kuninaka, Y.; Kawaguchi, M.; Yoshikawa, N.; Kimura, A.; et al. Sudden unexpected neonatal death due to late onset group B streptococcal sepsis—A case report. Leg. Med. 2013, 15, 260–263. [Google Scholar] [CrossRef]
- Frati, P.; Busardò, F.P.; Di Stefano, M.A.; Neri, M.; Sessa, F.; Fineschi, V. A fatal case of post-transfusion sepsis caused by Yersinia enterocolitica after delivery. Blood Transfus. 2015, 13, 528–531. [Google Scholar] [PubMed]
- Sárvári, K.P.; Vasas, B.; Kiss, I.; Lazar, A.; Horvath, I.; Simon, M.; Pető, Z.; Urbán, E. Fatal Clostridium perfringens sepsis due to emphysematous gastritis and literature review. Anaerobe 2016, 40, 31–34. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, C.; Pompilio, A.; Crocetta, V.; Gherardi, G.; Carnevale, A.; Di Bonaventura, G. Fatal sepsis by Klebsiella pneumoniae in a patient with systemic lupus erythematosus: The importance of postmortem microbiological examination for the ex post diagnosis of infection. Int. J. Leg. Med. 2015, 129, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, M. Immunohistochemical detection of sepsis-induced lung injury in human autopsy material. Leg. Med. 2003, 5, 73–86. [Google Scholar] [CrossRef]
- La Russa, R.; Maiese, A.; Viola, R.V.; De Matteis, A.; Pinchi, E.; Frati, P.; Fineschi, V. Searching for highly sensitive and specific biomarkers for sepsis: State-of-the-art in post-mortem diagnosis of sepsis through immunohistochemical analysis. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barranco, R.; Ventura, F. Immunohistochemistry in the Postmortem Diagnosis of Sepsis: A Systematic Review. Appl. Immunohistochem. Mol. Morphol. 2019. [Google Scholar] [CrossRef]
- Tsokos, M.; Fehlauer, F.; Püschel, K. Immunohistochemical expression of E-selectin in sepsis-induced lung injury. Int. J. Leg. Med. 2000, 113, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, M.; Fehlauer, F. Post-mortem markers of sepsis: An immunohistochemical study using VLA-4 (CD49d/CD29) and ICAM-1 (CD54) for the detection of sepsis-induced lung injury. Int. J. Leg. Med. 2001, 114, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, M.; Anders, S.; Paulsen, F.; Fehlauer, F.; Püschel, K. Comparative evaluation of pulmonary lactoferrin and lysozyme immunoreactivity for the postmortem diagnosis of death due to sepsis. Virchows Arch. 2001, 438, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, M.; Pufe, T.; Paulsen, F.; Anders, S.; Mentlein, R. Pulmonary expression of vascular endothelial growth factor in sepsis. Arch. Pathol. Lab. Med. 2003, 127, 331–335. [Google Scholar] [PubMed]
- Miyashita, T.; Kakimoto, N.; Ishida, Y.; Hayashi, T.; Kimura, A.; Tsokos, M.; Kondo, T. Immunohistochemical Expression of Tumor Necrosis Factor-α in Sepsis-Induced Lung Injury. Forensic Sci. Med. Pathol. 2006, 2, 103–108. [Google Scholar] [CrossRef]
- Müller, A.M.; Gruhn, K.M.; Herwig, M.C.; Tsokos, M. VE-cadherin and ACE: Markers for sepsis in post mortem examination? Leg. Med. 2008, 10, 257–263. [Google Scholar] [CrossRef]
- Herwig, M.C.; Tsokos, M.; Hermanns, M.I.; Kirkpatrick, C.J.; Müller, A.M. Vascular Endothelial Cadherin Expression in Lung Specimens of Patients with Sepsis-Induced Acute Respiratory Distress Syndrome and Endothelial Cell Cultures. Pathobiology 2013, 80, 245–251. [Google Scholar] [CrossRef] [PubMed]
- An, J.-L.; Ishida, Y.; Kimura, A.; Tsokos, M.; Kondo, T. Immunohistochemical detection of CCR2 and CX3CR1 in sepsis-induced lung injury. Forensic Sci. Int. 2009, 192, e21–e25. [Google Scholar] [CrossRef]
- Maiese, A.; Del Nonno, F.; Dell’Aquila, M.; Moauro, M.; Baiocchini, A.; Mastracchio, A.; Bolino, G. Postmortem diagnosis of sepsis: A preliminary immunohistochemical study with an anti-procalcitonin antibody. Leg. Med. 2017, 28, 1–5. [Google Scholar] [CrossRef]
- Spagnolo, E.V.; Mondello, C.; Di Mauro, D.; Vermiglio, G.; Asmundo, A.; Filippini, E.; Alibrandi, A.; Rizzo, G. Analysis on sarcoglycans expression as markers of septic cardiomyopathy in sepsis-related death. Int. J. Leg. Med. 2018, 132, 1685–1692. [Google Scholar] [CrossRef]
- Galassi, A.; Turatello, L.; De Salvia, A.; Neri, M.; Turillazzi, E.; La Russa, R.; Viola, R.V.; Frati, P.; Fineschi, V. Septic cardiomyopathy: The value of lactoferrin and CD15 as specific markers to corroborate a definitive diagnosis. Int. J. Immunopathol. Pharmacol. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Maiese, A.; Bolino, G.; Mastracchio, A.; Frati, P.; Fineschi, V. An immunohistochemical study of the diagnostic value of TREM-1 as marker for fatal sepsis cases. Biotech. Histochem. 2019, 94, 159–166. [Google Scholar] [CrossRef]
- Palmiere, C.; Augsburger, M. Markers for sepsis diagnosis in the forensic setting: State of the art. Croat. Med. J. 2014, 55, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Zhu, B.-L.; Ishikawa, T.; Quan, L.; Michiue, T. Significance of postmortem biochemistry in determining the cause of death. Leg. Med. 2009, 11, S46–S49. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, M.; Reichelt, U.; Jung, R.; Nierhaus, A.; Püschel, K. Interleukin-6 and C-reactive protein serum levels in sepsis-related fatalities during the early postmortem period. Forensic Sci. Int. 2001, 119, 47–56. [Google Scholar] [CrossRef]
- Reichelt, U.; Jung, R.; Nierhaus, A.; Tsokos, M. Serial monitoring of interleukin-1β, soluble interleukin-2 receptor and lipopolysaccharide binding protein levels after death. A comparative evaluation of potential postmortem markers of sepsis. Int. J. Leg. Med. 2005, 119, 80–87. [Google Scholar] [CrossRef]
- Schrag, B.; Roux-Lombard, P.; Schneiter, D.; Vaucher, P.; Mangin, P.; Palmiere, C. Evaluation of C-reactive protein, procalcitonin, tumor necrosis factor alpha, interleukin-6, and interleukin-8 as diagnostic parameters in sepsis-related fatalities. Int. J. Leg. Med. 2012, 126, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Schrag, B.; Iglesias, K.; Mangin, P.; Palmiere, C. Procalcitonin and C-reactive protein in pericardial fluid for postmortem diagnosis of sepsis. Int. J. Leg. Med. 2012, 126, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Augsburger, M.; Iglesias, K.; Bardy, D.; Mangin, P.; Palmiere, C. Diagnostic value of lipopolysaccharide-binding protein and procalcitonin for sepsis diagnosis in forensic pathology. Int. J. Leg. Med. 2013, 127, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Palmiere, C.; Bardy, D.; Mangin, P.; Augsburger, M. Value of sTREM-1, procalcitonin and CRP as laboratory parameters for postmortem diagnosis of sepsis. J. Infect. 2013, 67, 545–555. [Google Scholar] [CrossRef]
- Palmiere, C.; Mussap, M.; Bardy, D.; Cibecchini, F.; Mangin, P. Diagnostic value of soluble CD14 subtype (sCD14-ST) presepsin for the postmortem diagnosis of sepsis-related fatalities. Int. J. Leg. Med. 2013, 127, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Palmiere, C.; Egger, C. Usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis. J. Forensic Leg. Med. 2014, 28, 15–18. [Google Scholar] [CrossRef]
- Palmiere, C.; Augsburger, M. Copeptin as a diagnostic biomarker for sepsis-related deaths. Peptides 2014, 59, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Palmiere, C.; Augsburger, M. Endocan measurement for the postmortem diagnosis of sepsis. Leg. Med. 2014, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palmiere, C.; Augsburger, M. Pancreatic stone protein as a postmortem biochemical marker for the diagnosis of sepsis. Leg. Med. 2015, 17, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, C.; Hervet, T.; Grabherr, S.; Palmiere, C. Elevation of NT-proBNP and cardiac troponins in sepsis-related deaths: A forensic perspective. Int. J. Leg. Med. 2016, 130, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Unuma, K.; Makino, Y.; Sasaki, Y.; Iwase, H.; Uemura, K. Presepsin: A potential superior diagnostic biomarker for the postmortem differentiation of sepsis based on the Sepsis-3 criteria. Forensic Sci. Int. 2019, 299, 17–20. [Google Scholar] [CrossRef]
- Spagnolo, E.V.; Mondello, C.; Roccuzzo, S.; Cardia, L.; Raffino, C. A lethal Tick-Borne Encephalitis (TBE) due to TBE Virus in Sicily (Italy): A case of IgG + /IgM- response? Clin. Ther. 2018, 169, e145–e148. [Google Scholar]
- Gualniera, P.; Scurria, S.; Ventura Spagnolo, E.; Sapienza, D.; Asmundo, A. Post-cesarean necrotizing fasciitis caused by surgical site infection. Gazz. Med. Ital. 2017, 176, 565–569. [Google Scholar]
- Spagnolo, E.V.; Mondello, C.; Cardia, L.; Ventura-Spagnolo, E.; Bartoloni, G. Odontogenic abscess complicated by descending necrotizing mediastinitis: Evidence of medical and dental malpractice. Minerva Stomatol. 2016, 65, 412–415. [Google Scholar]
- Gualniera, P.; Mondello, C.; Scurria, S.; Oliva, A.; Grassi, S.; Pizzicannella, J.; Alibrandi, A.; Sapienza, D.; Asmundo, A. Experience of an Italian Hospital Claims Management Committee: A tool for extrajudicial litigations resolution. Leg. Med. 2020, 42, 101657. [Google Scholar] [CrossRef]
- Ventura-Spagnolo, E.; Mondello, C.; Roccuzzo, S.; Stassi, C.; Cardia, L.; Grieco, A.; Raffino, C. A unique fatal case of Waterhouse-Friderichsen syndrome caused by Proteus mirabilis in an immunocompetent subject: Case report and literature analysis. Medicine 2019, 98, e16664. [Google Scholar] [CrossRef] [PubMed]
- Laganà, P.; Delia, S.; Dattilo, G.; Mondello, C.; Spagnolo, E.V. A case of Infective Endocarditis due to Salmonella enterica phagetype 35. First report. Clin. Ther. 2017, 168, e397–e400. [Google Scholar]
- Minciullo, P.L.; Spagnolo, E.V.; Cascio, A.; Cardia, G.; Gangemi, S. Fatal anaphylactic shock and Taenia solium infestation: A possible link? Ann. Allergy Asthma Immunol. 2009, 103, 449–450. [Google Scholar] [CrossRef]
- Ventura Spagnolo, E.; Mondello, C.; Stassi, C.; Baldino, G.; D’Aleo, F.; Conte, M.; Argo, A.; Zerbo, S. Forensic microbiology: A case series analysis. EMBJ 2019, 14, 117–121. [Google Scholar]
- Mondello, C.; Cardia, L.; Ventura-Spagnolo, E. Immunohistochemical detection of early myocardial infarction: A systematic review. Int. J. Leg. Med. 2017, 131, 411–421. [Google Scholar] [CrossRef]
- Barranco, R.; Ventura, F.; Fracasso, T. Immunohistochemical renal expression of aquaporin 2, arginine-vasopressin, vasopressin receptor 2, and renin in saltwater drowning and freshwater drowning. Int. J. Leg. Med. 2020, 134, 1733–1740. [Google Scholar] [CrossRef]
- Ventura-Spagnolo, E.; Mondello, C.; Cardia, L.; Minutoli, L.; Puzzolo, D.; Asmundo, A.; Macaione, V.; Alibrandi, A.; Malta, C.; Baldino, G.; et al. Post-Mortem Immunohistochemical Evidence of β2-Adrenergic Receptor Expression in the Adrenal Gland. Int. J. Mol. Sci. 2019, 20, 3065. [Google Scholar] [CrossRef] [Green Version]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Sesta, L.; Mondello, C.; Cardia, L.; Mondello, E.; Baldino, G.; Ventura Spagnolo, E. COVID-19 in Italy. Clinical emergency and bioethical perspectives. EMBJ 2020, 15, 121–125. [Google Scholar]
- Baldino, G.; Argo, A.; Stassi, C.; Zerbo, S.; Spagnolo, E.V. Are there positive lessons for Italy’s NHS resulting from the Covid-19 pandemic? Med. Leg. J. 2020, 88. [Google Scholar] [CrossRef]
- Sessa, F.; Bertozzi, G.; Cipolloni, L.; Baldari, B.; Cantatore, S.; D’Errico, S.; Di Mizio, G.; Asmundo, A.; Castorina, S.; Salerno, M.; et al. Clinical-Forensic Autopsy Findings to Defeat COVID-19 Disease: A Literature Review. J. Clin. Med. 2020, 9, 2026. [Google Scholar] [CrossRef]
- Cipolloni, L.; Sessa, F.; Bertozzi, G.; Baldari, B.; Cantatore, S.; Testi, R.; D’Errico, S.; Di Mizio, G.; Asmundo, A.; Castorina, S.; et al. Preliminary Post-Mortem COVID-19 Evidence of Endothelial Injury and Factor VIII Hyperexpression. Diagnostics 2020, 10, 575. [Google Scholar] [CrossRef] [PubMed]
| Reference | Yang et al. [8] (2009) Case Series | Torgersen et al. [9] (2009) Retrospective Study | Sledzinska et al. [10] (2010) Case Report | |
| Findings | External Inspection
Focal and spotty necrosis (n = 4/4). Adrenal glands Widespread haemorrhage (n = 4/4). Brain Mild cerebral oedema with neuron degeneration, neuronophagia, and satellite phenomenon (n = 4/4). | Heart
Enlargement and softening (n = 68/235). Pancreas Necrotizing pancreatitis (n = 20/235). Kidneys and Urinary Tract
Oedema (n= 32/235). | Kidneys and Urinary Tract Macroscopic findings:
Macroscopic findings:
Focal contraction band necrosis. Brain
| |
| Reference | Rossi et al. [11] (2003) Case Report | Sinicina et al. [12] (2005) Case Report | Maiese et al. [13] (2019) Case Report | |
| Findings | Heart Macroscopic findings:
Congestion. Liver Congestion. Spleen Congestion. | Heart Macroscopic findings:
| Heart Macroscopic findings:
| |
| Reference | de Matos Soeiro et al. [14] (2008) Retrospective Study | Suzuki et al. [15] (2009) Case Report | Aslan et al. [16] (2018) Case Control Study | |
| Findings | Lungs Diffuse Alveolar Damage (DAD – n = 1374/3030):
| Serous Cavities Pleural effusion. Vessels Pneumohaemia of inner jugular vein, superior vena cava, and cardiac veins. Gastrointestinal Tract Macroscopic findings:
| Kidneys Glomeruli:
| |
| Reference | Garofalo et al. [17] (2019) Review | Gunia et al. [18] (2005) Case Control Study | Abernathy et al. [19] (2019) Case Report | Gioia et al. [20] (2016) Case Report |
| Findings | Kidneys and Urinary Tract
| Spleen
| External Inspection Multiple petechiae. Serous Cavities Cloudy yellow pericardial fluid. Heart Macroscopic findings:
Macroscopic findings:
Purulent and haemorrhagic lesions of the renal capsule. | External Inspection
Macroscopic findings:
Myoglobin detection within renal tubules. |
| Reference | Cultural Matrix | Microbial Isolate | Diagnosis |
|---|---|---|---|
| Yang et al. [8] (2009) Case Series | Obtained post mortem: Heart, liver, kidneys, cardiac blood | Streptococcus suis type 2 | Streptococcal Toxic Shock Syndrome (STSS) |
| Sledzinska et al. [10] (2010) Case Report | Obtained post mortem: Blood, kidney, lung, brain, spleen urine | Escherichia coli Dr + | Gestational interstitial tubulonephritis and chronic pyelitis |
| Suzuki et al. [15] (2009) Case Report | Obtained post mortem: Blood | Clostridium spp. Bacteroide fragilis | Anaerobic bacterial sepsis due to Pneumatosis Cystoides Intestinalis (PCI) |
| Abernathy et al. [19] (2019) Case Report | Obtained ante mortem: Blood Obtained post mortem: Pericardial effusion, i. v. tubing, bag of saline | Meticillin-Resistant Staphylococcus aureus (MRSA) Meticillin-Resistant Staphylococcus aureus (MRSA) | Sepsis following clandestine intravenous infusion |
| Gioia et al. [20] (2016) Case Report | Obtained post mortem: Aortic blood | Clostridium perfringens | Gas gangrene following polypectomy |
| Ploya et al. [22] (2005) Case Report | Obtained post mortem: Blood, urine, CSF, throat swabs, purpuric lesions, adrenal glands | Neisseria meningitidis serogroup C | Purpura fulminans |
| Kawaguchi et al. [23] (2013) Case Report | Obtained post mortem: Blood, faeces samples | Streptococcus agalactiae | Late onset group B streptococcal sepsis |
| Frati et al. [24] (2015) Case Report | Obtained post mortem: Blood samples | Y. enterocolitica | Post-transfusion sepsis |
| Sarvari et al. [25] (2016) Case Report | Obtained post mortem: Intestinal and subcutaneous tissues | Clostridium perfringens | Gas gangrene |
| D’Ovidio et al. [26] (2015) Case Report | Obtained post mortem: Cardiac tissue and valves, liver, CSF, blood | MDRK. Pneumoniae | Sepsis following an incision of a gluteal abscess |
| Reference | Tsokos et al. [30] (2000) Prospective Case Control Study | Tsokos et al. [31] (2001) Prospective Case Control Study | Tsokos et al. [32] (2001) Prospective Case Control Study | Tsokos et al. [33] (2003) Prospective Case Control Study |
| Markers Assessed | E-Selectin immunoreactivity tested on lung vessels:
| Immunoreactivity tested on intravascular, interstitial, and intra-alveolar leucocytes. VLA-4:
| Immunoreactivity tested on pulmonary leucocytes, and macrophages. Lactoferrin (LF):
| VEGF immunoreactivity tested on alveolar and bronchial epithelium, and glandular cells of the bronchi and bronchioles:
|
| Reference | Miyashita et al. [34] (2006) Prospective Case Control Study | Muller et al. [35] (2008) Prospective Case Control Study | Hervig et al. [36] (2013) Prospective Case Control Study | An et al. [37] (2009) Prospective Case Control Study |
| Markers Assessed | TNF-α immunoreactivity tested on lung macrophages:
| Immunoreactivity tested on lung vessels. VE-Cadherin:
| VE-Cadherin immunoreactivity tested on lung vessels:
| Immunoreactivity tested on pulmonary macrophages. CCR2:
|
| Reference | Maiese et al. [38] (2017) Prospective Case Control Study | Ventura Spagnolo et al. [39] (2018) Retrospective Case Control Study | Galassi et al. [40] (2018) Retrospective Case Control Study | Maiese et al. [41] (2019) Prospective Case Control Study |
| Markers Assessed | PCT immunoreactivity tested on brain, heart, lung, liver, and kidney samples:
| Sarcoglycan sub-complex tested on heart samples:
| Immunoreactivity tested on heart samples. LF:
| TREM-1 tested on brain, heart, lung, liver, and kidney samples:
|
| Reference | Tsokos et al. [44] (2001) Prospective Case Control Study | Reichelt et al. [45] (2005) Prospective Case Control Study | Schrag et al. [46] (2012) Prospective Case Control Study | Schrag et al. [47] (2012) Prospective Case Control Study | Augsburger et al. [48] (2013) Prospective Case Control Study |
| Cut-offs | CRP → 10 mg/L IL-6 → 10 pg/mL | sIL-1β → 5 pg/mL sIL-2R → 1.000 U/mL LBP → 10 µg/mL | CRP → 10 mg/L PCT→ 0.25 ng/mL TNF-α, IL-6 and IL-8 cut-offs not reported | CRP → 10 mg/L PCT → 2 µg/L | PCT → 2 µg/L LBP → 10 µg/mL |
| Findings | CRP Sepsis cases (n = 8):
Sepsis cases (n = 8):
| sIL-1β Sepsis cases (n = 8):
Sepsis cases (n = 8):
Sepsis cases (n = 8):
| CRP Sepsis cases (n = 8):
Sepsis cases (n = 8):
Sepsis cases (n = 8):
Sepsis cases (n = 8):
Sepsis cases (n = 8):
| CRP Sepsis cases (n = 12):
Sepsis cases (n = 12):
| PCT Sepsis cases (n = 12):
Sepsis cases (n = 12):
|
| Reference | Palmiere et al. [49] (2013) Prospective Case Control Study | Palmiere et al. [50] (2013) Prospective Case Control Study | Palmiere et al. [51] (2014) Prospective Case Control Study | Palmiere et al. [52] (2014) Prospective Case Control Study | |
| Cut-offs | CRP → 10 mg/L PCT → 2 µg/L sTREM-1 → 90 pg/mL | CRP → 10 mg/L PCT → 2 µg/L sCD14-ST → 600 pg/mL | CRP → 10 mg/L PCT → 2 µg/L sTREM-1 → 90 pg/mL sIL-2R → 5 ng/mL | CRP → 10 mg/L PCT → 2 µg/L IL-6 → 200 pg/mL Copeptin → 15.6 pg/mL | |
| Findings | CRP Sepsis cases (n = 16):
Sepsis cases (n = 16):
Sepsis cases (n = 16):
| CRP Sepsis cases (n = 19):
Sepsis cases (n = 19):
Sepsis cases (n = 19):
| CRP Sepsis cases (n = 12):
Sepsis cases (n = 12):
Sepsis cases (n = 12):
Sepsis cases (n = 12):
| CRP Sepsis cases (n = 28):
Sepsis cases (n = 28):
Sepsis cases (n = 28):
Sepsis cases (n = 28):
| |
| Reference | Palmiere et al. [53] (2014) Prospective Case Control Study | Palmiere et al. [54] (2015) Prospective Case Control Study | Tettamanti et al. [55] (2016) Retrospective Case Control Study | Unuma et al. [56] (2019) Retrospective Case Control Study | |
| Cut-offs | CRP → 10 mg/L PCT → 2 µg/L Endocan → 1.200 ng/mL | CRP → 10 mg/L PCT → 2 µg/L sTREM-1 → 90 pg/mL IL-6 → 200 pg/mL Psp/reg → 1 ng/mL | Troponin I → 0.03 µg/L Troponin T → 14 ng/L NT-proBNP → 738 pg/mL | CRP → 7 mg/dL PCT → 0.07 ng/mL PSEP → 1250 pg/mL | |
| Findings | CRP Sepsis cases (n = 16):
Sepsis cases (n = 16):
Sepsis cases (n = 16):
| CRP Sepsis cases (n = 20):
Sepsis cases (n = 20):
Sepsis cases (n = 20):
Sepsis cases (n = 20):
Sepsis cases (n = 20):
| Troponin I Sepsis cases (n = 16):
Sepsis cases (n = 16):
Sepsis cases (n = 16):
| CRP Sepsis cases (n = 19):
Sepsis cases (n = 19):
Sepsis cases (n = 19):
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stassi, C.; Mondello, C.; Baldino, G.; Ventura Spagnolo, E. Post-Mortem Investigations for the Diagnosis of Sepsis: A Review of Literature. Diagnostics 2020, 10, 849. https://doi.org/10.3390/diagnostics10100849
Stassi C, Mondello C, Baldino G, Ventura Spagnolo E. Post-Mortem Investigations for the Diagnosis of Sepsis: A Review of Literature. Diagnostics. 2020; 10(10):849. https://doi.org/10.3390/diagnostics10100849
Chicago/Turabian StyleStassi, Chiara, Cristina Mondello, Gennaro Baldino, and Elvira Ventura Spagnolo. 2020. "Post-Mortem Investigations for the Diagnosis of Sepsis: A Review of Literature" Diagnostics 10, no. 10: 849. https://doi.org/10.3390/diagnostics10100849
APA StyleStassi, C., Mondello, C., Baldino, G., & Ventura Spagnolo, E. (2020). Post-Mortem Investigations for the Diagnosis of Sepsis: A Review of Literature. Diagnostics, 10(10), 849. https://doi.org/10.3390/diagnostics10100849




