Gastrointestinal Applications of Iodine Quantification Using Dual-Energy CT: A Systematic Review
Abstract
1. Introduction
DECT and Iodine Quantification
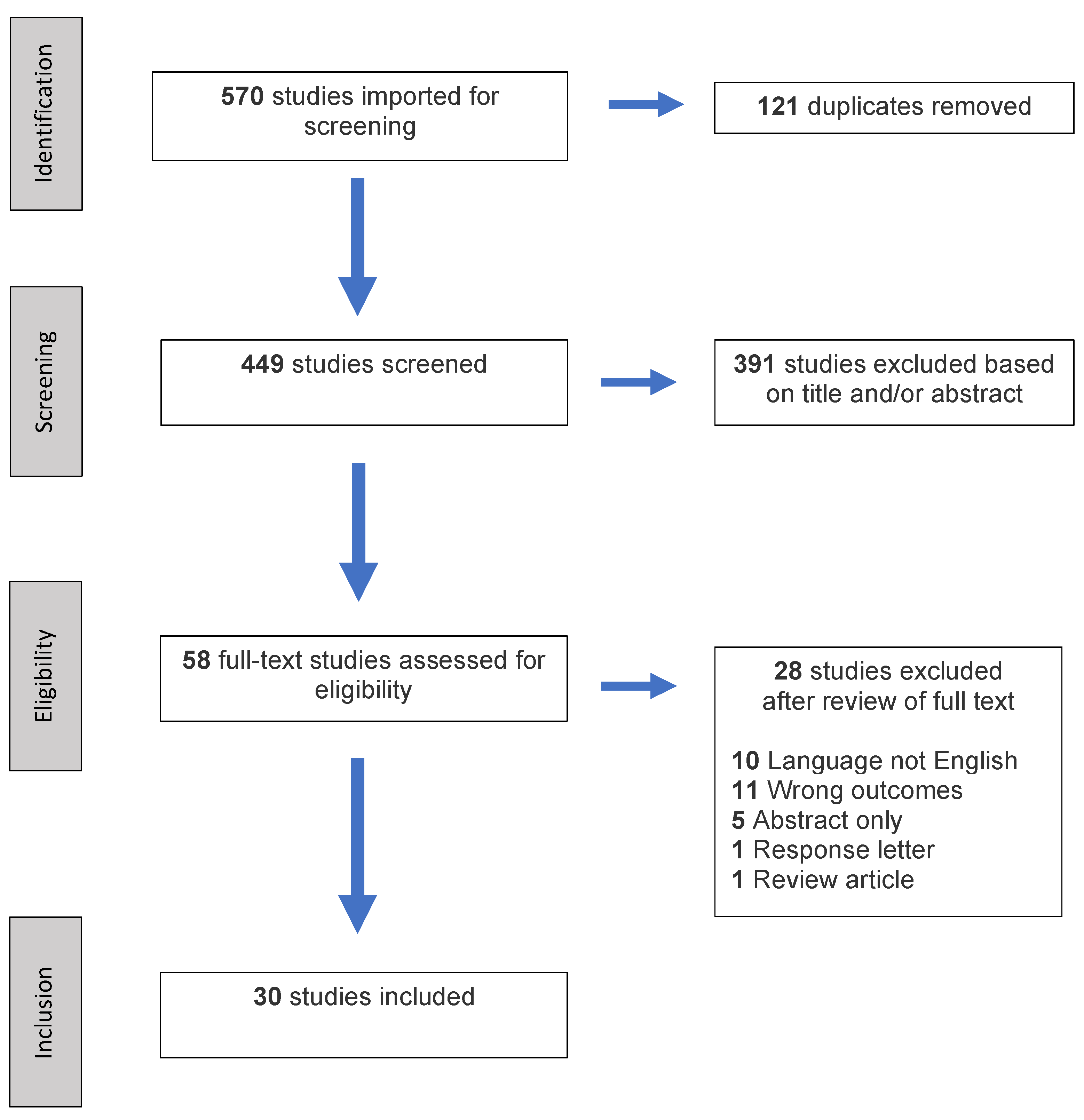
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
3. Results
3.1. Gastric Tumors
3.2. Colorectal Tumors
3.3. Crohn’s Disease
3.4. Miscellaneous Applications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP | Arterial phase |
| CDAI | Crohn’s disease activity index |
| CT | Computed tomography |
| DECT | Dual-energy Computed Tomography |
| DP | Delayed phase |
| GIST | Gastrointestinal stromal tumor |
| IC | Iodine concentration |
| IC-A | Iodine concentration in arterial phase |
| IC-V | Iodine concentration in venous phase |
| IQ | Iodine quantification |
| KeV | Kiloelectron volt |
| kVp | Peak kilovoltage |
| HU | Hounsfield unit |
| MDCT | Multi-detector computed tomography |
| MeSH | Medical subject heading |
| MSI | Microsatellite instability |
| MSS | Microsatellite stability |
| MVD | Microvascular density |
| nIC | Normalized iodine concentration |
| nIC-A | Normalized iodine concentration in arterial phase |
| nIC-V | Normalized iodine concentration in venous phase |
| nIC-D | Normalized iodine concentration in delayed phase |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies 2 |
| RCRG | Rectal cancer regression grade |
| RECIST | Response evaluation criteria in solid tumors |
| ROI | Region of interest |
| VM | Virtual monoenergetic |
| VP | Venous phase |
| VNC | Virtual non-contrast |
References
- Wells, M.; Hansel, S.L.; Bruining, D.H.; Fletcher, J.G.; Froemming, A.T.; Barlow, J.; Fidler, J.L. CT for Evaluation of Acute Gastrointestinal Bleeding. RadioGraphics 2018, 38, 1089–1107. [Google Scholar] [CrossRef]
- Moschetta, M.; Telegrafo, M.; Rella, L.; Ianora, A.A.S.; Angelelli, G. Multi-detector CT features of acute intestinal ischemia and their prognostic correlations. World J. Radiol. 2014, 6, 130–138. [Google Scholar] [CrossRef]
- Raman, S.P.; Horton, K.M.; Fishman, E.K. Computed tomography of Crohn’s disease: The role of three dimensional technique. World J. Radiol. 2013, 5, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Prakash, A.; Pradhan, G.; Vidholia, A.; Nagpal, N.; Saboo, S.S.; Kuehn, D.M.; Khandelwal, A. MDCT imaging of the stomach: Advances and applications. Br. J. Radiol. 2017, 90, 90. [Google Scholar] [CrossRef]
- Duman, M.; Tas, S.; Mecit, E.A.; Polat, E.; Duman, U.; Kurtulus, B.A.; Varolgunes, H.; Bostanci, E.B. Preoperative local staging of colorectal cancer patients with MDCT. Hepatogastroenterology 2012, 59, 1108–1112. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Martin, S.S.; Pfeifer, S.; Wichmann, J.L.; Albrecht, M.H.; Leithner, D.; Lenga, L.; Scholtz, J.-E.; Vogl, T.J.; Bodelle, B. Noise-optimized virtual monoenergetic dual-energy computed tomography: Optimization of kiloelectron volt settings in patients with gastrointestinal stromal tumors. Abdom. Radiol. 2016, 42, 718–726. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Hardie, A.D.; Schoepf, U.J.; Felmly, L.M.; Perry, J.D.; Varga-Szemes, A.; Mangold, S.; Caruso, D.; Canstein, C.; Vogl, T.J.; et al. Single- and dual-energy CT of the abdomen: Comparison of radiation dose and image quality of 2nd and 3rd generation dual-source CT. Eur. Radiol. 2016, 27, 642–650. [Google Scholar] [CrossRef]
- Chou, H.; Chin, T.Y.; Peh, W.C.G. Dual-energy CT in gout—A review of current concepts and applications. J. Med. Radiat. Sci. 2017, 64, 41–51. [Google Scholar] [CrossRef]
- Kaup, M.; Wichmann, J.L.; Scholtz, J.-E.; Beeres, M.; Kromen, W.; Albrecht, M.H.; Lehnert, T.; Boettcher, M.; Vogl, T.J.; Bauer, R.W. Dual-Energy CT–based Display of Bone Marrow Edema in Osteoporotic Vertebral Compression Fractures: Impact on Diagnostic Accuracy of Radiologists with Varying Levels of Experience in Correlation to MR Imaging. Radiology 2016, 280, 510–519. [Google Scholar] [CrossRef]
- Tijssen, M.P.M.; Hofman, P.A.M.; Stadler, A.A.R.; Van Zwam, W.; De Graaf, R.; Van Oostenbrugge, R.J.; Klotz, E.; Wildberger, J.E.; Postma, A.A. The role of dual energy CT in differentiating between brain haemorrhage and contrast medium after mechanical revascularisation in acute ischaemic stroke. Eur. Radiol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Darnell, A.; Macías, N.; Ayuso, J.R.; Rodriguez, S.; Rimola, J.; Pages, M.; García-Criado, Á.; Rengo, M.; Laghi, A.; et al. Virtual Unenhanced Images of the Abdomen With Second-Generation Dual-Source Dual-Energy Computed Tomography. Investig. Radiol. 2013, 48, 1–9. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Muscogiuri, G.; Schoepf, U.J.; Caruso, D.; Wichmann, J.L.; Cannaò, P.M.; Canstein, C.; Fuller, S.R.; Snider, L.; Varga-Szemes, A.; et al. Virtual unenhanced imaging of the liver with third-generation dual-source dual-energy CT and advanced modeled iterative reconstruction. Eur. J. Radiol. 2016, 85, 1257–1264. [Google Scholar] [CrossRef]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; Van Assen, M.; Tesche, C.; et al. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef]
- Schabel, C.; Bongers, M.; Sedlmair, M.; Korn, A.; Grosse, U.; Mangold, S.; Claussen, C.; Thomas, C. Assessment of the Hepatic Veins in Poor Contrast Conditions using Dual Energy CT: Evaluation of a Novel Monoenergetic Extrapolation Software Algorithm. RöFo Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2014, 186, 591–597. [Google Scholar] [CrossRef]
- Martin, S.S.; Wichmann, J.L.; Weyer, H.; Scholtz, J.-E.; Leithner, D.; Spandorfer, A.; Bodelle, B.; Jacobi, V.; Vogl, T.J.; Albrecht, M.H. Endoleaks after endovascular aortic aneurysm repair: Improved detection with noise-optimized virtual monoenergetic dual-energy CT. Eur. J. Radiol. 2017, 94, 125–132. [Google Scholar] [CrossRef]
- Meier, A.; Wurnig, M.C.; Desbiolles, L.; Leschka, S.; Frauenfelder, T.; Alkadhi, H. Advanced virtual monoenergetic images: Improving the contrast of dual-energy CT pulmonary angiography. Clin. Radiol. 2015, 70, 1244–1251. [Google Scholar] [CrossRef]
- Chang, S.; Han, K.; Youn, J.-C.; Im, D.J.; Kim, J.Y.; Suh, Y.J.; Hong, Y.; Hur, J.; Kim, Y.J.; Choi, B.W.; et al. Utility of Dual-Energy CT-based Monochromatic Imaging in the Assessment of Myocardial Delayed Enhancement in Patients with Cardiomyopathy. Radiology 2018, 287, 442–451. [Google Scholar] [CrossRef]
- Husarik, D.B.; Gordic, S.; Desbiolles, L.; Krauss, B.; Leschka, S.; Wildermuth, S.; Alkadhi, H. Advanced Virtual Monoenergetic Computed Tomography of Hyperattenuating and Hypoattenuating Liver Lesions. Investig. Radiol. 2015, 50, 695–702. [Google Scholar] [CrossRef]
- Mileto, A.; Nelson, R.C.; Samei, E.; Choudhury, K.R.; Jaffe, T.A.; Wilson, J.M.; Marin, D. Dual-Energy MDCT in Hypervascular Liver Tumors: Effect of Body Size on Selection of the Optimal Monochromatic Energy Level. Am. J. Roentgenol. 2014, 203, 1257–1264. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Caruso, D.; Schoepf, U.J.; De Santis, D.; Muscogiuri, G.; Albrecht, M.H.; Meinel, F.G.; Wichmann, J.L.; Burchett, P.F.; Varga-Szemes, A.; et al. A noise-optimized virtual monoenergetic reconstruction algorithm improves the diagnostic accuracy of late hepatic arterial phase dual-energy CT for the detection of hypervascular liver lesions. Eur. Radiol. 2018, 28, 3393–3404. [Google Scholar] [CrossRef]
- Bongers, M.N.; Schabel, C.; Thomas, C.; Raupach, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Comparison and Combination of Dual-Energy- and Iterative-Based Metal Artefact Reduction on Hip Prosthesis and Dental Implants. PLoS ONE 2015, 10, e0143584. [Google Scholar] [CrossRef]
- Tanaka, R.; Hayashi, T.; Ike, M.; Noto, Y.; Goto, T.K. Reduction of dark-band-like metal artifacts caused by dental implant bodies using hypothetical monoenergetic imaging after dual-energy computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 833–838. [Google Scholar] [CrossRef]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Radiol. 2017, 42, 702–709. [Google Scholar] [CrossRef]
- Jamali, S.; Michoux, N.; Coche, E.; Dragean, C. Virtual unenhanced phase with spectral dual-energy CT: Is it an alternative to conventional true unenhanced phase for abdominal tissues? Diagn. Interv. Imaging 2019, 100, 503–511. [Google Scholar] [CrossRef]
- Meyer, M.; Nelson, R.C.; Vernuccio, F.; González, F.; Farjat, A.E.; Patel, B.N.; Samei, E.; Henzler, T.; Schoenberg, S.O.; Marin, D. Virtual Unenhanced Images at Dual-Energy CT: Influence on Renal Lesion Characterization. Radiology 2019, 291, 381–390. [Google Scholar] [CrossRef]
- Slebocki, K.; Kraus, B.; Chang, D.-H.; Hellmich, M.; Maintz, D.; Bangard, C. Incidental Findings in Abdominal Dual-Energy Computed Tomography. J. Comput. Assist. Tomogr. 2017, 41, 294–297. [Google Scholar] [CrossRef]
- Yan, W.-Q.; Xin, Y.-K.; Jing, Y.; Li, G.-F.; Wang, S.-M.; Rong, W.-C.; Xiao, G.; Lei, X.-B.; Li, B.; Hu, Y.-C.; et al. Iodine Quantification Using Dual-Energy Computed Tomography for Differentiating Thymic Tumors. J. Comput. Assist. Tomogr. 2018, 42, 873–880. [Google Scholar] [CrossRef]
- Deniffel, D.; Sauter, A.P.; Fingerle, A.; Rummeny, E.J.; Makowski, M.R.; Pfeiffer, D. Improved differentiation between primary lung cancer and pulmonary metastasis by combining dual-energy CT–derived biomarkers with conventional CT attenuation. Eur. Radiol. 2020, 1–9. [Google Scholar] [CrossRef]
- Peng, J.C.; Feng, Q.; Zhu, J.; Shen, J.; Qiao, Y.Q.; Xu, J.R.; Ran, Z.H. Usefulness of spectral computed tomography for evaluation of intestinal activity and severity in ileocolonic Crohn’s disease. Ther. Adv. Gastroenterol. 2016, 9, 795–805. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, P.; Zha, K.; Liu, D.; Wang, F.; Zhou, K.; Gao, J. Spectral Computed Tomography for the Quantitative Assessment of Patients With Carcinoma of the Gastroesophageal Junction. J. Comput. Assist. Tomogr. 2019, 43, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, L.; Wang, D.; Huang, X.; Wei, J.; Zhang, W.; Zhang, Z.; Zhou, J. Gastrointestinal stromal tumor risk classification: Spectral CT quantitative parameters. Abdom. Radiol. 2019, 44, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Whiting, P.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Pan, Z.; Pang, L.; Ding, B.; Yan, C.; Zhang, H.; Du, L.; Wang, B.; Song, Q.; Chen, K.; Yan, F. Gastric Cancer Staging with Dual Energy Spectral CT Imaging. PLoS ONE 2013, 8, e53651. [Google Scholar] [CrossRef]
- Liang, P.; Ren, X.-C.; Gao, J.-B.; Chen, K.-S.; Xu, X. Iodine Concentration in Spectral CT: Assessment of Prognostic Determinants in Patients With Gastric Adenocarcinoma. Am. J. Roentgenol. 2017, 209, 1033–1038. [Google Scholar] [CrossRef]
- Chen, X.-H.; Ren, K.; Liang, P.; Chai, Y.-R.; Chen, K.-S.; Gao, J.-B. Spectral computed tomography in advanced gastric cancer: Can iodine concentration non-invasively assess angiogenesis? World J. Gastroenterol. 2017, 23, 1666–1675. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Wang, X.; Liang, P.; Gao, J. Detection of gastric cancer and its histological type based on iodine concentration in spectral CT. Cancer Imaging 2018, 18, 42. [Google Scholar] [CrossRef]
- Yang, L.; Shi, G.; Zhou, T.; Li, Y.; Li, Y. Quantification of the Iodine Content of Perigastric Adipose Tissue by Dual-Energy CT: A Novel Method for Preoperative Diagnosis of T4-Stage Gastric Cancer. PLoS ONE 2015, 10, e0136871. [Google Scholar] [CrossRef]
- Xie, Z.-Y.; Chai, R.-M.; Ding, G.-C.; Liu, Y.; Ren, K. T and N Staging of Gastric Cancer Using Dual-Source Computed Tomography. Gastroenterol. Res. Pr. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Y.; Shi, G.-F.; Zhou, T.; Tan, B.-B. The Concentration of Iodine in Perigastric Adipose Tissue: A Novel Index for the Assessment of Serosal Invasion in Patients with Gastric Cancer after Neoadjuvant Chemotherapy. Digestion 2018, 98, 87–94. [Google Scholar] [CrossRef]
- Kupeli, A.; Bulut, E.; Cansu, A.; Guner, A.; Soyturk, M.; Danişan, G.; Danışan, G. Contribution of DECT in detecting serosal invasion of gastric cancer. Turk. J. Med Sci. 2019, 49, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-M.; Ling, W.; Zhu, J.; Xu, J.-R.; Wu, L.-M.; Gong, H.-X. Dual Energy Spectral CT Imaging in the assessment of Gastric Cancer and cell proliferation: A Preliminary Study. Sci. Rep. 2018, 8, 17619. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, Z.-Y.; Zhang, X.P.; Li, Y.-L.; Li, X.-T.; Wang, Z.-L.; Ji, J.-F.; Sun, Y.-S.; Li, Z.-W. Evaluating the response of gastric carcinomas to neoadjuvant chemotherapy using iodine concentration on spectral CT: A comparison with pathological regression. Clin. Radiol. 2015, 70, 1198–1204. [Google Scholar] [CrossRef]
- Liu, J.; Chai, Y.; Zhou, J.; Dong, C.; Zhang, W.; Liu, B. Spectral Computed Tomography Imaging of Gastric Schwannoma and Gastric Stromal Tumor. J. Comput. Assist. Tomogr. 2017, 41, 417–421. [Google Scholar] [CrossRef]
- Meng, X.; Ni, C.; Shen, Y.; Hu, X.; Chen, X.; Li, Z.; Hu, D. Differentiating malignant from benign gastric mucosal lesions with quantitative analysis in dual energy spectral computed tomography. Medicine 2017, 96, e5878. [Google Scholar] [CrossRef]
- Gong, H.-X.; Zhang, K.-B.; Wu, L.-M.; Baigorri, B.F.; Yin, Y.; Geng, X.-C.; Xu, J.-R.; Zhu, J. Dual Energy Spectral CT Imaging for Colorectal Cancer Grading: A Preliminary Study. PLoS ONE 2016, 11, e0147756. [Google Scholar] [CrossRef]
- Chuang-Bo, Y.; Tai-Ping, H.; Hai-Feng, D.; Yong-Jun, J.; Xi-Rong, Z.; Guang-Ming, M.; Chenglong, R.; Jun, W.; Yong, Y. Quantitative assessment of the degree of differentiation in colon cancer with dual-energy spectral CT. Abdom. Radiol. 2017, 42, 2591–2596. [Google Scholar] [CrossRef]
- Al-Najami, I.; Drue, H.C.; Steele, R.J.C.; Baatrup, G. Dual energy CT—A possible new method to assess regression of rectal cancers after neoadjuvant treatment. J. Surg. Oncol. 2017, 116, 984–988. [Google Scholar] [CrossRef]
- Al-Najami, I.; Sheta, H.M.; Baatrup, G. Differentiation between malignant and benign rectal tumors by dual-energy computed tomography—A feasibility study. Acta Oncol. 2019, 58, S55–S59. [Google Scholar] [CrossRef]
- Sun, K.; Han, R.; Han, Y.; Shi, X.; Hu, J.; Lu, B. Accuracy of Combined Computed Tomography Colonography and Dual Energy Iiodine Map Imaging for Detecting Colorectal masses using High-pitch Dual-source CT. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Fan, S.; Li, X.; Zheng, L.; Hu, D.; Ren, X.; Ye, Z. Correlations between the iodine concentrations from dual energy computed tomography and molecular markers Ki-67 and HIF-1α in rectal cancer: A preliminary study. Eur. J. Radiol. 2017, 96, 109–114. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kim, S.H.; Bae, J.S.; Jeon, S.K.; Han, J.K. Can quantitative iodine parameters on DECT replace perfusion CT parameters in colorectal cancers? Eur. Radiol. 2018, 28, 4775–4782. [Google Scholar] [CrossRef]
- Wu, J.; Lv, Y.; Wang, N.; Zhao, Y.; Zhang, P.; Liu, Y.; Chen, A.; Li, J.; Li, X.; Guo, Y.; et al. The value of single-source dual-energy CT imaging for discriminating microsatellite instability from microsatellite stability human colorectal cancer. Eur. Radiol. 2019, 29, 3782–3790. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.H.; Ryu, H.S.; Han, J.K. Iodine Quantification on Spectral Detector-Based Dual-Energy CT Enterography: Correlation with Crohn’s Disease Activity Index and External Validation. Korean J. Radiol. 2018, 19, 1077–1088. [Google Scholar] [CrossRef]
- Dane, B.; Duenas, S.; Han, J.; Oʼdonnell, T.; Ream, J.; Chang, S.; Megibow, A. Crohnʼs Disease Activity Quantified by Iodine Density Obtained From Dual-Energy Computed Tomography Enterography. J. Comput. Assist. Tomogr. 2020, 44, 242–247. [Google Scholar] [CrossRef]
- De Kock, I.; Delrue, L.; Lecluyse, C.; Hindryckx, P.; De Vos, M.; Villeirs, G. Feasibility study using iodine quantification on dual-energy CT enterography to distinguish normal small bowel from active inflammatory Crohn’s disease. Acta Radiol. 2018, 60, 679–686. [Google Scholar] [CrossRef]
- Ge, X.; Yu, J.; Wang, Z.; Xu, Y.; Pan, C.; Jiang, L.; Yang, Y.; Yuan, K.; Liu, W. Comparative study of dual energy CT iodine imaging and standardized concentrations before and after chemoradiotherapy for esophageal cancer. BMC Cancer 2018, 18, 1120. [Google Scholar] [CrossRef]
- Yang, C.-B.; Yu, N.; Jian, Y.-J.; Yu, Y.; Duan, H.-F.; Zhang, X.-R.; Ma, G.-M.; Guo, Y.; Duan, X. Spectral CT Imaging in the Differential Diagnosis of Small Bowel Adenocarcinoma From Primary Small Intestinal Lymphoma. Acad. Radiol. 2019, 26, 878–884. [Google Scholar] [CrossRef]
- Fletcher, C.D.M.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of Gastrointestinal Stromal Tumors:A Consensus Approach. Int. J. Surg. Pathol. 2002, 10, 81–89. [Google Scholar] [CrossRef]
- Lourenco, P.D.M.; Rawski, R.; Mohammed, M.F.; Khosa, F.; Nicolaou, S.; McLaughlin, P. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia. Am. J. Roentgenol. 2018, 211, 564–570. [Google Scholar] [CrossRef]
- Winklhofer, S.; Lambert, J.W.; Wang, Z.J.; Sun, Y.; Gould, R.G.; Zagoria, R.J.; Yeh, B.M. Reduction of peristalsis-related gastrointestinal streak artifacts with dual-energy CT: A patient and phantom study. Abdom. Radiol. 2016, 41, 1456–1465. [Google Scholar] [CrossRef]
- Al-Najami, I.; Lahaye, M.; Beets-Tan, R.G.H.; Baatrup, G. Dual-energy CT can detect malignant lymph nodes in rectal cancer. Eur. J. Radiol. 2017, 90, 81–88. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Bagnacci, G.; Gentili, F.; Nigri, A.; Pelini, V.; Vindigni, C.; Mazzei, F.G.; Baiocchi, G.L.; Pittiani, F.; Morgagni, P.; et al. Gastric Cancer Maximum Tumour Diameter Reduction Rate at CT Examination as a Radiological Index for Predicting Histopathological Regression after Neoadjuvant Treatment: A Multicentre GIRCG Study. Gastroenterol. Res. Pr. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Patel, B.N.; Vernuccio, F.; Meyer, M.; Godwin, B.; Rosenberg, M.; Rudnick, N.; Harring, S.; Nelson, R.; Ramirez-Giraldo, J.C.; Farjat, A.; et al. Dual-Energy CT Material Density Iodine Quantification for Distinguishing Vascular From Nonvascular Renal Lesions: Normalization Reduces Intermanufacturer Threshold Variability. Am. J. Roentgenol. 2019, 212, 366–376. [Google Scholar] [CrossRef]
- Uhrig, M.; Sedlmair, M.; Schlemmer, H.; Hassel, J.; Ganten, M. Monitoring targeted therapy using dual-energy CT: Semi-automatic RECIST plus supplementary functional information by quantifying iodine uptake of melanoma metastases. Cancer Imaging 2013, 13, 306–313. [Google Scholar] [CrossRef]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, F.; Caravetta, A.; Mollo, F.; Spinoso, A.; Peluso, L.; Guarino, R. Sorafenib, Risk of Bleeding and Spontaneous Rupture of Hepatocellular Carcinoma. A Clinical Case. Acta Medica 2011, 54, 177–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dimitrakopoulou-Strauss, A.; Ronellenfitsch, U.; Cheng, C.; Pan, L.; Sachpekidis, C.; Hohenberger, P.; Henzler, T. Imaging therapy response of gastrointestinal stromal tumors (GIST) with FDG PET, CT and MRI: A systematic review. Clin. Transl. Imaging 2017, 5, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Belmouhand, M.; Löfgren, J.; Johannesen, H.H.; Baeksgaard, L.; Gutte, H.; Tariq, K.; Achiam, M.P. Early response evaluation of neoadjuvant therapy with PET/MRI to predict resectability in patients with adenocarcinoma of the esophagogastric junction. Abdom. Radiol. 2018, 44, 836–844. [Google Scholar] [CrossRef]
- Lordick, F.; Ott, K.; Krause, B.-J.; A Weber, W.; Becker, K.; Stein, H.J.; Lorenzen, S.; Schuster, T.; Wieder, H.; Herrmann, K.; et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007, 8, 797–805. [Google Scholar] [CrossRef]
- Furukawa, A.; Kanasaki, S.; Kono, N.; Wakamiya, M.; Tanaka, T.; Takahashi, M.; Murata, K. CT Diagnosis of Acute Mesenteric Ischemia from Various Causes. Am. J. Roentgenol. 2009, 192, 408–416. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Gentili, F.; Volterrani, L. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia: A Necessary Clarification. Am. J. Roentgenol. 2019, 212, W93–W94. [Google Scholar] [CrossRef]
- Darras, K.E.; McLaughlin, P.D.; Kang, H.; Black, B.; Walshe, T.; Chang, S.D.; Harris, A.C.; Nicolaou, S. Virtual monoenergetic reconstruction of contrast-enhanced dual energy CT at 70keV maximizes mural enhancement in acute small bowel obstruction. Eur. J. Radiol. 2016, 85, 950–956. [Google Scholar] [CrossRef]
- Jacobsen, M.C.; Schellingerhout, D.; Wood, C.A.; Tamm, E.P.; Godoy, M.C.; Sun, J.; Cody, D.D. Intermanufacturer Comparison of Dual-Energy CT Iodine Quantification and Monochromatic Attenuation: A Phantom Study. Radiology 2018, 287, 224–234. [Google Scholar] [CrossRef]
| Author | Year | Focus | Population | DECT Scanner | kV Range | Contrast | Flow Rate | Total Iodine | ROI Placement | Reference | Phase | Normalization | Outcome Measure | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pan [36] | 2013 | Degree of differentiation gastric cancer | 96 | Discovery CT750 HD, GE Healthcare | 80/140 | Ultravist 300 mg I/mL | 3 mL/s | 85–110 mL | Lesion and the normal gastric wall. | Pathology | AP/VP | Aorta | nIC-A, nIC-V | Significant difference between well-differentiated and poorly differentiated adenocarcinoma in both phases (nIC-A: p < 0.02, nIC-V: p < 0.05). Significant difference between metastatic and non-metastatic lymph nodes. nIC had no correlation with cancer subtypes. |
| Tang [45] | 2015 | Evaluating the response of gastric carcinomas to neoadjuvant chemotherapy | 20 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 3.5 mL/s | 1.5 mL/kg | N/A | Pathology | AP/VP | N/A | IC-A, IC-V, | % decrease in IC-A was significantly different in good response group vs. poor response group (p = 0.012) |
| Yang [40] | 2015 | Assessment of IC in perigastric fat in gastric cancer patients with and without serosal invasion (Stage T4a) | 54 | SOMATOM Definition Flash, Siemens Healthcare | 100/140 | Omnipaque 300 mg I/mL | 3.0 mL/s | 2 mL/kg | Perigastric fat adjacent to tumor | Pathology | AP/VP | N/A | IC-A, IC-V | Significant difference between patients with and without serosal invasion in both phases (p < 0.001) |
| Liang [37] | 2017 | Correlation with clinicopathologically determined prognostic factors in gastric adenocarcinoma (TNM, MVD) | 34 | Discovery CT750 HD, GE Healthcare | N/A | Optiray 320 mg I/mL | 3 mL/s | 1.5 mL/kg | Area that encompassed the entire tumor, away from any peripheral fat and necrotic areas. | Pathology | AP/VP | Aorta | nIC-A, nIC-V | Significant difference between moderately and poorly differentiated adenocarcinoma (nIC-A: p = 0.005, nIC-V: p = 0.013). Positive correlation between nIC and MVD (nIC-A: r = 0.423, nIC-V: r = 0.542). No correlation with lymphatic metastasis or TNM stage (nIC-A: r = 0.119, nIC-V: r = 0.097) |
| Liu [46] | 2017 | Value of DECT in Gastric schwanomma and GIST | 12 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 3–4 mL/s | 1.0 mL/kg | Tumor; avoiding necrosis and cystic areas, calcification, and larger vessels | Pathology | AP/VP | N/A | IC-A, IC-V | Significant difference in IC betweengastric schwanommas and GIST (p < 0.001) |
| Chen [38] | 2017 | Correlation with MVD in gastric cancer patients | 34 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 350 mg I/mL | 2.5–4.5 mL/s | 60–110 mL | Lesion; avoiding artifacts, necrosis, and vessels | Pathology | AP/VP | Aorta | nIC-A, nIC-V | Significant difference between well and poorly differentiated adenocarcinoma (nIC-A: p < 0.003, nIC-V: p < 0.001). Positive correlation between nIC and MVD (nIC-A: r = 0.423, nIC-V: r = 0.606). |
| Meng [47] | 2017 | Differentiation between malignant and benign gastric lesions (Cancer, Inflammation, normal) | 161 | Discovery CT750 HD, GE Healthcare | 80/140 | Ultravist 370 mg I/mL | 3–4 mL/s | 1.0 mL/kg | Lesion; avoiding cystic, necrosis, and hemorrhage | Pathology | AP/VP | Aorta | nIC-A, nIC-V, IC-A, IC-V | nIC and IC in gastric cancer differed significantly from normal mucosa and gastric inflammation (p < 0.05, aside from nIC-A: p = 0.116) |
| Xie [41] | 2018 | T and N staging of gastric cancer | 71 | SOMATOM Definition Flash, Siemens Healthcare | 100/140 | Omnipaque 350 mg I/mL | 2.5–3 mL/s | 70 mL | Tumor and extraserosal fat | Pathology | AP/VP/DP | Aorta | nIC-A, nIC-V, nIC-D | Significant difference between T3 and T4 in extraserosal fat in arterial and dealyed phase (nIC-A: p = 0.004, nIC-D: p = 0.001). No significant findings between differentiated vs. Undifferetiated adenocarcinoma (nIC-A: p = 0.06, nIC-V: p = 0.07, nIC-D: p = 0.09) with ROI placement on tumor |
| Yang [42] | 2018 | IC in perigastric adipose tissue in the assessment of Serosal Invasion in Patients with Gastric Cancer after Neoadjuvant Chemotherapy | 43 | SOMATOM Definition Flash, Siemens Healthcare | 100/140 | Omnipaque 300 mg I/mL | 3.0 mL/s | 2 mL/kg | Perigastric adipose tissue without blood vessels or other tissues | Pathology | AP/VP | Aorta | nIC-V, IC-V | Significant difference between patients with and without serosal invasion pre- and post neoadjuvant chemotherapy (p < 0.05) aside from nIC in patients with serosal invasion prior to chemotherapy (p = 0.10) |
| Cheng [44] | 2018 | Correlation with Ki-67 protein level expression in advanced & early Gastric cancer | 162 | Discovery CT750 HD, GE Healthcare | 80/140 | Iopamidol 370 mg I/mL | 3.0 mL/s | 1.8 mL/kg | Solid tumor; avoiding necrotic and fat areas | Pathology | AP/VP/DP | Aorta | nIC-A, nIC-V, nIC-D, IC-A, IC-V, IC-D | Significant difference between early (confined to mucosa/submucosa) vs. advanced gastric cancer (invasion of the submucosa) in nIC-V/D (p = 0.002, p = 0.000) and IC-V/D (p = 0.029, p = 0.002). Ki/67 correlates well with nIC-V/D (r = 0.753, r = 0.745) and IC-V/D (r = 0.818, r = 0.730) |
| Li [39] | 2018 | Discrimination between benign and malignant & correlation to degree of differention | 87 | Discovery CT750 HD, GE Healthcare | 80/140 | Ultravist 370 mg I/mL | 3.0 mL/s | 1.5 mL/kg | Solid part of the tumor; avoiding peripheral fat, visible vessel, calcification and cystic/necrotic areas. | Pathology | AP/VP | Aorta | nIC-A, nIC-V, IC-A, IC-V | Significant difference between well-differentiated and poorly differentiated adenocarcinoma in all phases (p < 0.0001, except nIC-A: p = 0.0445). |
| Küpeli [43] | 2019 | IC measurements in the perigastric fat and its’ correlation with gastric cancer TNM staging | 41 | Aquilion, Toshiba Medical Systems | 80/130 | (nonionic contrast agent) | 4.0 mL/s | 2 mL/kg | Normal gastric tissue, tumor and perigastric fat | Pathology | AP/VP | N/A | IC-A, IC-V | Significant difference in IC-A (p < 0.001) and IC-V (p < 0.001) between patients with serosal invasion (T4) vs. Absent(T1-3) |
| Author | Year | Focus | Population | DECT Scanner | kVp Range | Contrast | Flow Rate | Total Iodine | ROI Placement | Reference | Phase | Normalization | Outcome Measure | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gong [48] | 2016 | Colorectal cancer differentiation degree | 81 | Discovery CT750 HD, GE Healthcare | N/A | Iopamidol 370 mg I/mL | 3.5 mL/s | 1.8 mL/kg | Solid tumor regions avoiding areas with obvious features of cystic or necrotic change | Pathology | VP | Psoas muscle | nIC-A, nIC-V, IC-A, IC-V | Significant difference between well and moderately differentiated vs. Poorly differentiated colonic adenocarcinoma in all phases (p = 0.000) |
| Al-Najami [50] | 2017 | Regression assessment in rectal cancer patients following neoadjuvant chemoradiotherapy treatment | 11 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 3 mL/s | 1 mL/kg | Tumor; based on a macroscopic evaluation of the most representative images of associated MRI scan | Pathology (RCRG) | N/A | N/A | IC | Significant difference in IC in partial and complete response group following neoadjuvant chemoradiotherapy treatment (p < 0.05) |
| Chaung-bo [49] | 2017 | Colon cancer differentiation degree | 47 | Discovery CT750 HD, GE Healthcare | N/A | Iohexol 300 mg I/mL | 3–4 mL/s | 0.8–1 mL/kg | Tumor tissue; avoiding areas of necrosis, calcification, and artifacts caused by the gas and liquid interface | Pathology | AP/VP | Aorta or iliac artery | nIC-A, nIC-V, IC-A, IC-V | Significant difference in nIC-A (p = 0.02) and IC-A (p = 0.001) between poorly and well-dffierentiated colon cancer |
| Fan [53] | 2017 | Correlation with Ki-67 and HIF-1α in rectal cancer | 80 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 2.5 mL/s | 1.2 mL/kg | Solid tumor regions avoiding areas with obvious features of cystic or necrotic change | Ki-67 and HIF-1α | 70 s | External Iliac artery | nIC-V | Postively correlated with Ki-67 value (r = 0.344, p = 0.002) and HIF-1α levels (r = 0.598, p < 0.001) in rectal cancer patients |
| Sun [52] | 2018 | Accuracy of Combined CT Colonography and DECT iodine mapping for Detecting Colorectal masses | 28 | SOMATOM Definition Flash, Siemens Healthcare | 100/140 | Omnipaque 350 mg I/mL | 4.0 mL/s | 60 mL | Tumor | Optical colonoscopy and Pathology | 4 s (post bolus tracking) | N/A | IC-A | Significant difference in IC between stool and colonic neoplasia (p < 0.01). Significant difference nIC between colonic adenomas and adenocarcinomas (p < 0.01) |
| Kang [54] | 2018 | Correlation with perfusion CT parameters in colorectal cancer | 41 | SOMATOM Definition Flash, Siemens Healthcare | 80/140 | Bonorex 350 mg I/mL | 4–5 mL/s | 1.125 mL/kg | Tumor | Perfusion CT measurements (Blood flow, blood volume, permeability, mean transit time) | 50 s | Aorta and inferior vena cava | nIC-V, IC-V | IC-V correlates with some perfusion CT parameteres (Blood volume: r = 0.32, p = 0.04; permeability: r = 0.34, p = 0.03; mean transit time: r = −0.38, p = 0.02) |
| Wu [55] | 2019 | Discriminating MSI from MSS in human colorectal cancer | 114 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 3–3.5 mL/s | 1.2 mL/kg | Solid tumor; avoiding bleeding, necrosis, and cystic portions | Pathology (Immunohistochemical staining) | AP/VP/DP | External Iliac artery | nIC-A, nIC-V, nIC-D | Significant difference in nIC between MSS and MSI in all phases (p < 0.001) |
| Al-Najami [51] | 2019 | Differentiation between malignant and benign rectal tumors | 16 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 3 mL/s | 1 mL/kg | Tumor; most representative areas of evident tumor tissue | Pathology | N/A | N/A | IC | Z-effective was significant between malignant * and benign group (p = 0.03), however IC was nonsignificant (p > 0.05) |
| Author | Year | Focus | Population | kVp Range | DECT Scanner | Contrast | Flow Rate | Total Iodine | ROI Placement | Reference | Phase | Normalization | Outcome Measure | Findings: |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peng [30] | 2016 | Disease activity in ileocolonic crohns disease | 50 | 80/140 | Discovery CT750 HD, GE Healthcare | Iopamidol 370 mg I/mL | 4 mL/s | 1.5 mL/kg | Placed on iodine concentration maps and encompassed the high-enhancing areas | Endoscopy (Simple Endoscopic Score for Crohn’s Disease) | 45 s | Artery (not specified) | nIC-V | Significant differences in nIC-V between endoscopic normal and mild (p = 0.002) as well as mild and severe lesions (p < 0.001) |
| Kim [56] | 2018 | Correlation with disease activity index | 39 | 120 | IQon Spectral CT, Philips Healthcare | Iohexol, 350 mg I/mL | 3–5 mL/s | 1.6 mL/kg | Bowel wall with strongest enhancement on iodine concentration maps | Crohn’s disease activity index (CDAI) | VP | N/A | IC-V | Iodine concentrations correlates well with CDAI score (r = 0.744, p < 0.001) |
| DeKock [58] | 2019 | Distinguishing normal small bowel from active inflammatory crohns | 40 | 80/140 | SOMATOM Definition Flash, Siemens Healthcare | Visipaque, GE healthcare | 3.5 mL/s | 100 mL | Normal bowel wall = ROI over entire bowel wall. Crohns = ROI placed on mucosa (brightest area) | Endoscopy, biochemistry and clinical symptoms | 70 s | Aorta | nIC-V, IC-V | Significant difference between disease and control group (p < 0.001) |
| Dane [57] | 2020 | Correlation with disease activity | 22 | 80/150 | SOMATOM FORCE, Siemens Healthcare | Ultravist 300 mg I/mL | 3–4 mL/s | 1.5 mL/kg | Brightest involved bowel wall segment | Crohn’s disease activity index (CDAI) | 60 s | Aorta | IC-V (Min, max and weighted average) | The ICmax and ICmin of affected bowel differed significantly from normal bowel (p < 0.0001) |
| Author | Year | Focus | Population | DECT Scanner | kVp Range | Contrast | Flow Rate | Total Iodine | ROI Placement | Reference | Phase | Normalization | Outcome Measure | Findings: |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winklhofer [63] | 2016 | Reduction of peristalsis-related gastrointestinal streak artifacts | 100 | Discovery CT750 HD, GE Healthcare | 80/140 | N/A | N/A | N/A | The most visibly bright area of streak artifact in the 70 keV axial images | ROI measurements in streak artifacts vs. non artifact in 70 keV, 120 keV and water (iodine) | VP | N/A | N/A | ROI measurements in areas with and without streak artifcts were non-significant in iodine/water (p = 0.088) compared to monoenergetic images and water/iodine (p < 0.001). Streak artifacts are reduced in iodine/water images |
| Lourenco [62] | 2018 | Applications in Acute Bowel ischemia | 60 | SOMATOM Definition Flash, Siemens Healthcare | 100/140 | Omnipaque 350 mg I/mL | 3.5 mL/s | 90 mL | Ischemic and normalbowel | Electronic medical record, procedrual and pathology reports | VP | N/A | IC-V | 65% reduction in IC among patients with confirmed ischemia * |
| Ge [59] | 2018 | Iodine concentrations in esophageal cancer before and after chemoradiotherapy | 45 | SOMATOM Definition Flash, Siemens Healthcare | 100/140 | Iohexol 300 mg I/mL | 3 mL/s | 70 mL | Tumor; avoiding tumor margins and necrotic areas | RECIST criteria | AP/VP | Aorta | nIC-A, nIC-V | Significantly lower nIC-A and nIC-V in effective group vs. ineffective group post-chemoradiotherapy (p < 0.05) |
| Zhou [31] | 2019 | Differentiation between squamous cell carcinoma and adenocarcinoma in the gastroesophageal junction | 61 | Discovery CT750 HD, GE Healthcare | 80/140 | Iobitrido 350 mg I/mL | 3 mL/s | 1.5 mL/kg | Around the entire lesion | Pathology | AP/VP | Arota | nIC-A, nIC-V (nIC difference, nIC ratio) | Significant difference between squamous cell carcinoma and adenocarcinoma in both phases (nIC-A: p = 0.02, nIC-V: p = 0.00) |
| Yang [60] | 2019 | Differetiation between small bowel adenocarcinoma and primary small intestinal lymphoma | 42 | Discovery CT750 HD, GE Healthcare | 80/140 | Omnipaque 300 mg I/mL | 3–4 mL/s | 0.8–1.0 mL/kg | Tumor; avoiding focal necrosis, calcification, and blood vessels | Pathology | AP/VP | Aorta | nIC-A, nIC-V, IC-A, IC-V | Significant difference between small bowel adenocarcinoma and primary small intestinal lymphoma in nIC-A (p = 0.001), nIC-V (p = 0.002) and IC-A (p = 0.003) |
| Zhang [32] | 2019 | Value of IC paramenters in gastrointestinal stromal tumor risk stratification | 86 | Discovery CT750 HD, GE Healthcare | N/A | Omnipaque 300 mg I/mL | 3.5–4.0 mL/s | 1.2 mL/kg | Primary lesion and normal intestinal wall | Pathology (GIST recurrence risk stratification criteria) | AP/VP/DP | Aorta | nIC-A, nIC-V, nIC-D | Significant difference between high risk and intermediate/low risk GIST patients in all phases (p < 0.001) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.J.; Taudorf, M.; Ulriksen, P.S.; Achiam, M.P.; Resch, T.A.; Nielsen, M.B.; Lönn, L.B.; Hansen, K.L. Gastrointestinal Applications of Iodine Quantification Using Dual-Energy CT: A Systematic Review. Diagnostics 2020, 10, 814. https://doi.org/10.3390/diagnostics10100814
Xu JJ, Taudorf M, Ulriksen PS, Achiam MP, Resch TA, Nielsen MB, Lönn LB, Hansen KL. Gastrointestinal Applications of Iodine Quantification Using Dual-Energy CT: A Systematic Review. Diagnostics. 2020; 10(10):814. https://doi.org/10.3390/diagnostics10100814
Chicago/Turabian StyleXu, Jack Junchi, Mikkel Taudorf, Peter Sommer Ulriksen, Michael Patrick Achiam, Timothy Andrew Resch, Michael Bachmann Nielsen, Lars Birger Lönn, and Kristoffer Lindskov Hansen. 2020. "Gastrointestinal Applications of Iodine Quantification Using Dual-Energy CT: A Systematic Review" Diagnostics 10, no. 10: 814. https://doi.org/10.3390/diagnostics10100814
APA StyleXu, J. J., Taudorf, M., Ulriksen, P. S., Achiam, M. P., Resch, T. A., Nielsen, M. B., Lönn, L. B., & Hansen, K. L. (2020). Gastrointestinal Applications of Iodine Quantification Using Dual-Energy CT: A Systematic Review. Diagnostics, 10(10), 814. https://doi.org/10.3390/diagnostics10100814







