Liquid Biopsy in Pediatric Renal Cancer: Stage I and Stage IV Cases Compared
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients’ Clinical Characteristics
2.1.1. Informed Consent
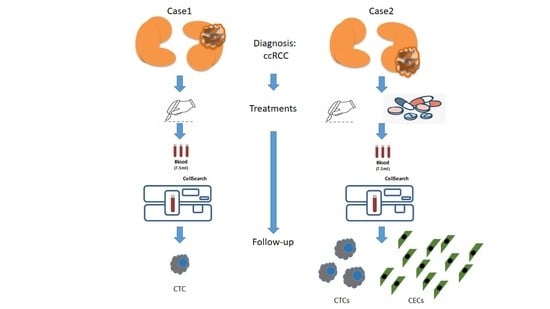
2.1.2. Case 1—Presentation
2.1.3. Case 2—Presentation
2.2. Total, M30-Positive and MET-Positive CTC and CEC Enumeration
2.3. Genomic Analysis
3. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
References
- Stiller, C.A.; Marcos-Gragera, R.; Ardanaz, E.; Pannelli, F.; Almar Marques, E.; Canada Martinez, A.; Steliarova-Foucher, E. Geographical patterns of childhood cancer incidence in Europe, 1988–1997. Report from the automated childhood cancer information system project. Eur. J. Cancer 2006, 42, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Brok, J.; Treger, T.D.; Gooskens, S.L.; van den Heuvel-Eibrink, M.M.; Pritchard-Jones, K. Biology and treatment of renal tumours in childhood. Eur. J. Cancer 2016, 68, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Selle, B.; Furtwangler, R.; Graf, N.; Kaatsch, P.; Bruder, E.; Leuschner, I. Population-based study of renal cell carcinoma in children in Germany, 1980–2005: More frequently localized tumors and underlying disorders compared with adult counterparts. Cancer 2006, 107, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, P.; Terenziani, M.; Casale, F.; Carli, M.; Bisogno, G.; Schiavetti, A.; Mancini, A.; Rondelli, R.; Pession, A.; Jenkner, A.; et al. Renal cell carcinoma in children: A clinicopathologic study. J. Clin. Oncol. 2003, 21, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Malkan, A.D.; Loh, A.; Bahrami, A.; Navid, F.; Coleman, J.; Green, D.M.; Davidoff, A.M.; Sandoval, J.A. An approach to renal masses in pediatrics. Pediatrics 2015, 135, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Dome, J.S.; Fernandez, C.V.; Mullen, E.A.; Kalapurakal, J.A.; Geller, J.I.; Huff, V.; Gratias, E.J.; Dix, D.B.; Ehrlich, P.F.; Khanna, G.; et al. Children’s oncology group’s 2013 blueprint for research: Renal tumors. Pediatric Blood Cancer 2013, 60, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; Miller, M.C.; et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Basso, U.; Facchinetti, A.; Rossi, E.; Maruzzo, M.; Conteduca, V.; Aieta, M.; Massari, F.; Fraccon, A.P.; Mucciarini, C.; Sava, T.; et al. Prognostic role of circulating tumor cells-CTCs in metastatic renal cell carcinoma. J. Clin. Oncol. 2017, 35 (Suppl. 15), 4568. [Google Scholar] [CrossRef]
- Rossi, E.; Fassan, M.; Aieta, M.; Zilio, F.; Celadin, R.; Borin, M.; Grassi, A.; Troiani, L.; Basso, U.; Barile, C.; et al. Dynamic changes of live/apoptotic circulating tumour cells as predictive marker of response to sunitinib in metastatic renal cancer. Br. J. Cancer 2012, 107, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.G.; Shusterman, S.; Ingle, A.M.; Ahern, C.H.; Reid, J.M.; Wu, B.; Baruchel, S.; Glade-Bender, J.; Ivy, P.; Grier, H.E.; et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: A children’s oncology group study. Clin. Cancer Res. 2011, 17, 5113–5122. [Google Scholar] [CrossRef]
- Rowand, J.L.; Martin, G.; Doyle, G.V.; Miller, M.C.; Pierce, M.S.; Connelly, M.C.; Rao, C.; Terstappen, L.W.M.M. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytom. A 2007, 71, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Basso, U.; Celadin, R.; Zilio, F.; Pucciarelli, S.; Aieta, M.; Barile, C.; Sava, T.; Bonciarelli, G.; Tumolo, S.; et al. M30 neoepitope expression in epithelial cancer: Quantification of apoptosis in circulating tumor cells by CellSearch analysis. Clin. Cancer Res. 2010, 16, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, P.; Pratap, J.; Tiwari, P.; Bera, M.K.; Kundu, A.K. Renal cell carcinoma in children and adolescence: Our experience. Afr. J. Paediatr. Surg. 2014, 11, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Boominathan, R.; Foulk, B.; Rao, C.; Kemeny, G.; Strickler, J.H.; Abbruzzese, J.L.; Harrison, M.R.; Hsu, D.S.; Healy, P.; et al. Development of a Novel c-MET-based CTC detection platform. Mol. Cancer Res. 2016, 14, 539–547. [Google Scholar] [CrossRef]
- Shojaei, F.; Lee, J.H.; Simmons, B.H.; Wong, A.; Esparza, C.O.; Plumlee, P.A.; Feng, J.; Stewart, A.E.; Hu-Lowe, D.D.; Christensen, J.G. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010, 70, 10090–10100. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Spellman, P.T.; Ricketts, C.J.; Creighton, C.J.; Fei, S.S.; Davis, C.; Wheeler, D.A.; Murray, B.A.; Schmidt, L.; Vocke, C.D.; et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 2016, 374, 135–145. [Google Scholar] [PubMed]
| pt | Date of Diagnosis | Date of Follow-Up/Treatments | Blood Draw Date | CTCs/7.5 mL | M30+/Sample | MET+/Sample | CECs/4 mL | Treatments | IMAGING/Pathological Evaluation | Report | Objective Response |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19-Sep-17 | MRI | renal lesion | ||||||||
| 5-Oct-17 | needle biopsy | ccRCC | |||||||||
| 13-Oct-17 | nephrectomy | ||||||||||
| 3-Nov-17 | 1 | nd | 1 | 28 | |||||||
| 21-Feb-18 | 0 | nd | 0 | nd | CR | ||||||
| 2 | 5-May-17 | 3 | 3 | CT scan | renal lesion 4.4 cm × 4.2cm × 5.5 cm; LN and lung invasion | ||||||
| 9-May-17 | nephrectomy + lymphadenectomy | histology of surgical sample | ccRCC + LN invasion | ||||||||
| 22-Jun-17 | sunitinib (1st cycle) | ||||||||||
| 19-Jul-17 | 1 | nd | 1 | 91 | |||||||
| 28-Jul-17 | CT scan | PD | |||||||||
| 2-Aug-17 | sunitinib (2nd cycle) | ||||||||||
| 13-Sep-17 | sunitinib (3th cycle) | ||||||||||
| 6-Oct-17 | further sunitinib cycles | CT scan | reduction of lung lesions | PR | |||||||
| 19-Oct-17 | 2 | 2 | nd | 10 | |||||||
| 1-Jan-18 | resection of brain metastasis | MRI | 4.5 cm cerebral metastasis | PD | |||||||
| 16-Jan-18 | 16-Jan-18 | 0 | 0 | nd | 323 | CT scan | increase of lung lesions | PD | |||
| 29-Jan-18 | CT scan | lung carcinosis, visceral metastasis | PD | ||||||||
| 31-Jan-18 | 1 | 0 | nd | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, E.; Zin, A.; Facchinetti, A.; Poggiana, C.; Tombolan, L.; Affinita, M.C.; Bonvini, P.; Santoro, L.; Schiavi, F.; Bisogno, G.; et al. Liquid Biopsy in Pediatric Renal Cancer: Stage I and Stage IV Cases Compared. Diagnostics 2020, 10, 810. https://doi.org/10.3390/diagnostics10100810
Rossi E, Zin A, Facchinetti A, Poggiana C, Tombolan L, Affinita MC, Bonvini P, Santoro L, Schiavi F, Bisogno G, et al. Liquid Biopsy in Pediatric Renal Cancer: Stage I and Stage IV Cases Compared. Diagnostics. 2020; 10(10):810. https://doi.org/10.3390/diagnostics10100810
Chicago/Turabian StyleRossi, Elisabetta, Angelica Zin, Antonella Facchinetti, Cristina Poggiana, Lucia Tombolan, Maria Carmen Affinita, Paolo Bonvini, Luisa Santoro, Francesca Schiavi, Gianni Bisogno, and et al. 2020. "Liquid Biopsy in Pediatric Renal Cancer: Stage I and Stage IV Cases Compared" Diagnostics 10, no. 10: 810. https://doi.org/10.3390/diagnostics10100810
APA StyleRossi, E., Zin, A., Facchinetti, A., Poggiana, C., Tombolan, L., Affinita, M. C., Bonvini, P., Santoro, L., Schiavi, F., Bisogno, G., & Zamarchi, R. (2020). Liquid Biopsy in Pediatric Renal Cancer: Stage I and Stage IV Cases Compared. Diagnostics, 10(10), 810. https://doi.org/10.3390/diagnostics10100810