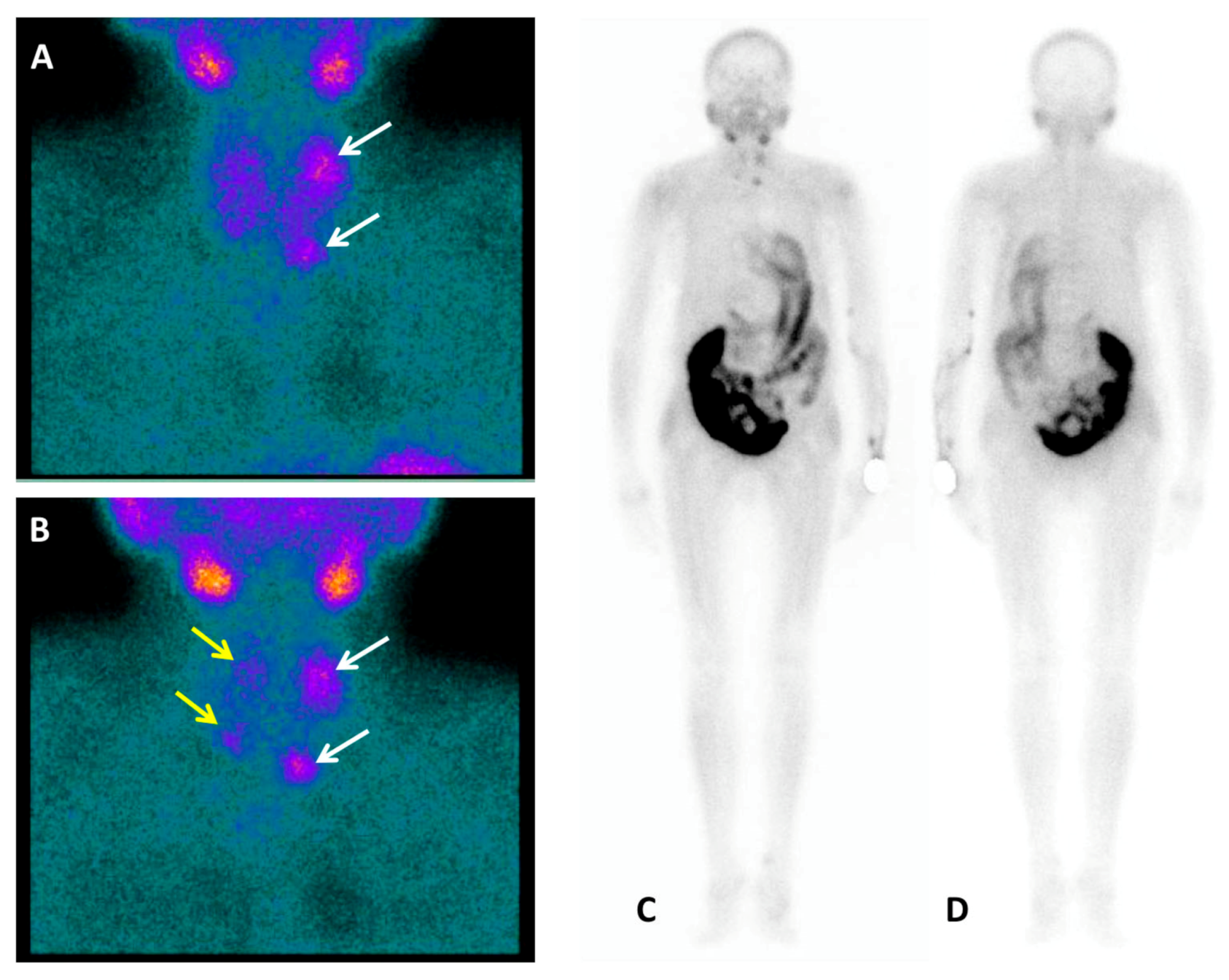
Figure 1.
A nephrectomised 62-year-old woman with chronic renal failure (stage 5D, Kidney Disease Improving Global Outcomes (KDIGO)) [
1] in dialysis treatment since 2012. Listed for kidney transplantation, laboratory tests were consistent with severe hyperparathyroidism: Parathyroid hormone (PTH) level 2068 pg/mL (normal range 15–57 pg/mL), total calcium level of 7.7 mg/dL (normal range 8.5–10.1 mg/dL), ionized calcium level of 3.49 mg/dL (normal range 4.6–5.3 mg/dL) and inorganic phosphate level of 3.7 mg/dL (normal range 2.5–4.9 mg/dL). Then, patient performed neck ultrasound (US) and, for the recent occurrence of dorsal back pain, a whole-spine magnetic resonance (MRI). According to tertiary hyperparathyroidism (HPT) diagnosis [
2] the nuclear medicine imaging workup before surgery consisted of Tc-99m-methoxyisobutylisonitrile (Tc-99m-MIBI) and Tc-99m-methylenediphosphonate (Tc-99m-MDP) planar scintigraphy, completed with Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT), and
18F-FCH PET/CT. Tc-99m-MIBI parathyroid dual-phase planar scintigraphy (anterior view) showed on the early acquisition (
A) a left superior hyperfunctioning parathyroid gland together with a left inferior one slightly under the lower third of the left thyroid lobe (white arrows). The delayed acquisition (
B) demonstrated, after thyroid gland radiotracer washout, persistent uptake in left parathyroid glands and a mild Tc-99m-MIBI concentration also in the other two right glands (yellow arrows). Planar scintigraphy was integrated with a whole-body acquisition [
3] because of the detection of lytic bone lesions on the whole-spine MRI, performed a few days before, doubtful for renal osteodystrophy, giant cell bone tumour, or carcinoma. No Tc-99m-MIBI uptake was found in the bone as showed in the whole body anterior (
C) and posterior (
D) planar views. Then, patient was directed to Tc-99m-MDP bone scan.
Figure 1.
A nephrectomised 62-year-old woman with chronic renal failure (stage 5D, Kidney Disease Improving Global Outcomes (KDIGO)) [
1] in dialysis treatment since 2012. Listed for kidney transplantation, laboratory tests were consistent with severe hyperparathyroidism: Parathyroid hormone (PTH) level 2068 pg/mL (normal range 15–57 pg/mL), total calcium level of 7.7 mg/dL (normal range 8.5–10.1 mg/dL), ionized calcium level of 3.49 mg/dL (normal range 4.6–5.3 mg/dL) and inorganic phosphate level of 3.7 mg/dL (normal range 2.5–4.9 mg/dL). Then, patient performed neck ultrasound (US) and, for the recent occurrence of dorsal back pain, a whole-spine magnetic resonance (MRI). According to tertiary hyperparathyroidism (HPT) diagnosis [
2] the nuclear medicine imaging workup before surgery consisted of Tc-99m-methoxyisobutylisonitrile (Tc-99m-MIBI) and Tc-99m-methylenediphosphonate (Tc-99m-MDP) planar scintigraphy, completed with Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT), and
18F-FCH PET/CT. Tc-99m-MIBI parathyroid dual-phase planar scintigraphy (anterior view) showed on the early acquisition (
A) a left superior hyperfunctioning parathyroid gland together with a left inferior one slightly under the lower third of the left thyroid lobe (white arrows). The delayed acquisition (
B) demonstrated, after thyroid gland radiotracer washout, persistent uptake in left parathyroid glands and a mild Tc-99m-MIBI concentration also in the other two right glands (yellow arrows). Planar scintigraphy was integrated with a whole-body acquisition [
3] because of the detection of lytic bone lesions on the whole-spine MRI, performed a few days before, doubtful for renal osteodystrophy, giant cell bone tumour, or carcinoma. No Tc-99m-MIBI uptake was found in the bone as showed in the whole body anterior (
C) and posterior (
D) planar views. Then, patient was directed to Tc-99m-MDP bone scan.
![Diagnostics 10 00851 g001 Diagnostics 10 00851 g001]()
Figure 2.
Tc-99m-MDP bone scintigraphy, performed to investigate the unknown bone lesion, showed diffusely increased radiotracer uptake throughout the axial and appendicular skeleton (anterior (
A) and posterior (
B) planar views). Especially intense activity is noted in the mandible and maxilla with absent renal and bladder activity, suggesting a “metabolic super-scan” [
4]. Of note, the mild uptake in the posterior arch of 11th–12th right ribs (blue arrow) is to be referred to a recent trauma reported in the medical patient history. Additional SPECT/CT acquisition (
C–
F) demonstrated no osteo-tropic radiopharmaceutical uptake in the right D5 costovertebral joint (white arrows) and in the left 6th rib (yellow arrows), as well as in the right iliac wing (red arrows) lytic lesions detectable on co-registration CT images.
Figure 2.
Tc-99m-MDP bone scintigraphy, performed to investigate the unknown bone lesion, showed diffusely increased radiotracer uptake throughout the axial and appendicular skeleton (anterior (
A) and posterior (
B) planar views). Especially intense activity is noted in the mandible and maxilla with absent renal and bladder activity, suggesting a “metabolic super-scan” [
4]. Of note, the mild uptake in the posterior arch of 11th–12th right ribs (blue arrow) is to be referred to a recent trauma reported in the medical patient history. Additional SPECT/CT acquisition (
C–
F) demonstrated no osteo-tropic radiopharmaceutical uptake in the right D5 costovertebral joint (white arrows) and in the left 6th rib (yellow arrows), as well as in the right iliac wing (red arrows) lytic lesions detectable on co-registration CT images.
Figure 3.
18F-FCH PET/CT was performed as the last preoperative imaging. (
A) A static image acquisition was started 5 min after injection of 2.5 MBq/Kg of
18F-FCH from the skull to the diaphragm in order to include any ectopic foci, based on the literature evidence [
5,
6]. (
B) A late acquisition at 60 min was subsequently performed, choosing a whole-body protocol because of the evidence of
18F-FCH uptake in bones in the early scan. PET/CT clearly showed
18F-FCH uptake in the four parathyroid glands, particularly in the left superior one that appeared also enlarged (red arrows).
Figure 3.
18F-FCH PET/CT was performed as the last preoperative imaging. (
A) A static image acquisition was started 5 min after injection of 2.5 MBq/Kg of
18F-FCH from the skull to the diaphragm in order to include any ectopic foci, based on the literature evidence [
5,
6]. (
B) A late acquisition at 60 min was subsequently performed, choosing a whole-body protocol because of the evidence of
18F-FCH uptake in bones in the early scan. PET/CT clearly showed
18F-FCH uptake in the four parathyroid glands, particularly in the left superior one that appeared also enlarged (red arrows).
Figure 4.
18F-FCH PET/CT delayed whole-body acquisition (C) demonstrated radiotracer uptake in multiple bone lesions, the most evident in the right D5 costovertebral joint (D) (white arrow) and in the left 6th rib (D) (yellow arrow), as well as in the right iliac wing (E) (red arrow). Their metabolic and morphological (F,G) aspect were compatible with renal osteodystrophy. A few days later, patient underwent subtotal parathyroidectomy. Three parathyroid glands were removed: two of 20 mm size each at the upper and lower poles of the left thyroid lobe respectively, the third of 15 mm size at the lower pole of the right lobe. The histological examination reported a definitive diagnosis of “parathyroid hyperplasia with chief and oxyphilic cells, nodular pattern”. The day after subtotal parathyroidectomy the biochemical values were: PTH level 16 pg/mL (normal range 15–57 pg/mL), total calcium level of 6.2 mg/dL (normal range 8.5–10.1 mg/dL), ionized calcium level of 2.88 mg/dL (normal range 4.6–5.3 mg/dL). The patient achieved HPT biochemical resolution. The two-week follow-up demonstrated blood values normalization and a further significant reduction of PHT (<6 pg/mL).
Figure 4.
18F-FCH PET/CT delayed whole-body acquisition (C) demonstrated radiotracer uptake in multiple bone lesions, the most evident in the right D5 costovertebral joint (D) (white arrow) and in the left 6th rib (D) (yellow arrow), as well as in the right iliac wing (E) (red arrow). Their metabolic and morphological (F,G) aspect were compatible with renal osteodystrophy. A few days later, patient underwent subtotal parathyroidectomy. Three parathyroid glands were removed: two of 20 mm size each at the upper and lower poles of the left thyroid lobe respectively, the third of 15 mm size at the lower pole of the right lobe. The histological examination reported a definitive diagnosis of “parathyroid hyperplasia with chief and oxyphilic cells, nodular pattern”. The day after subtotal parathyroidectomy the biochemical values were: PTH level 16 pg/mL (normal range 15–57 pg/mL), total calcium level of 6.2 mg/dL (normal range 8.5–10.1 mg/dL), ionized calcium level of 2.88 mg/dL (normal range 4.6–5.3 mg/dL). The patient achieved HPT biochemical resolution. The two-week follow-up demonstrated blood values normalization and a further significant reduction of PHT (<6 pg/mL).
![Diagnostics 10 00851 g004 Diagnostics 10 00851 g004]()
In recent years, many studies have been performed on the use of
18F-FCH PET/CT in primary hyperparathyroidism with promising results [
7,
8]. However, its performance in secondary and tertiary hyperparathyroidism is limited [
9,
10]. Our report showed how nuclear medicine imaging can provide effective and complete diagnostic information, essential for HPT patient management. In particular,
18F-FCH PET/CT was demonstrated to be a reliable multimodality imaging technique that, integrating biomolecular and morphologic data, is able to detect with high sensitivity both the hyperfunctioning parathyroid glands and bone involvement, frequently observed in patients with tertiary HPT.
Author Contributions
Conceptualization, C.F. and G.S.; data curation, F.I. and M.T.F.; writing—original draft preparation, G.S.; writing—review and editing, C.F. and V.L.; supervision, G.R. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Giampaolo Sigrisi for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Consent for Publication
The patient provided written informed consent for publication.
References
- Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [CrossRef] [PubMed]
- Jamal, S.A.; Miller, P.D. Secondary and Tertiary Hyperparathyroidism. J. Clin. Densitom. 2013, 16, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Reczek, J.; Elgazzar, A. Prominent Tc-99m MIBI Skeletal Uptake in Renal Osteodystrophy: A Possible Role for Whole-Body Scanning. Clin. Nucl. Med. 2003, 28, 775–777. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, P.; Schicht, I.M.; Pauwels, E.K.J.; te Velde, J.; de Graeff, J. Bone scintigraphy in renal osteodystrophy. J. Nucl. Med. 1978, 19, 1289–1296. [Google Scholar] [PubMed]
- Zajíčková, K.; Zogala, D.; Kubinyi, J. Parathyroid imaging by 18F-fluorocholine PET/CT in patients with primary hyperparathyroidism and inconclusive conventional methods: Clinico-pathological correlations. Physiol. Res. 2018, 67, S551–S557. [Google Scholar] [CrossRef] [PubMed]
- Broos, W.A.M.; Wondergem, M.; van der Zant, F.M.; Knol, R.J.J. Dual-time-point 18F-fluorocholine PET/CT in parathyroid imaging. J. Nucl. Med. 2019, 60, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Thanseer, N.; Bhadada, S.K.; Sood, A.; Mittal, B.R.; Behera, A.; Gorla, A.K.R.; Kalathoorakathu, R.R.; Singh, P.; Dahiya, D.; Saikia, U.N.; et al. Comparative Effectiveness of Ultrasonography, 99mTc-Sestamibi, and 18F-Fluorocholine PET/CT in Detecting Parathyroid Adenomas in Patients With Primary Hyperparathyroidism. Clin. Nucl. Med. 2017, 42, e491–e497. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Hehenwarter, L.; Paymani, Z.; Rendl, G.; Imamovic, L.; Rettenbacher, R.; Tsybrovskyy, O.; Langsteger, W.; Pirich, C. 18F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with 99mTc-MIBI or 99mTc-tetrofosmin SPECT/CT: A prospective dual-centre study in 100 patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Burgess, A.; Huchet, V.; Lefèvre, M.; Tassart, M.; Ohnona, J.; Kerrou, K.; Balogova, S.; Talbot, J.N.; Périé, S. Is 18F-fluorocholine-positron emission tomography/computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J. Clin. Endocrinol. Metab. 2014, 99, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- Broos, W.A.M.; Wondergem, M.; Van Der Zant, F.M.; Knol, R.J.J. Tertiary hyperparathyroidism with renal osteodystrophy on 18F-fluorocholine PET/CT. Clin. Nucl. Med. 2018, 43, 766–768. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).