Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty
Abstract
1. Introduction
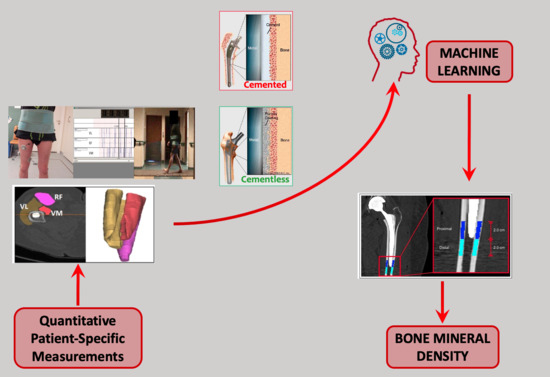
1.1. An Introduction to the Methodology
1.2. Aim of the Work
2. Materials and Methods
2.1. The Dataset
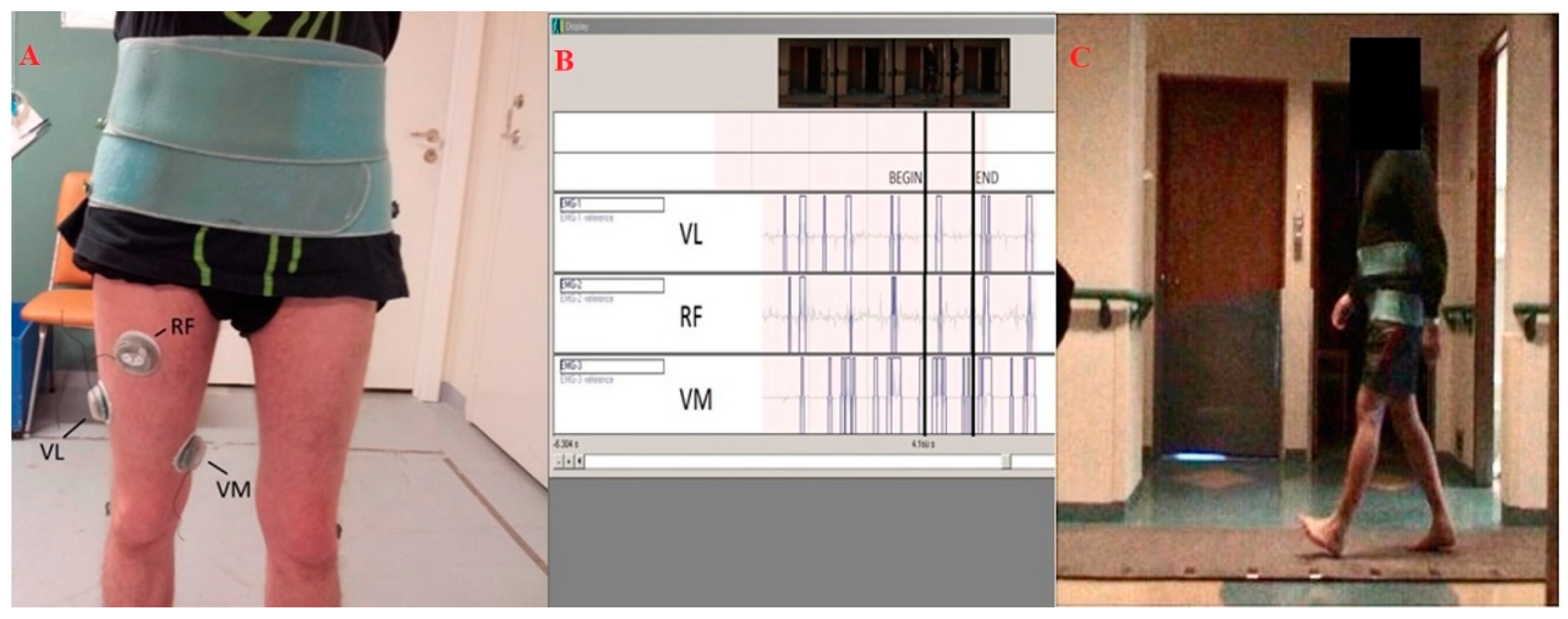
2.2. Gait Analysis and EMG Assessment
2.3. Imaging Acquisition
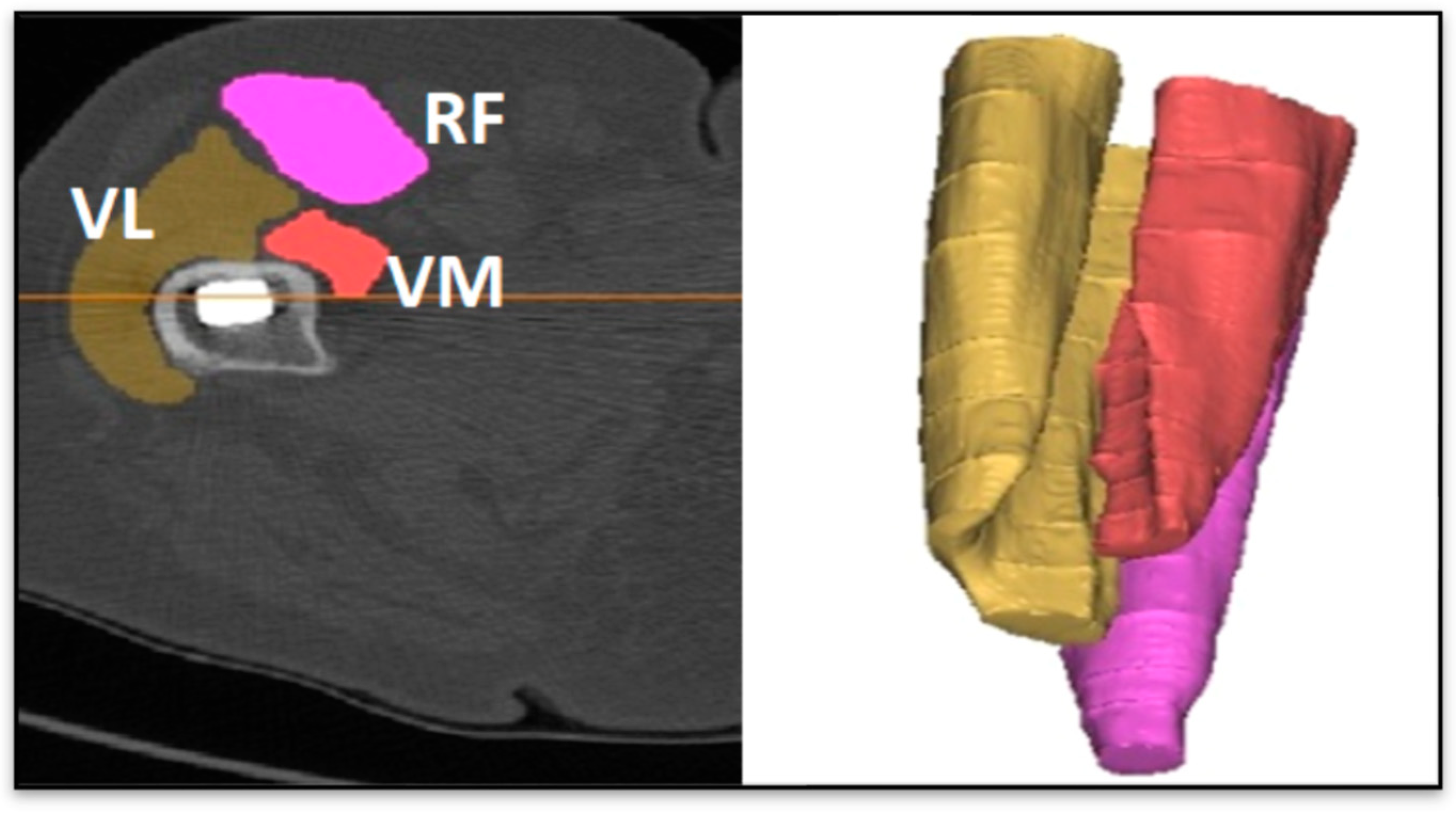
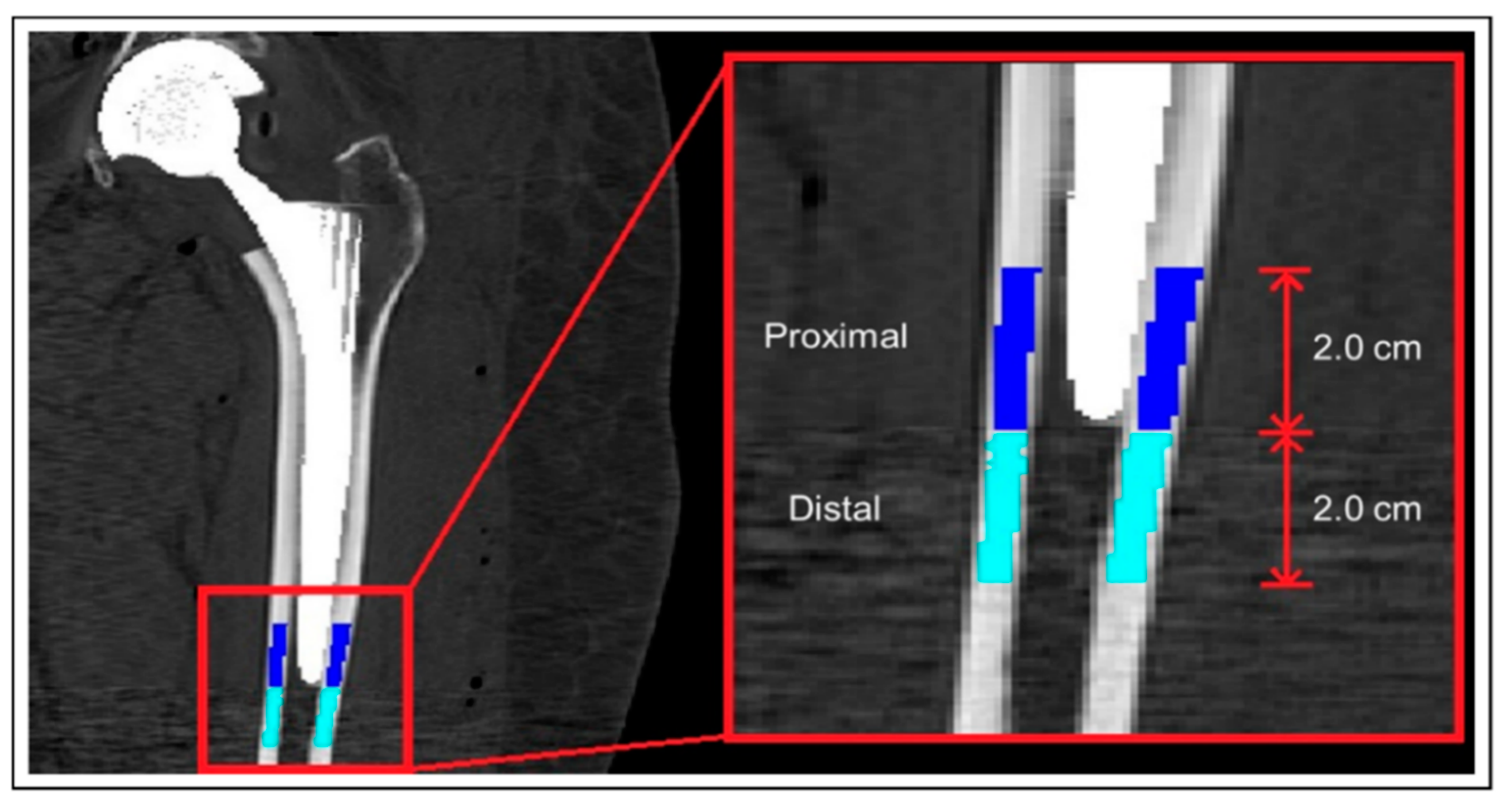
2.4. Muscle and Bone Mineral Density Calculation
2.5. Tools and Algorithms
2.6. Metrics and Workflow of the Machine Learning Analysis
- Accuracy: the number of correct predictions over the total.
- Precision: a measure of the positive patterns correctly predicted from the total predicted patterns in a positive class.
- Recall: a measure of the fraction of positive patterns that are correctly classified.
- Specificity: better known as the “true negative rate”.
- Sensitivity: better known as the “true positive rate”.
3. Results
3.1. Classification Task
3.2. Regression Task
4. Discussion
Limitations and Future Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bishop, N.E.; Ferguson, S.; Tepic, S. Porosity Reduction in Bone Cement at the Cement-Stem Interface. J. Bone Jt. Surg. Br. Vol. 1996, 78, 349–356. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshihara, Y.; Henmi, O.; Morita, M.; Shiromoto, Y.; Kawano, T.; Kanaji, A.; Ando, K.; Nakagawa, M.; Kosaki, N.; et al. Cementless total hip replacement: Past, present, and future. J. Orthop. Sci. 2009, 14, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Tyson, Y.; Rolfson, O.; Kärrholm, J.; Hailer, N.P.; Mohaddes, M. Uncemented or cemented revision stems? Analysis of 2,296 first-time hip revision arthroplasties performed due to aseptic loosening, reported to the Swedish Hip Arthroplasty Register. Acta Orthop. 2019, 90, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Venesmaa, P.K.; Kröger, H.P.J.; Miettinen, H.J.A.; Jurvelin, J.S.; Suomalainen, O.T.; Alhava, E.M. Monitoring of Periprosthetic BMD After Uncemented Total Hip Arthroplasty with Dual-Energy X-Ray Absorptiometry—A 3-Year Follow-Up Study. J. Bone Miner. Res. 2001, 16, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Engh, C.A.; Macalino, G.E.; Lauro, G.R. Outcome of revision hip arthroplasty done without cement. J. Bone Jt. Surg. Am. 1994, 76, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Moreland, J.R.; Bernstein, M.L. Femoral revision hip arthroplasty with uncemented, porous-coated stems. Clin. Orthop. 1995, 319, 141–150. [Google Scholar] [CrossRef]
- Hailer, N.P.; Garellick, G.; Kärrholm, J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010, 81, 34–41. [Google Scholar] [CrossRef]
- Radl, R.; Aigner, C.; Hungerford, M.; Pascher, A.; Windhager, R. Proximal femoral bone loss and increased rate of fracture with a proximally hydroxyapatite-coated femoral component. J. Bone Jt. Surg. Br. Vol. 2000, 82, 1151–1155. [Google Scholar] [CrossRef]
- Magnússon, B.; Pétursson, Þ.; Edmunds, K.; Magnúsdóttir, G.; Halldórsson, G.; Jonsson, H.J.; Gargiulo, P. Improving Planning and Post-Operative Assessment for Total Hip Arthroplasty. Eur. J. Transl. Myol. 2015, 25, 101. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Cauley, J.A.; Danielson, M.; Nevitt, M.C.; Lang, T.; Bauer, U.C.; Harris, T.B. Race and Sex Effects on the Association Between Muscle Strength, Soft Tissue, and Bone Mineral Density in Healthy Elders: The Health, Aging, and Body Composition Study. J. Bone Miner. Res. 2001, 16, 1343–1352. [Google Scholar] [CrossRef]
- Izzo, G.M. Support for total hip replacement surgery: Structures modeling, Gait Data Analysis and Report system. Eur. J. Trans. Myol. 2012, 22, 69–121. [Google Scholar] [CrossRef]
- Gargiulo, P.; Pétursson, T.; Magnússon, B.; Bifulco, P.; Cesarelli, M.; Izzo, G.M.; Magnúsdóttir, G.; Halldórsson, G.; Ludvigsdóttir, G.K.; Tribel, J.; et al. Assessment of Total Hip Arthroplasty by Means of Computed Tomography 3D Models and Fracture Risk Evaluation. Artif. Organs 2013, 37, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Edmunds, K.J.; Gíslason, M.K.; Latour, C.; Hermannsson, Þ.; Esposito, L.; Bifulco, P.; Cesarelli, M.; Fraldi, M.; Cristofolini, L.; et al. Patient-specific mobility assessment to monitor recovery after total hip arthroplasty. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, A.; Hejazi, H.; Kamali, A.; Hadi, H. Investigation of the outcome of patients with hip fractures using vitamin D3. Eur. J. Transl. Myol. 2018, 28. [Google Scholar] [CrossRef]
- Norouzi, A.; Behrouzibakhsh, F.; Kamali, A.; Yazdi, B.; Ghaffari, B. Short-term complications of anesthetic technique used in hip fracture surgery in elderly people. Eur. J. Transl. Myol. 2018, 28. [Google Scholar] [CrossRef]
- Improta, G.; Balato, G.; Ricciardi, C.; Russo, M.A.; SantaLucia, I.; Triassi, M.; Cesarelli, M. Lean Six Sigma in healthcare. TQM J. 2019, 31, 526–540. [Google Scholar] [CrossRef]
- Ricciardi, C.; Balato, G.; Romano, M.; SantaLucia, I.; Cesarelli, M.; Improta, G. Fast track surgery for knee replacement surgery: A lean six sigma approach. TQM J. 2020, 32, 461–474. [Google Scholar] [CrossRef]
- Ricciardi, C.; Improta, G.; Amato, F.; Cesarelli, G.; Romano, M. Classifying the type of delivery from cardiotocographic signals: A machine learning approach. Comput. Methods Programs Biomed. 2020, 105712, 105712. [Google Scholar] [CrossRef]
- Stanzione, A.; Ricciardi, C.; Cuocolo, R.; Romeo, V.; Petrone, J.; Sarnataro, M.; Mainenti, P.P.; Improta, G.; De Rosa, F.; Insabato, L.; et al. MRI Radiomics for the Prediction of Fuhrman Grade in Clear Cell Renal Cell Carcinoma: A Machine Learning Exploratory Study. J. Digit. Imaging 2020, 33, 879–887. [Google Scholar] [CrossRef]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach. Anticancer. Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Ricciardi, C.; Edmunds, K.J.; Recenti, M.; Sigurdsson, S.; Gudnason, V.; Carraro, U.; Gargiulo, P. Assessing cardiovascular risks from a mid-thigh CT image: A tree-based machine learning approach using radiodensitometric distributions. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Cantoni, V.; Improta, G.; Iuppariello, L.; Latessa, I.; Cesarelli, M.; Triassi, M.; Cuocolo, A. Application of data mining in a cohort of Italian subjects undergoing myocardial perfusion imaging at an academic medical center. Comput. Methods Programs Biomed. 2020, 189, 105343. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, H.S.; Helm, J.M.; Navarro, S.M.; Karnuta, J.M.; Schaffer, J.L.; Callaghan, J.J.; Mont, M.A.; Kamath, A.F.; Krebs, V.E.; Ramkumar, P.N. Artificial Intelligence and Machine Learning in Lower Extremity Arthroplasty: A Review. J. Arthroplast. 2019, 34, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Cabitza, F.; Locoro, A.; Banfi, G. Machine Learning in Orthopedics: A Literature Review. Front. Bioeng. Biotechnol. 2018, 6, 75. [Google Scholar] [CrossRef]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef]
- Navarro, S.M.; Wang, E.Y.; Haeberle, H.S.; Mont, M.A.; Krebs, V.E.; Patterson, B.M.; Ramkumar, P.N. Machine Learning and Primary Total Knee Arthroplasty: Patient Forecasting for a Patient-Specific Payment Model. J. Arthroplast. 2018, 33, 3617–3623. [Google Scholar] [CrossRef]
- Ramkumar, P.N.; Navarro, S.M.; Haeberle, H.S.; Karnuta, J.M.; Mont, M.A.; Iannotti, J.P.; Patterson, B.M.; Krebs, V.E. Development and Validation of a Machine Learning Algorithm After Primary Total Hip Arthroplasty: Applications to Length of Stay and Payment Models. J. Arthroplast. 2019, 34, 632–637. [Google Scholar] [CrossRef]
- Ramkumar, P.; Navarro, S.; Wang, E.Y. Small data forecasting of length of stay after primary total hip arthroplasty in the value-based payment era: Validation of a predictive big data machine learning model. In Orthopaedic Proceedings; Bone & Joint Publishing: London, UK, 2019; Volume 101, p. 89, No. SUPP_4. [Google Scholar]
- Harris, A.H.S.; Kuo, A.C.; Weng, Y.; Trickey, A.W.; Bowe, T.; Giori, N.J. Can Machine Learning Methods Produce Accurate and Easy-to-use Prediction Models of 30-day Complications and Mortality After Knee or Hip Arthroplasty? Clin. Orthop. Relat. Res. 2019, 477, 452–460. [Google Scholar] [CrossRef]
- Recenti, M.; Ricciardi, C.; Aubonnet, R.; Esposito, L.; Jonsson, H.; Gargiulo, P. A Regression Approach to Assess Bone Mineral Density of Patients undergoing Total Hip Arthroplasty through Gait Analysis. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020; pp. 1–6. [Google Scholar]
- Wolff, J. Das Gesetz der Transformation der Knochen. DMW-Dtsch. Med. Wochenschr. 1893, 19, 1222–1224. [Google Scholar] [CrossRef]
- Christen, P.P.; Ito, K.K.; Galis, F.; Van Rietbergen, B.B. Determination of hip-joint loading patterns of living and extinct mammals using an inverse Wolff’s law approach. Biomech. Model. Mechanobiol. 2014, 14, 427–432. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The Relationship Between Stress Shielding and Bone Resorption Around Total Hip Stems and the Effects of Flexible Materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Tougui, I.; Jilbab, A.; El Mhamdi, J. Heart disease classification using data mining tools and machine learning techniques. Health Technol. 2020, 10, 1137–1144. [Google Scholar] [CrossRef]
- D’Addio, G.; Ricciardi, C.; Improta, G.; Bifulco, P.; Cesarelli, M. Feasibility of Machine Learning in Predicting Features Related to Congenital Nystagmus. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing–MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; Volume 76, pp. 907–913. [Google Scholar]
- Improta, G.; Ricciardi, C.; Amato, F.; D’Addio, G.; Cesarelli, M.; Romano, M. Efficacy of Machine Learning in Predicting the Kind of Delivery by Cardiotocography. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing–MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; Volume 76, pp. 793–799. [Google Scholar]
- Recenti, M.; Ricciardi, C.; Edmunds, K.; Gislason, M.K.; Gargiulo, P. Machine learning predictive system based upon radiodensitometric distributions from mid-thigh CT images. Eur. J. Transl. Myol. 2020, 30, 8892. [Google Scholar] [CrossRef]
- Chawla, N.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Kohavi, R.; John, G.H. Wrappers for feature subset selection. Artif. Intell. 1997, 97, 273–324. [Google Scholar] [CrossRef]
- Burgess, L.; Swain, I.D.; Taylor, P.; Wainwright, T.W. Strengthening Quadriceps Muscles with Neuromuscular Electrical Stimulation Following Total Hip Replacement: A Review. Curr. Phys. Med. Rehabil. Rep. 2019, 7, 275–283. [Google Scholar] [CrossRef]
- Robbins, S.M.; Gomes, S.K.; Huk, O.L.; Zukor, D.J.; Antoniou, J. The Influence of Lateral and Posterior Total Hip Arthroplasty Approaches on Muscle Activation and Joint Mechanics During Gait. J. Arthroplast. 2020, 35, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Fraldi, M.; Cutolo, A.; Esposito, L.; D’Amore, A. Visco-elastic and thermal-induced damaging in time-dependent reshaping of human cornea after conductive keratoplasty. Mech. Time-Depend. Mater. 2016, 21, 45–59. [Google Scholar] [CrossRef]
- Fraldi, M.; Esposito, L.; Perrella, G.; Cutolo, A.; Cowin, S.C. Topological optimization in hip prosthesis design. Biomech. Model. Mechanobiol. 2009, 9, 389–402. [Google Scholar] [CrossRef]
- Minutolo, V.; Esposito, L.; Sacco, E.; Fraldi, M. Designing stress for optimizing and toughening truss-like structures. Meccanica 2020, 55, 1603–1622. [Google Scholar] [CrossRef]
- Esposito, L.; Bifulco, P.; Gargiulo, P.; Fraldi, M. Singularity-free finite element model of bone through automated voxel-based reconstruction. Comput. Methods Biomech. Biomed. Eng. 2015, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Bifulco, P.; Gargiulo, P.; Gíslason, M.; Cesarelli, M.; Iuppariello, L.; Jónsson, H.; Cutolo, A.; Fraldi, M. Towards a patient-specific estimation of intra-operative femoral fracture risk. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Gislason, M.K.; Edmunds, K.J.; Pitocchi, J.; Carraro, U.; Esposito, L.; Fraldi, M.; Bifulco, P.; Cesarelli, M.; Jónsson, H. CT-Based Bone and Muscle Assessment in Normal and Pathological Conditions. Encycl. Biomed. Eng. 2019, 3, 119–134. [Google Scholar] [CrossRef]
- Gislason, M.K.; Lupidio, F.; Jónsson, H.; Cristofolini, L.; Esposito, L.; Bifulco, P.; Fraldi, M.; Gargiulo, P. Three dimensional bone mineral density changes in the femur over 1 year in primary total hip arthroplasty patients. Clin. Biomech. 2020, 78, 105092. [Google Scholar] [CrossRef] [PubMed]
- Pétursson, Þ.; Edmunds, K.J.; Gislason, M.K.; Magnússon, B.; Magnúsdóttir, G.; Halldórsson, G.; Jónsson, H.; Gargiulo, P. Bone Mineral Density and Fracture Risk Assessment to Optimize Prosthesis Selection in Total Hip Replacement. Comput. Math. Methods Med. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Taylor, M.J.; Schils, S.J.; Ruys, A.J. Home FES: An Exploratory Review. Eur. J. Transl. Myol. 2019, 29. [Google Scholar] [CrossRef]
- Cvecka, J.; Tirptakova, V.; Sedliak, M.; Kern, H.; Mayr, W.; Hamar, D. Physical activity in elderly. Eur. J. Transl. Myol. 2015, 25, 249–252. [Google Scholar] [CrossRef]
- Šarabon, N.; Smajla, D.; Kozinc, Ž.; Kern, H. Speed-power based training in the elderly and its potential for daily movement function enhancement. Eur. J. Transl. Myol. 2020, 30, 8898. [Google Scholar] [CrossRef]
- Skyttä, E.T.; Jarkko, L.; Antti, E.; Huhtala, H.; Ville, R. Increasing incidence of hip arthroplasty for primary osteoarthritis in 30-to 59-year-old patients: A population-based study from the Finnish Arthroplasty Register. Acta Orthop. 2011, 82, 1–5. [Google Scholar] [CrossRef]
- Esposito, L.; Minutolo, V.; Gargiulo, P.; Jonsson, H.J.; Gislason, M.K.; Fraldi, M. Towards an App to Estimate Patient-Specific Perioperative Femur Fracture Risk. Appl. Sci. 2020, 10, 6409. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Training Set | Test Set | |||||||
|---|---|---|---|---|---|---|---|---|
| N° Features | Accuracy (%) | Accuracy (%) | Recall (%) | Precision (%) | Sensitivity (%) | Specificity (%) | AUCROC | |
| RF | 9 | 91.7 | 78.6 | 71.4 | 83.3 | 71.4 | 85.7 | 0.735 |
| GB | 22 | 75.0 | 92.9 | 85.7 | 100 | 85.0 | 100 | 0.857 |
| RF | GB | |
|---|---|---|
| Base of support (cm) | Base of support (cm) | Stop Healthy VM |
| BMD of the Proximal region of femur | Toe In Out Operated (angle) | Start Healthy RFe |
| SD of the BMD of the Proximal region of femur | Velocity (m/s) | Stop Healthy RFe |
| VM Operated HU | Healthy leg BMD | Start Healthy VL |
| VM Healthy HU | RFe Operated HU | Stop Healthy VL |
| Stop Healthy VM | RFe Operated Dev. Std. | Start Operated VM |
| Stop Healthy RFe | RFe Healthy Dev. Std. | Stop Operated VM |
| Start Healthy VL | VL Operated HU | Start Operated RFe |
| Start Operated VL | VL Healthy HU | Stop Operated RFe |
| VM Operated HU | Start Operated VL | |
| Start Healthy VM | Stop Operated VL | |
| R2 | Mean Absolute Error | Mean Squared Error | Root Mean Squared Deviation | Mean Signed Difference | ||
|---|---|---|---|---|---|---|
| Proximal BMD | RF | 0.634 | 0.017 | 0.001 | 0.026 | −0.004 |
| GB | 0.539 | 0.018 | 0.001 | 0.029 | −0.007 | |
| Distal BMD | RF | 0.591 | 0.02 | 0.001 | 0.029 | 0.002 |
| GB | 0.621 | 0.016 | 0.001 | 0.024 | −0.004 |
| Features | |
|---|---|
| Type of prosthesis | Start Healthy VM |
| Base of support (cm) | Stop Healthy VM |
| Double Support (% of gait cycle) | Start Healthy VL |
| Toe In Out Healthy (angle) | Stop Healthy VL |
| Toe In Out Operated (angle) | Stop Operated VM |
| SD of the BMD of the Proximal region of femur | Start Operated RFe |
| Healthy leg BMD | Stop Operated RFe |
| RFe Operated HU | Start Operated VL |
| VL Operated HU | Stop Operated VL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, C.; Jónsson, H., Jr.; Jacob, D.; Improta, G.; Recenti, M.; Gíslason, M.K.; Cesarelli, G.; Esposito, L.; Minutolo, V.; Bifulco, P.; et al. Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty. Diagnostics 2020, 10, 815. https://doi.org/10.3390/diagnostics10100815
Ricciardi C, Jónsson H Jr., Jacob D, Improta G, Recenti M, Gíslason MK, Cesarelli G, Esposito L, Minutolo V, Bifulco P, et al. Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty. Diagnostics. 2020; 10(10):815. https://doi.org/10.3390/diagnostics10100815
Chicago/Turabian StyleRicciardi, Carlo, Halldór Jónsson, Jr., Deborah Jacob, Giovanni Improta, Marco Recenti, Magnús Kjartan Gíslason, Giuseppe Cesarelli, Luca Esposito, Vincenzo Minutolo, Paolo Bifulco, and et al. 2020. "Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty" Diagnostics 10, no. 10: 815. https://doi.org/10.3390/diagnostics10100815
APA StyleRicciardi, C., Jónsson, H., Jr., Jacob, D., Improta, G., Recenti, M., Gíslason, M. K., Cesarelli, G., Esposito, L., Minutolo, V., Bifulco, P., & Gargiulo, P. (2020). Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty. Diagnostics, 10(10), 815. https://doi.org/10.3390/diagnostics10100815